Efficacy and safety of fibrinogen concentrate for on-demand treatment of bleeding and surgical prophylaxis in paediatric patients with congenital fibrinogen deficiency
Abstract
Background
Congenital fibrinogen deficiency (CFD) is a rare, inherited disorder affecting normal blood clotting function, where patients can experience severe and/or frequent bleeding episodes (BEs). Treatment with human fibrinogen concentrate (HFC) can prevent/arrest bleeding. There is a need for more data on the efficacy, pharmacokinetics (PK) and safety of HFC treatment in paediatric patients with CFD.
Methods
Haemostatic efficacy of HFC (Fibryga®, Octapharma AG) for on-demand treatment of bleeding and surgical prophylaxis in patients <12 years old was assessed by investigators and an Independent Data Monitoring and Endpoint Adjudication Committee (IDMEAC) based on an objective 4-point efficacy scale. Maximum clot firmness (MCF; surrogate marker of haemostatic efficacy), single-dose PK and safety were also assessed.
Results
Of 14 patients receiving HFC (median [range] age 6.0 years [1.0–10.0]), eight received HFC for 10 BEs, three for surgical prophylaxis and 13 for PK. The IDMEAC rated haemostatic efficacy as 100% successful for on-demand BE treatment (95% CI 69.15–100.00) and surgical prophylaxis (95% CI 29.24–100.00). After a mean first dose of 70.78 mg/kg for BEs, mean (±SD) MCF significantly increased from pre-treatment to 1-hour post-infusion (3.3 mm [±1.77]; P = 0.0002), coinciding with haemostatic efficacy. PK parameters were favourable. Two possibly related adverse events occurred, including one serious (portal vein thrombosis). No allergic/hypersensitivity reactions or deaths were observed.
Conclusion
HFC treatment for on-demand treatment of BEs and surgical prophylaxis was efficacious for this ultra-rare paediatric population with congenital afibrinogenaemia and showed a favourable PK and safety profile.
1 INTRODUCTION
Congenital fibrinogen deficiency (CFD) is a rare disorder affecting 1–2 per million people in the general population.1 The two major types of CFD are classified as afibrinogenaemia (complete absence of fibrinogen) and hypofibrinogenaemia (proportional decrease of functional and antigenic fibrinogen levels).2 Fibrinogen deficiency can lead to inadequate blood clot formation, resulting in excessive bleeding even after minor tissue injuries, and frequent spontaneous bleeding episodes (BEs) of varying frequencies/severities.3
Historically, CFD treatment consisted of fibrinogen replacement therapy with cryoprecipitate or fresh frozen plasma (FFP) 4, 5; however, these both have several limitations as they contain several other plasma proteins, have variable fibrinogen levels, carry potential risks of pathogen transmission due to lack of virus inactivation, and require crossmatching and preparation/thawing before infusion.6, 7
Virus-inactivated products, such as solvent/detergent-treated plasma or methylene blue-treated cryoprecipitate, address the risk of pathogen transmission but their potential to correct plasma fibrinogen levels in patients with CFD is limited compared to human fibrinogen concentrate (HFC). HFC represents the current standard of fibrinogen replacement in CFD; it undergoes virus inactivation and has rapid preparation times without the requirement of blood group matching or thawing, a well-defined fibrinogen content allowing accurate and standardised dosing, few extraneous proteins and smaller infusion volumes.5, 6, 8, 9 Plasma-derived HFCs are proven to be efficacious with favourable safety profiles in CFD treatment.10-13
We previously reported data from a Phase 3 study on the efficacy and safety of HFC (Fibryga®, Octapharma AG) in adult/adolescent patients with CFD (FORMA-02).12 However, there is a need for more prospective data on efficacy and safety of HFC in paediatric patients with CFD. Here, we present the results of a Phase 3 study of this HFC for on-demand acute BE treatment, surgical prophylaxis and single-dose pharmacokinetics (PK) in paediatric patients with CFD.
2 METHODS
2.1 Study design
FORMA-04 (NCT02408484) was a multinational, multicentre, prospective, open-label, uncontrolled Phase 3 study to assess efficacy, single-dose PK and safety of HFC for on-demand acute BE treatment and surgical prophylaxis in paediatric patients with CFD.14 The provided HFC included a transfer device, promoting rapid reconstitution.15 Patients received individually dosed HFC for on-demand acute BE treatment, or surgical prophylaxis, as previously described 12 and outlined further in the Appendix S1. Data were analysed for the full study population of paediatric patients, as well as two age subgroups (<6 years and ≥6–<12 years).
