Dataset for the reporting of carcinoma of the exocrine pancreas: recommendations from the International Collaboration on Cancer Reporting (ICCR)
Abstract
Aims
Current guidelines for pathology reporting on pancreatic cancer differ in certain aspects, resulting in divergent reporting practices and a lack of comparability of data. Here, we report on a new international dataset for pathology reporting on resection specimens with cancer of the exocrine pancreas (ductal adenocarcinoma and acinar cell carcinoma). The dataset was produced under the auspices of the International Collaboration on Cancer Reporting (ICCR), which is a global alliance of major (inter)national pathology and cancer organisations.
Methods and results
According to the ICCR’s rigorous process for dataset development, an international expert panel consisting of pancreatic pathologists, a pancreatic surgeon and an oncologist produced a set of core and non-core data items based on a critical review and discussion of current evidence. Commentary was provided for each data item to explain the rationale for selecting it as a core or non-core element and its clinical relevance, and to highlight potential areas of disagreement or lack of evidence, in which case a consensus position was formulated. Following international public consultation, the document was finalised and ratified, and the dataset, which includes a synoptic reporting guide, was published on the ICCR website.
Conclusions
This first international dataset for cancer of the exocrine pancreas is intended to promote high-quality, standardised pathology reporting. Its widespread adoption will improve the consistency of reporting, facilitate multidisciplinary communication, and enhance the comparability of data, all of which will help to improve the management of pancreatic cancer patients.
Graphical Abstract
Introduction
Pathology reporting on cancer resection specimens provides information that is essential to the management of the individual patient, is used for clinical trials and tissue-based research, and is recorded in cancer registries. Given this central role of pathology data in cancer care at an individual level and a population level, standardised and structured pathology reporting is essential to ensure that relevant information is complete, unambiguous, and delivered in a user-friendly format. Several organisations worldwide have independently developed datasets for pathology reporting on cancer of the exocrine pancreas.1-4 Although these are broadly similar, differences in content, structure and terminology may affect the comparability of data between countries. Moreover, existing datasets are mainly limited to the content of the pathology report, and lack guidance regarding practical aspects of the examination process that are essential for the provision of accurate data. These concerns pertain especially to reporting on pancreatic cancer specimens, which is often perceived as challenging, owing to the complexity of the surgical specimens and the divergence of recommendations issued by national and international organisations.
The International Collaboration on Cancer Reporting (ICCR) coordinates the production of evidence-based international pathology reporting datasets that have a consistent style and contain all of the parameters needed to guide patient management. The ICCR is a collaboration of multiple pathology organisations, and has alliances with international cancer organisations, including the International Agency for Research on Cancer, Union for International Cancer Control (UICC), and American Joint Committee on Cancer (AJCC). The ICCR datasets are freely available from the ICCR website (http://www.iccr-cancer.org).
Here, we report on the development of the dataset for pathology reporting on resection specimens with cancer of the exocrine pancreas, discuss the rationale for the inclusion of data items, and propose a consensus position in areas of controversy and where there is limited evidence to assist pathologists in their diagnostic practice.
Methods
In accordance with the ICCR procedure for the development of cancer datasets, the Dataset Steering Committee (DSC) appointed a Series Champion (K.W.) and a Chair (C.V.). The responsibility of the former was to coordinate the development of a series of datasets for hepatopancreatobiliary cancer and ensure harmonisation across datasets, and the Chair oversaw the development of the dataset for pancreatic cancer. Together, they identified 10 other expert pancreatic pathologists who, together with the Chair, two clinicians, and Project Managers (F.W. and Christina Selinger), formed the Dataset Authoring Committee (DAC). The expert panel included pathologists from the USA (A.M.K. and S.K.), the UK (F.C. and R.F.), Europe (I.E. and L.B.), Canada (D.F.S.), Australia (A.G. and J.K.) and Japan (N.F.), as well as a pancreatic surgeon (M.D.C, USA) and oncologist (J.-L.vL., Europe).