2.2 Study population and inclusion/exclusion criteria
Patients from the full analysis set (FAS) were recruited from five sites; India (4 patients from 3 sites), Lebanon (8 patients from 1 site) and the Islamic Republic of Iran (2 patients from 1 site). All patients were aged <12 years at the start of treatment, had documented congenital afibrinogenaemia/severe hypofibrinogenaemia and were expected to undergo on-demand acute BE treatment, or planning to undergo elective surgery. Informed consent was obtained from each patients’ legal guardian. Key inclusion/exclusion criteria are presented in Appendix S1: Table S1.
2.3 Haemostatic efficacy evaluation
Haemostatic efficacy evaluation for on-demand BE treatment and surgical prophylaxis was conducted using an objective 4-point efficacy scale (excellent, good, moderate and none), as previously described 12 and outlined in Appendix S1: Tables S2–S4. All clinical efficacy assessments were adjudicated by an Independent Data Monitoring and Endpoint Adjudication Committee (IDMEAC) which was specified as the final efficacy analysis. Overall haemostatic efficacy was assessed using a 2-point efficacy scale: treatment success (rating of excellent/good) or treatment failure (rating of moderate/none).
2.4 Clot strength—maximum clot firmness (MCF)
MCF (measured by the ROTEM® analyser) was quantified as a surrogate marker for haemostatic efficacy, as previously described.16 MCF was measured immediately before (≤30 min) and at 1-hour (±15 minutes) post-infusion for the first infusion to treat each BE.
2.5 Assessment of in vivo recovery (IVR), fibrinogen and haemoglobin levels
IVR was calculated as the maximum increase in plasma fibrinogen activity at 3 hours post-infusion compared to pre-infusion levels. IVR was calculated following the first infusion for each BE and the loading dose of HFC for each surgery. Plasma fibrinogen activity levels were measured by the Clauss assay before the first HFC infusion for each BE. Plasma fibrinogen antigen levels were determined using a fibrinogen-specific enzyme-linked immunosorbent assay. Following HFC infusion, fibrinogen levels were measured at 1 hour, 3 hours and daily, including before and within 1 hour after each additional HFC infusion, if required. Changes in plasma haemoglobin in relation to fibrinogen plasma level changes were also measured.
2.6 PK measurements
PK endpoints were assessed after a single infusion of 70 mg/kg per body weight of HFC as described previously16; PK analysis was performed at 8 time points up to 14 days post-infusion and are described in the Appendix S1. Fibrinogen activity and antigen levels were assessed at all time points.
2.7 Safety
All adverse events (AEs) and serious AEs (SAEs) were recorded. Any AE observed between the start of the first HFC infusion, the end of each 30-day observation and follow-up periods was recorded as a treatment-emergent AE (TEAE). Non-TEAEs were all AEs not occurring in follow-up periods post-treatment.
2.8 Statistical analysis
Statistical analysis was performed for all BEs in each patient. Confidence intervals (CI, 95%) of mean data were calculated using a t test, and P-values were calculated using a paired t test. A P-value <0.01 was considered statistically significant.
3 RESULTS
3.1 Patient characteristics
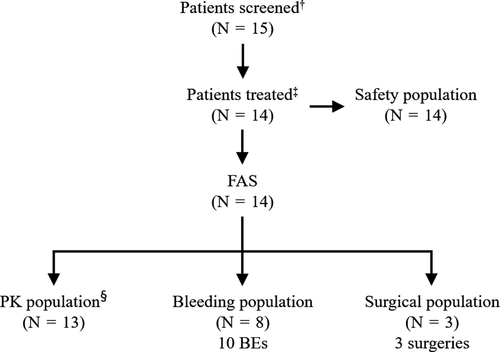
Fifteen paediatric patients were screened, all of whom met enrolment criteria. All patients had a diagnosis of afibrinogenaemia. One patient was not treated with HFC during the study (Figure 1). A total of 11 patients completed the study; three patients discontinued prematurely (withdrawal of consent [n =2]; SAE [n =1]). Eight patients received HFC for treatment of 10 BEs. Of these, two also received HFC for surgery. One patient received HFC for surgery only. PK assessments were performed on 13 patients. Patient demographics for the FAS and PK populations are shown in Table 1. The median age of both the FAS and PK populations was 6 years (range 1–10). In the FAS population, six patients were <6 years old and eight were aged 6–<12 years. In the PK population, five patients were aged <6 years and eight were aged 6–<12 years.
| Parameter | FAS Population (N = 14) | PK Population (N = 13a) | ||
|---|---|---|---|---|
| Mean±SD | Median (range) | Mean±SD | Median (range) | |
| Age at informed consent signed (years) | 6.0 ± 2.57 | 6.0 (1.0–10.0) | 6.2 ± 2.61 | 6.0 (1.0–10.0) |
| Height (cm) | 111.3 ± 15.37 | 111.0 (82.0–139.0) | 112.1 ± 15.70 | 111.0 (82.0–139.0) |
| Weight (kg) | 19.8 ± 6.93 | 17.0 (11.8–35.0) | 20.2 ± 7.06 | 17.5 (11.8–35.0) |
| BMI (kg/m2) | 15.7 ± 2.82 | 15.3 (12.0–22.8) | 15.8 ± 2.91 | 15.9 (12.0–22.8) |
| N | % | N | % | |
| Age category | ||||
| <6 years | 6 | 42.9 | 5 | 38.5 |
| ≥6–<12 years | 8 | 57.1 | 8 | 61.5 |
| Gender | ||||
| Female | 8 | 57.1 | 7 | 53.8 |
| Male | 6 | 42.9 | 6 | 46.2 |
| Race | ||||
| Asian | 4 | 28.6 | 4 | 30.8 |
| White | 10 | 71.4 | 9 | 69.2 |
- Abbreviations: BMI, body mass index; FAS, full analysis set; N, number of patients; PK, pharmacokinetic; SD, standard deviation.
- a One patient from the FAS did not undergo PK analysis.
3.2 Haemostatic efficacy for on-demand treatment of BEs
Eight patients received HFC for treatment of 10 BEs; 5 BEs (50%) were spontaneous and 5 (50%) were due to trauma. Eight BEs (80%) were minor and 2 (20%) were major. A single HFC infusion was administered for all minor BEs, while two major BEs received three and four infusions, respectively. A summary of BE characteristics is shown in Appendix S1: Table S5. The median (range) total dose per BE was 73.91 mg/kg (47.75–262.50), and dose per infusion was 70.21 mg/kg (23.13–98.44; Table 2).
| Dose mg/kg | Mean (± SD) | Median | Range |
|---|---|---|---|
| BEs (n = 10) | |||
| Total dose per BE | 93.78 (±64.60) | 73.91 | 47.45–262.50 |
| Total dose per minor BEa (n = 8) | 68.20 (±19.31) | 71.90 | 47.40–98.40 |
| Total dose per major BEb (n = 2) | 196.50 (±95.46) | 196.50 | 129.00–264.00 |
| Dose per infusion for all BEs (n = 15 infusions) | 62.52 (±22.56) | 70.21 | 23.13–98.44 |
| Dose for first infusion per BE | 70.78 (±17.88) | 73.91 | 47.45–98.44 |
| Dose for first infusion per minor BE | 68.20 (±19.31) | 71.90 | 47.40–98.40 |
| Dose for first infusion per major BE | 80.90 (±3.05) | 80.90 | 78.80–83.10 |
| Surgery (n = 3) | |||
| Total dose per surgery | 211.16 (±207.84) | 108.09 | 75.00–450.39 |
| Total dose per minor surgeryc (n = 2) | 91.55 (±23.40) | 91.55 | 75.00–108.09 |
| Total dose per major surgeryd (n = 1) | 450.40 (n/a) | 450.40 | n/a |
| Dose per infusion for all surgeries (n = 8 infusions) | 79.19 (±20.56) | 78.75 | 52.50–108.09 |
| Pre-operative loading dose (first infusion) per all surgeries | 78.53 (±27.96) | 75.00 | 52.50–108.09 |
| Pre-operative loading dose (first infusion) per minor surgeryc | 91.50 (±23.40) | 91.50 | 75.00–108.09 |
| Pre-operative loading dose (first infusion) per major surgery | 52.50 (n/a) | 52.50 | n/a |
- Abbreviations: BE, bleeding episode; SD, standard deviation.