In line with other ICCR datasets, the pancreatic cancer dataset included a number of elements, categorised as core or non-core, with a reporting guide accompanied by a commentary for each element. Core elements were determined on the basis of whether they were considered to be essential for clinical management, staging, or prognosis, and whether they had evidentiary support at Level III-2 or above (based on prognostic factors in the National Health and Medical Research Council levels of evidence5). In the absence of such evidence, an element was considered to be core if there was unanimous agreement by the DAC. Non-core elements were elements categorised as lacking Level III-2 evidence but that were unanimously considered to be clinically important and part of good practice, albeit not yet sufficiently validated or regularly used in patient management.
The initial working draft of the dataset was developed by the Project Managers on the basis of a review of all published, relevant pathology datasets and guidelines. Following editing by the Chair, the draft was circulated to the DAC and discussed in a series of teleconferences. On the basis of these discussions, the Chair edited the dataset and recirculated it to the DAC for further review via e-mail communications until consensus was reached. The dataset was posted on the ICCR website for open international consultation for a period of 8 weeks. The dataset was reviewed in response to feedback received, approved by the DAC, and ratified by the DSC.
Results
SCOPE
The dataset has been developed for resection specimens with carcinoma of the exocrine pancreas, i.e. ductal adenocarcinoma or acinar cell carcinoma. Neuroendocrine neoplasia, lymphoma, sarcoma and secondary malignancy were excluded. Also excluded were carcinomas of the ampulla of Vater, common bile duct and duodenum, because the criteria for assignment of T category and N category, and treatment and prognosis, differ from those for pancreatic cancer.
Correct identification of cancer origin is important and is primarily based on detailed macroscopic and microscopic assessment, in particular the location of the centre of the tumour mass.6-8 The presence of precursor lesions [e.g. dysplasia in the ampulla or duodenum, high-grade pancreatic intraepithelial neoplasia (PanIN), and intraductal papillary mucinous neoplasia (IPMN) with high-grade dysplasia] may be helpful in identifying cancer origin, although these are often lacking or, in the case of low-grade PanIN, of no evidentiary support.9 Furthermore, intestinal/pancreatobiliary-type tumour morphology and marker expression (cytokeratin 20, CDX2, mucin 1, and mucin 2) may be useful.10 In duodenal adenocarcinoma, morphology and immunohistochemical phenotype are heterogeneous and may overlap with pancreatobiliary cancer.11
CORE AND NON-CORE ELEMENTS
The agreed core and non-core elements are summarised in Table 1 and described in further detail below.
| Core | Non-core |
|---|---|
| Neoadjuvant therapy |
Tumour dimensions
|
| Operative procedure |
Extent of invasion
|
| Tumour focality |
Lymphatic and venous invasion
|
| Tumour site |
Margin status
|
|
Tumour dimensions
|
Additional findings |
| Histological tumour type | Ancillary studies |
| Histological tumour grade | |
| Extent of invasion | |
| Lymphatic and venous invasion | |
| Perineural invasion | |
| Response to neoadjuvant therapy | |
| Margin status | |
| Lymph node status | |
| Histologically confirmed distant metastases | |
| Pathological staging |
Neoadjuvant therapy
Information regarding the administration of neoadjuvant chemo(radio)therapy is essential for the pathologist, as it can have a profound effect on morphology, has implications for specimen sampling and histological interpretation, and requires assessment of the effect of treatment (core element).
Operative procedure
As a range of surgical procedures are used, depending on the tumour site, size, and extent, the type of surgical specimen should be recorded (core element). For extended resection specimens, the tissue(s) or organ(s) that are resected en bloc, e.g. the left adrenal gland, should be clearly indicated.
Tumour focality
Truly multifocal pancreatic cancer is rare, but may occur, for example, in the context of IPMN. Tumour focality is determined with a combination of macroscopic and microscopic assessment (core element). If there are multiple tumours in a specimen, it is recommended to complete a single dataset, in which the number of individual tumours and their sites and dimensions are recorded.