- a All minor BEs (n = 8) were treated with a single infusion of HFC.
- b For the two major BEs, one was treated with three infusions and the other was treated with four infusions.
- c For minor surgeries (n = 2), no further post-operative infusions were administered following the loading dose.
- d The one major surgery was treated with 6 infusions.
Haemostatic efficacy in all BE treatments is shown in Table 3. Overall haemostatic efficacy assessed by the IDMEAC was 100% successful for treatment of all 10 BEs (95% CI 69.15–100.00, Table 3), including in both age subgroups.
| All Bleed Efficacy rating | Investigator | IDMEAC | |||
|---|---|---|---|---|---|
| 4-point Efficacy Scale | N (%) | N (%) | |||
| Excellent | 7 (70.0%) | 8 (80.0%) | |||
| Good | 1 (10.0%) | 2 (20.0%) | |||
| Moderate | 1 (10.0%) | 0 (0.0%) | |||
| None | 1 (10.0%)b | 0 (0.0%) | |||
| 2-Point Efficacy Scale a | N (%) | 95% CI c | N (%) | 95% CI c | |
| Success | 8 (80.0%) | 44.39–97.48 | 10 (100.0%) | 69.15–100.00 | |
| Failure | 2 (20.0%) | 0 (0.0%) | |||
| All Bleed Efficacy rating (age subgroups) | Investigator | IDMEAC | |||
|
Age <6 years (N = 5) |
4-point Efficacy Scale | N (%) | N (%) | ||
| Excellent | 3 (60.0%) | 4 (80.0) | |||
| Good | 1 (20.0%) | 1 (20.0) | |||
| Moderate | 0 (0.0%) | 0 (0.0) | |||
| None | 1 (20.0%)b | 0 (0.0) | |||
| 2-Point Efficacy Scale a | N (%) | 95% CI c | N (%) | 95% CI c | |
| Success | 4 (80.0) | 28.36–99.49 | 5 (100.0) | 47.82–100.00 | |
| Failure | 1 (20.0) | 0 (0.0) | |||
|
Age ≥6–12 years (N = 5) |
4-point Efficacy Scale | Investigator | IDMEAC | ||
| Excellent | 4 (80.0) | 4 (80.0) | |||
| Good | 0 (0.0) | 1 (20.0) | |||
| Moderate | 1 (20.0) | 0 (0.0) | |||
| None | 0 (0.0) | 0 (0.0) | |||
| 2-Point Efficacy Scale a | N (%) | 95% CI c | N (%) | 95% CI c | |
| Success | 4 (80.0) | 28.36–99.49 | 5 (100.0) | 47.82–100.00 | |
| Failure | 1 (20.0) | 0 (0.0) | |||
- Abbreviations: BE, bleeding episode; CI, confidence interval; FAS, full analysis set; IDMEAC, Independent Data Monitoring & Endpoint Adjudication Committee; N, number of BEs.
- a Efficacy rating of excellent or good indicated success and efficacy rating of moderate or none indicated failure.
- b For one patient, the haemostatic efficacy assessment was missing. As per the statistical analysis plan, the rating by the investigator was considered as ‘None’.
- c 95% CI for the success rate was calculated by Clopper Pearson Method.
For all 10 BEs, mean (±SD) IVR was 1.5 mg/dL[mg/kg] (±0.34) with a median (range) of 1.4 mg/dL[mg/kg] (1.1–2.1). For patients <6 years, mean (±SD) IVR was 1.3 mg/dL[mg/kg] (±0.14), and 1.8 mg/dL[mg/kg] (±0.28) for patients ≥6–<12 years.
3.3 MCF
After a mean (±SD) first HFC dose of 70.78 mg/kg (±17.19) for the 10 BEs, there was a significant increase in average MCF measured from baseline (0.0 mm), with a mean (±SD) increase of 3.3 mm (±1.77) and a median (range) change of 4.0 mm (0.0–5.0; P = 0.0002) at 1-hour post-infusion, which was identical between age subgroups. This data included two patients with an MCF of 0.0 mm 1 hour after HFC administration, likely due to a pre-analytical sample issue, as these patients had measurable fibrinogen levels. With the 0.0 mm MCF values excluded, mean (±SD) change in MCF from baseline to 1-hour post-infusion was 4.1 mm (±0.35), with a median (range) of 4.0 (4.0–5.0; P = <0.001). The statistically significant overall increase in MCF from baseline coincided with haemostatic efficacy in the bleeding population, as adjudicated by the IDMEAC.
3.4 Fibrinogen and haemoglobin plasma concentrations
In the bleeding population, fibrinogen plasma level increased from baseline in all patients after the first HFC infusion. At 1-hour post-infusion, the mean (±SD) fibrinogen level was 98.1 mg/dL (±13.33), with a median (range) of 100.0 mg/dL (79.0–118.0). This was comparable for fibrinogen level in patients <6 years (mean [±SD] 99.2 mg/dL [±12.83]; median [range] 106.0 mg/dL [79.0–110.0]) and patients ≥6–<12 years (mean [±SD] 97.0 mg/dL [±15.23]; median [range] 90.0 mg/dL [83.0–118.0]). Fibrinogen level coincided with clinical efficacy, which was rated as 100% successful by the IDMEAC for on-demand BE treatment. For 1 BE, a decrease during one of the days of the follow-up period of more than 20% in haemoglobin was observed, for 2 BEs the decrease was between 10% and 20%, and for the remaining 7 BEs the decrease in haemoglobin was <10%.
3.5 Haemostatic efficacy for surgical prophylaxis
Haemostatic efficacy of HFC in the prevention of bleeding during and after surgery was assessed in three patients for a total of three surgeries, one of which was major (splenectomy) and two were minor (circumcision and pulpectomy for two teeth). All three patients undergoing surgery were <6 years of age. A mean (±SD) loading dose of 78.50 mg/kg (±27.96) was administered prior to the three surgeries (Table 2). No further post-operative infusions were administered for the two minor surgeries. The patient undergoing major surgery received a total of six infusions. The mean (±SD) total dose per surgery was 211.16 mg/kg (±207.84), and the mean dose per infusion was 79.19 mg/kg (±20.56; Table 2).
Intra-and post-operative haemostatic efficacy is shown in Table 4. For all three surgeries, overall haemostatic efficacy was rated 100% by both the investigator and IDMEAC (95% CI 29.24–100.00; Table 4). Mean (±SD) IVR value for the loading dose of the first infusion for each of the three surgeries was 1.3 mg/dL/[mg/kg] (±0.22) with a median (range) of 1.3 mg/dL/[mg/kg] (1.0–1.4).
| Efficacy rating | Investigator | IDMEAC | ||
|---|---|---|---|---|
| Intra-operative efficacy | ||||
| 4-Point Efficacy Scale | N (%) | N (%) | ||
| Excellent | 3 (100.0) | 3 (100.0) | ||
| Good | 0 (0.0) | 0 (0.0) | ||
| Moderate | 0 (0.0) | 0 (0.0) | ||
| None | 0 (0.0) | 0 (0.0) | ||
| 2-Point Efficacy Scalea | N (%) | 95% CI b | N (%) | 95% CI b |
| Success | 3 (100.0) | 29.24–100.00 | 3 (100.0) | 29.24–100.00 |
| Failure | 0 (0.0) | 0 (0.0) | ||
| Post-operative efficacy | ||||
| 4-Point Efficacy Scale | N (%) | N (%) | ||
| Excellent | 3 (100.0) | 3 (100.0) | ||
| Good | 0 (0.0) | 0 (0.0) | ||
| Moderate | 0 (0.0) | 0 (0.0) | ||
| None | 0 (0.0) | 0 (0.0) | ||
| 2-Point Efficacy Scalea | N (%) | 95% CI b | N (%) | 95% CI b |
| Success | 3 (100.0) | 29.24–100.00 | 3 (100.0) | 29.24–100.00 |
| Failure | 0 (0.0) | 0 (0.0) | ||
- Abbreviations: CI, confidence interval; IDMEAC, Independent Data Monitoring & Endpoint Adjudication Committee; N, number of surgeries.
- a Efficacy rating of excellent or good indicated success and efficacy rating of moderate or none indicated failure.
- b 95% CI for the success rate was calculated by Clopper Pearson Method.