Tumour site
Determination of the tumour site (core element)—pancreatic head (including the uncinate process), body, and tail—is based on clinical information combined with macroscopic specimen assessment. If a tumour involves more than one anatomical region, each site should be recorded. For multifocal cancer, the location of the largest tumour should be selected, and the sites of smaller tumours should be specified under ‘other’. On rare occasions, tumour may not be visible macroscopically following neoadjuvant treatment with complete tumour regression.
Tumour dimensions
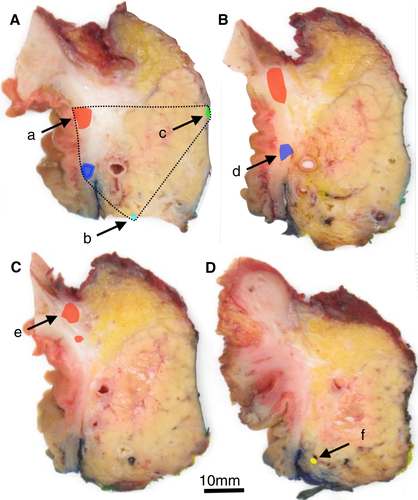
Because pT-category assignment is based on tumour size,12 the maximum dimension of the tumour is a core element. Tumours are usually not perfectly spherical, so identification of the largest tumour diameter requires three-dimensional measurement. The additional tumour dimensions and the sizes of possible smaller tumours may be recorded as non-core data. Assessment is based on macroscopic evaluation and microscopic confirmation/correction. The latter is important, because the dispersed growth pattern of ductal adenocarcinoma of the pancreas13 or the presence of peritumoral fibrosis may result in macroscopic underestimation or overestimation, respectively. A tumour should be measured in three dimensions such that the largest dimension can be correctly identified. Because tumours in the pancreatic body or tail often have their largest dimension along the length of the pancreas, tumour size must also be assessed across the sagittal specimen slices. Similar considerations apply to the measurement of tumours in the pancreatic head.
Measurement of tumour size may be challenging following neoadjuvant treatment, especially in cases of good treatment effect, when only a few scattered residual cancer foci are present (Figures 1 and 2). Currently, there is no international consensus on how best to measure tumour size in this setting. Two approaches can be used: (i) summation of the maximum dimensions of the different individual tumour foci; or (ii) measurement of the greatest linear dimension of the entire area involved by cancer cells, including non-cancerous tissue. Both approaches have disadvantages that may result in imperfect and, at times, considerably divergent size assessment. Hence, it is recommended to record the approach that is used (non-core).
Histological tumour type
Tumour type determined according to the World Health Organization (WHO) classification of tumours of the gastrointestinal tract, 5th edition, 2019 (Table 2) is a core element.14 Ductal adenocarcinoma accounts for 90% of all pancreatic malignancies, whereas acinar cell carcinoma represents <2% of all pancreatic cancers in adults. Correct diagnosis of the various subtypes of ductal adenocarcinoma is important, as they may differ in terms of prognosis, treatment response, and molecular profile. Invasive carcinoma that has arisen from a neoplastic precursor lesion, e.g. from a mucinous cystic neoplasm, should be recorded under the corresponding histological tumour type in accordance with the WHO classification.14
| Descriptor | ICD-O codes |
|---|---|
| Ductal adenocarcinoma NOS | 8500/3 |
| Colloid carcinoma | 8480/3 |
| Poorly cohesive carcinoma | 8490/3 |
| Signet-ring cell carcinoma | 8490/3 |
| Medullary carcinoma NOS | 8510/3 |
| Adenosquamous carcinoma | 8560/3 |
| Hepatoid carcinoma | 8576/3 |
| Large cell carcinoma with rhabdoid phenotype | 8014/3 |
| Carcinoma, undifferentiated, NOS | 8020/3 |
| Undifferentiated carcinoma with osteoclast-like giant cells | 8035/3 |
| Acinar cell carcinoma | 8550/3 |
| Acinar cell cystadenocarcinoma | 8551/3 |
| Mixed acinar–neuroendocrine carcinoma | 8154/3 |
| Mixed acinar–endocrine–ductal carcinoma | 8154/3 |
| Mixed acinar–ductal carcinoma | 8552/3 |
- © World Health Organization/International Agency for Research on Cancer. Reproduced with permission.