3.6 PK analysis
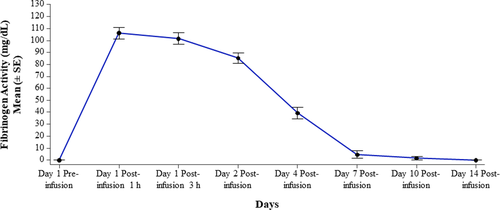
Thirteen patients underwent PK analysis with a single infusion of HFC with an actual dose of 73.5 mg/kg. Before HFC administration, fibrinogen plasma level was at/below the limit of detection of the Clauss fibrinogen activity assay for all patients. Fibrinogen level increased by a mean (±SD) of 106.1 mg/dL (±17.04) at 1-hour post-infusion and 101.5 mg/dL (±17.36) at 3-hours post-infusion. By 7 days post-infusion, mean fibrinogen plasma level had decreased to nearly baseline (Figure 2). PK parameters determined by fibrinogen activity are presented in Table 5 and were comparable between age subgroups (Appendix S1: Table S6). Mean (±SD) values for AUCnorm, incremental IVR and T1/2 were 1.314 h*kg*g/L/mg (±0.286), 1.459 mg/dL/(mg/kg) (± 0.229) and 63.339 hours (±11.975), respectively.
| Parameter | N | Mean | SD | Median | Range |
|---|---|---|---|---|---|
| AUC, g*h/L | 10a | 96.595 | 21.003 | 92.514 | 73.207–140.949 |
| AUCnorm h*kg*g/L/mg | 10a | 1.314 | 0.286 | 1.259 | 0.996–1.918 |
| AUC standardised to 70 mg/kg g*h/L | 10a | 91.987 | 20.010 | 88.100 | 69.689–134.237 |
| Cmax, g/L | 13 | 1.072 | 0.168 | 1.020 | 0.930–1.540 |
| Cmaxnorm, kg*g/L/mg | 13 | 0.015 | 0.002 | 0.014 | 0.013–0.021 |
| Cmax, standardised to 70 mg/kg (g*h/L) | 13 | 1.021 | 0.160 | 0.971 | 0.886–1.467 |
| Incremental IVR, mg/dL/(mg/kg) | 13 | 1.459 | 0.229 | 1.387 | 1.265–2.095 |
| Tmax, h | 13 | 1.462 | 0.877 | 1.000 | 1.000–3.000 |
| T1/2, h | 10a | 63.339 | 11.975 | 59.635 | 45.574–91.649 |
| MRT, h | 10a | 88.031 | 16.818 | 82.317 | 63.625–126.656 |
| CL, mL/h/kg | 10a | 0.790 | 0.151 | 0.796 | 0.521–1.004 |
| Vss, mL/kg | 10a | 67.632 | 7.069 | 67.749 | 52.783–76.803 |
- PK parameters were determined from the actual values for fibrinogen activity measured pre- and post-infusion. Comparable data were also obtained for PK parameters determined by fibrinogen antigen for the full PK population (data not shown). The exact doses, calculated by use of actual activity potencies, were used for the PK analysis. Abbreviations: AUC, area under the curve; Cmax, maximum plasma concentration; Cmaxnorm, maximum plasma concentration normalised to the dose administered; CL, clearance; HFC, human fibrinogen concentrate; IVR, in vivo recovery; MRT, mean residence time; PK, pharmacokinetics; SD, standard deviation; T1/2, half-life; Tmax, time to reach maximum plasma concentration; Vss, volume of distribution at steady state.
- a PK parameters for three subjects were not calculated because of insufficient number of quantifiable values. Fibrinogen concentrations below the limit of quantification were set to missing.
3.7 Safety
Ten AEs occurred in four (28.6%) patients (Table 6). Of these, seven AEs in three patients were TEAEs and three AEs in two patients were non-TEAEs. There were no deaths following HFC administration, and no severe/serious allergic reactions were observed. Two AEs were deemed possibly related to treatment, portal vein thrombosis (PVT) and fever (pyrexia), which both occurred in the same patient. The PVT, which was rated as an SAE of severe intensity, occurred after splenectomy for a spontaneous spleen rupture. The SAE was assessed as possibly related to HFC, while taking in consideration that PVT is a regular complication of splenectomy. The SAE led to discontinuation of the patient from the study. There were no clinical signs of neutralising anti-fibrinogen antibodies in any patients during the study.