Histological tumour grade
Histological tumour grade is a core element, as it has prognostic significance.15-17 Currently, the WHO and the UICC/AJCC propose different systems for the grading of differentiation,12, 14, 18-20 but the results are highly concordant and have similar predictive values.21 The UICC/AJCC system, which is based on the proportion of the tumour that is composed of glands, is recommended because it is more widely used and less complex than the WHO system. By consensus, acinar cell carcinoma is not graded for histological tumour differentiation, and neither is pancreatic ductal adenocarcinoma following neoadjuvant treatment.
Extent of invasion
According to the 8th edition of the UICC TNM classification of malignant tumours12 and the 8th edition of the AJCC cancer staging manual,18 tumour size is the exclusive criterion for T categories pT1–pT3. Only pT4 tumours remain defined by the extent of tumour invasion (involvement of the common hepatic artery, superior mesenteric artery, and/or coeliac axis), but these are, with very few exceptions,22 considered to be unresectable. Although most features related to the extent of tumour invasion have become irrelevant for T-category assignment, they are still considered to be core elements, because they represent information that is important for correlation with preoperative imaging, evaluation of resectability, and intraoperative assessment. This pertains particularly to tumours that infiltrate named blood vessels or neighbouring organs, and require resection with an extended surgical procedure.23
The presence or absence of tumour invasion in these additionally resected structures should therefore be recorded (core element). In contrast, the depth of tumour invasion into the wall of a resected blood vessel is considered to be a non-core element, because its prognostic value is insufficiently supported by evidence.24, 25
Lymphatic and venous invasion
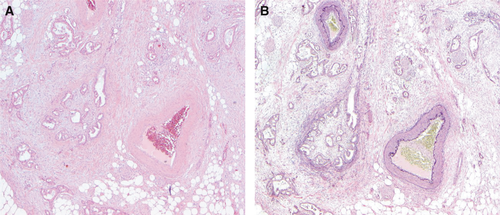
The presence of invasion of lymph channels or veins is a core element, because it correlates with survival, albeit less strongly than tumour stage.14, 26-29 As tumour invasion of lymphatic and tumour invasion of venous vessels represent different biological processes with different outcomes, i.e. lymph node metastasis or distant, blood-borne spread, these features should be recorded separately, as recommended by the 8th edition of the UICC TNM classification of malignant tumours,12 and not as combined ‘lymphovascular invasion’. Because it may be difficult to distinguish between lymphatic and venous invasion, both data elements are considered to be non-core. In practice, the ‘orphan arteriole’ sign and elastin staining (Figure 3) or immunohistochemistry (caldesmon and podoplanin/D2-40) may be helpful.29
Perineural invasion
Involvement of intrapancreatic and extrapancreatic nerves, which is a common finding in pancreatic ductal adenocarcinoma, is an adverse prognostic factor30-32 and should be recorded as a core element.
Response to neoadjuvant therapy
The response to neoadjuvant treatment should be recorded as a core element, as it reflects the tissue-based result of clinical intervention. Moreover, complete and nearly complete response correlate with better prognosis.33
Various scoring systems exist, but comparative data regarding interobserver variability and prognostic significance are largely lacking; therefore, further investigation is required before an optimal scheme is identified.34-38 Until there is a clear consensus on the best scheme, the modified Ryan scheme (included in the College of American Pathologists guidelines for reporting pancreatic carcinomas3) is recommended, because the four-point scoring scale39 is based on non-numeric criteria and the evaluation of the residual cancer (not the proportion of tumour that has been destroyed), which makes it easier to use.35 Although current evidence does not show a difference in prognosis between score 2 and score 3,40 it is deemed important to retain these scores, in order to risk-stratify the majority of patients (>80%) who fall into both categories. For that reason, the modified system proposed by Chatterjee,33 which has merged score 2 and 3 into a single group, is not recommended. Other recently proposed regression grading schemes may have merit but require further validation studies in independent cohorts.34, 36, 41-43
Accurate evaluation of tumour regression requires extensive sampling of lesional tissue. In cases of complete tumour regression, the entire tumour bed and any adjacent abnormal-looking tissues should be processed for histological examination.
Given the lack of a validated scoring system for the regression of lymph node metastases, assessment of treatment effect is currently limited to the primary tumour.
Margin status
Margin assessment is based on combined macroscopic and microscopic measurement. Because margin involvement may be a focal, macroscopically indiscernible finding, extensive sampling is important for accurate assessment of the margin status.44, 45
If a transection margin was submitted for intraoperative examination, the frozen section findings should be taken into consideration when the final pathology report is issued.
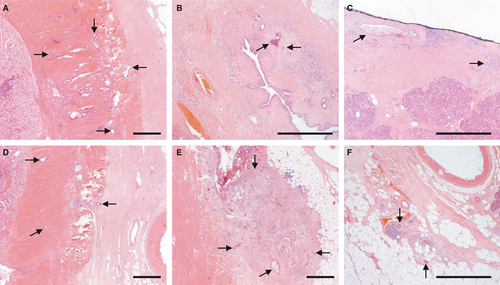
‘R1’ is defined by the UICC12 and AJCC18 as microscopic residual disease, i.e. irrespective of whether tumour is left behind at a surgical resection margin or at a non-surgical tissue plane. Assessment of the margin status should therefore encompass evaluation of all surfaces of the specimen, including the anterior pancreatic surface and the surface of the superior mesenteric vein groove (Figure 4). Studies based on a standardised examination protocol that includes evaluation of all surfaces reported a high R1 rate (>70%) that correlates with survival.46-49 The margin status is therefore considered to be a core element.
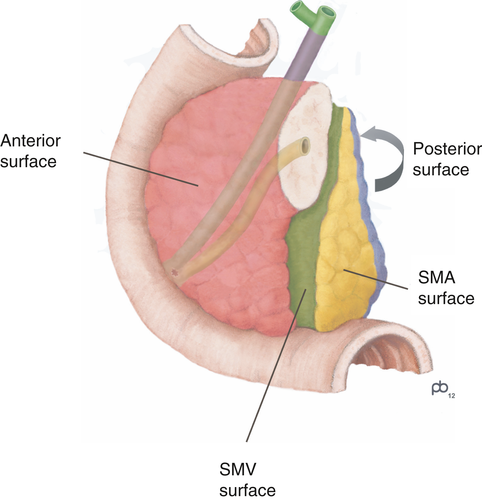
A margin is generally considered to be positive if the tumour is ≤1 mm from the margin. However, this definition of microscopic margin involvement is somewhat contentious, as larger clearances of up to 2 mm were found to be prognostically relevant.50-52 Because the anterior pancreatic surface is a peritonealised anatomical surface, involvement is defined by breaching of the surface, i.e. a clearance of 0 mm. While further evidence is awaited, assessment of the margin status based on R1 defined as a clearance of 1 mm (or 0 mm for the anterior surface) is recommended by the AJCC and other professional bodies.1-3, 18 If the minimum clearance is found to be <1 mm, more accurate measurement to the tenth of a millimetre is not required, as this may vary between section levels.