| AEs | |||
|---|---|---|---|
| AE, n | 10 | ||
| Patients with AE, N (%) | 4 (28.6) | ||
| Severity of AE, n | |||
| Mild | 7 | ||
| Moderate | 2 | ||
| Severe | 1 | ||
| Probably or possibly related AE, n | 2 | ||
| AE leading to discontinuation, n | 1a | ||
| SAE, n | 1 | ||
| Death, n | 0 | ||
| AE severity (PT) | |||
| Mild | Moderate | Severe | |
| Abdominal pain | Procedural pain | PVTb | |
| Pyrexiab | Haemarthrosis | ||
| Ecchymosis (n = 3) | |||
| Influenza-like illness | |||
| Tonsillitis | |||
- Abbreviations: AE, adverse event; N, number of patients; n, number of AEs; PT, preferred term; PVT, portal vein thrombosis; Abbreviation: SAE, serious adverse event.
- a AE leading to discontinuation was an SAE.
- b Assessed as possibly related to treatment.
4 DISCUSSION
This prospective, open-label, uncontrolled study of HFC in paediatric patients with congenital fibrinogen deficiency demonstrated 100% efficacy for on-demand treatment of acute BEs and surgical prophylaxis in this ultra-rare disease setting. Assessments of efficacy made using robust objective 4-point efficacy criteria are further supported by significant increases in MCF and fibrinogen plasma concentration. Furthermore, data presented herein demonstrate favourable PK and safety profiles of HFC in paediatric patients with CFD.
Data from this paediatric study are comparable to data previously reported in adult/adolescent patients (≥12–54 years) using the same HFC.12 In the FORMA-02 study, a subgroup analysis of six patients aged ≥12–18 years demonstrated haemostatic efficacy to be 100% successful for the on-demand treatment of 11 BEs.12 This was comparable to the data presented herein, as well as the full FORMA-02 data set (98.9% treatment success for 89 BEs).12 Furthermore, the mean HFC dose administered per infusion for BE treatment in FORMA-02 (65.51 mg/kg for the full data set) was comparable to the dose administered in this paediatric study (62.52 mg/kg); this was expected, as the same dosing recommendations were used in both studies. Haemostatic efficacy in this study also coincided with a statistically significant increase in MCF. This was also observed in adults/adolescents, although the overall mean increase in MCF in this study at one-hour post-infusion (3.3 mm) was lower than the mean increase in MCF reported for the adult patients in FORMA-02 (5.8 mm) and the subgroup of six adolescent patients (4.0 mm).12 However, this difference was less pronounced when using the calculation that excluded the unlikely values of 0.0 mm increase in MCF.
Results from this paediatric patient study demonstrated a 100% overall success rate for surgical prophylaxis. This is comparable to data from FORMA-02, which demonstrated 100% intra- and post-operative efficacy of HFC in adult/adolescent patients.12 In this paediatric population, the mean (±SD) dose of HFC required per surgery (78.53 mg/kg [±27.96]) was lower than that required in adult/adolescent patients in FORMA-02 (104.49 mg/kg [±54.86]), but this is likely due to differences in the number of surgeries and dose per surgery between the two studies; both report data for one major surgery each but there was a lower median dose per surgery in the paediatric population (median dose per surgery; 75.00 mg/kg, N = 3, two of which were minor) compared to the adult/adolescent population (median dose per surgery; 85.80 mg/kg, N = 12, 11 of which were minor).12
The high success rate for haemostatic efficacy in treating BEs in paediatric patients with CFD reported herein is also comparable to that reported for other fibrinogen concentrates in several mixed age and paediatric studies. However, defined objective criteria were not used in these studies to assess haemostatic efficacy. In a study of 12 patients (mean age 11.5 years), Haemocomplettan® P demonstrated very good efficacy (highest rating) for on-demand BE treatment (N = 26) and surgical prophylaxis (N = 11), with the exception of one procedure (pylorotomy), where efficacy was considered moderate.11 However, efficacy assessments were based on the participating physician's clinical judgement; no objective criteria were used. Studies using FibCLOT/Clottafact® have also demonstrated haemostatic efficacy ranging from 73 to 100% for on-demand BE treatment in three mixed aged studies,10, 13, 17 and in a study of 11 paediatric patients aged 1–12 years, demonstrated 96.9% success for BE treatment and 100% success for surgical prophylaxis using a 4-point efficacy scale, although this did not include objective criteria such as drop in haemoglobin levels as part of the clinical assessment of efficacy.18