Recording of the distance of the tumour to the margins if the clearance is >1 mm is recommended (non-core). This is particularly important for ductal adenocarcinomas following neoadjuvant treatment, because, in these tumours, a clearance of >1 mm does not necessarily reflect an absence of microscopic residual disease. An appropriate definition of R1 in this setting has not been established.53 Similarly, recording of the minimum distance to the closest margin(s) is also recommended for acinar cell carcinoma, which has a less dispersed growth pattern than ductal adenocarcinoma, such that R1 based on clearance of 1 mm is inappropriate.
By consensus, diagnosing macroscopic residual disease (R2) is the surgeon’s responsibility, so these data are not included in the dataset.
Lymph node status
Regional lymph node status is one of the most potent predictors of survival for patients with ductal adenocarcinoma of the pancreas (core element).27, 54-58 All lymph nodes in the resection specimen should be examined histologically. The lymph node yield for Whipple’s resection specimens should be at least 12.59-61 For distal pancreatectomy specimens, this has not been established. Separately submitted regional lymph nodes should be included in the overall number.
Currently, there is insufficient evidence to include lymph node micrometastasis (<2 mm or isolated tumour cells) or extranodal tumour extension in the dataset.62, 63
Histologically confirmed distant metastases
Distant metastasis, including spread to extraregional lymph nodes (e.g. para-aortic), is associated with a poor prognosis.64-66 Both the presence and site(s) of distant/extraregional metastasis should be recorded (core element).
Pathological staging
The tumour stage of ductal adenocarcinoma and acinar cell carcinoma should be assigned according to the criteria of the 8th editions of the UICC12 and AJCC18 staging systems (core element).
If there are multiple synchronous cancers, stage should be based on the largest tumour (recorded as ‘pTm’) and overall lymph node status. The prefix ‘y’ should be used in cases of preoperative treatment.
Additional findings
The information recorded in this non-core element refers to any diagnostic lesion that is found in addition to the index lesion.
Ancillary studies
Ancillary immunohistochemical or molecular analyses are considered to be non-core, as they are not recommended for routine diagnostics.
Discussion
It is well established that structured pathology reporting ensures that data are complete and leads to better multidisciplinary communication, greater clinician satisfaction, and easier data extraction by cancer registries.67 Currently, several national datasets and reporting checklists exist for pancreatic cancer. However, they show important differences and lack guidance on key aspects of pathology examination, which may reduce the international comparability of data, e.g. regarding margin status.68, 69 Here, we describe the development of the first internationally agreed dataset for reporting on resection specimens with cancer of the exocrine pancreas. To promote widespread uptake with the aim of improving the quality of pancreatic cancer reporting globally, the dataset and structured reporting template are freely available at the ICCR website.
The process of developing the dataset revealed divergent practices related to a number of core data elements, the cause of which is essentially a lack of evidence. In particular, the use of neoadjuvant chemo(radio)therapy as part of the standard treatment for pancreatic cancer has resulted in areas of uncertainty, especially those related to the evaluation of tumour size, margin status, and treatment effect.36, 37, 70, 71 This illustrates the need for research that focuses on concrete diagnostic challenges, and the importance of regular review of the dataset to align routine pathology reporting with cancer care. Last but not least, worldwide standardised reporting will allow the establishment of benchmarking metrics that define good practice. Although this is an essential part of quality assessment in diagnostic pathology more generally, it is currently inadequate for the reporting of pancreatic cancer.
Conflicts of interest
C. Verbeke has received honoraria for consulting for FibroGen Inc. M. Del Chiaro has been awarded an industry grant from Haemonetics Inc. and is a co-PI of a Boston Scientific-sponsored multicentre international study on the use of intraoperative pancreatoscopy in patients with IPMN. The funders had no role in the design of the study, the results, the writing of the manuscript, or the decision to publish the results.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Acknowledgement
The authors are extremely grateful to Christina Selinger for her expert and invaluable assistance in the preparation of this manuscript, along with the wider project team at ICCR.