The current study also demonstrates a favourable PK profile of HFC in paediatric patients with CFD. In this study, lower IVR, Cmax, AUC and AUCnorm, shorter half-life and faster clearance were observed compared to adult/adolescent patients (FORMA-01 [N = 22 patients aged 12–53 years]).16 Mean values for several parameters reported here (such as AUC, Cmax, MRT, Tmax and T1/2) were also numerically lower in this paediatric population than those reported for other fibrinogen concentrates in mixed aged studies.10, 19 Lower PK parameters are expected owing to physiological differences in body size and pharmacodynamics between paediatrics and adults/adolescents,20 which have also been reported for factor VIII concentrates for haemophilia A treatment,21, 22 as well as in a recently published study of 16 paediatric patients 18 and a mixed aged study of 31 patients (aged 17 months–48 years) with afibrinogenaemia.23 In the latter study, modelling using patient body weight best described the PK of fibrinogen concentrate in the treatment of afibrinogenaemia.23
Treatment with HFC in this paediatric population showed a favourable safety profile; of the 10 AEs reported in this study, only two were assessed as possibly related to HFC; pyrexia and a case of PVT, which was classified as an SAE and led to discontinuation of the patient from the study. PVT is a regular complication of splenectomy, and other studies with and without HFC treatment have also reported thromboembolic events.11, 17, 24, 25 Patients with CFD have been documented to experience thromboembolic events in the absence of treatment with HFC.25 In the absence of fibrinogen, other adhesive proteins such as fibronectin, vitronectin and von Willebrand Factor are likely to serve as a ligand in afibrinogenaemia, supporting the formation of platelet-based clots that are frailer than fibrin clots. At the same time, in the absence of fibrin formation, there is excessive circulating thrombin. Both aspects may be considered related to the risk for thromboembolic events, but the underlying pathogenesis remains to be confirmed.26 The pyrexia AE was also experienced in the same patient with PVT and was most likely a clinical manifestation of the PVT.27 A favourable safety profile has also been observed with other fibrinogen concentrates in paediatric patients.18
This prospective study evaluating treatment of CFD, an ultra-rare condition, showed that HFC is efficacious in treating BEs and in perioperative prophylaxis, leading to significant increases in fibrinogen plasma level and markers of global haemostasis. This study used robust objective criteria for efficacy in addition to adjudication by an independent committee of experts to assess haemostatic efficacy for BE treatment and surgical prophylaxis. Our assessment showed success of this treatment in paediatric patients. However, a potential limitation includes the low numbers of patients studied in this indication and age group. Also, the small number of major BEs and surgeries recorded is a limitation in evaluating dosing and response in more severe episodes, although low frequency of bleeding symptoms reflects the variable presentation and ultra-rare setting of CFD. However, this data is consistent with published studies of other HFC in paediatric patients,18 and together, these data support that HFC is efficacious with a favourable safety profile for the treatment of bleeding and surgical prophylaxis in paediatric patients.
5 CONCLUSIONS
This study of HFC efficacy, PK assessment and safety in paediatric patients with CFD demonstrated HFC to be 100% efficacious for on-demand treatment of bleeding and surgical prophylaxis. There were no severe allergic/hypersensitivity reactions, or clinical evidence of neutralising anti-fibrinogen antibodies. PK parameters demonstrated a satisfactory PK profile, with an expected difference of some PK values as compared to adult/adolescent patients, probably due to physiological differences in this population. There are no additional safety concerns for use of this HFC in paediatric patients, and haemostatic efficacy for BEs and surgical prophylaxis is comparable to that in adults/adolescents. Together, the data demonstrate an efficacious and favourable safety profile of the HFC used in this study for treating paediatric patients with CFD.
6 DATA SHARING AND DATA ACCESSIBILITY
The data that support the findings of this study are available from Octapharma. Restrictions apply to the availability of these data, which were used under licence for this study. Data are available from the authors with the permission of Octapharma.
ACKNOWLEDGEMENTS
B.S., F.P. and S.K. conceived the study and contributed to data interpretation. C.S. and I.K. contributed to data interpretation. C.K., S.L., F.D’S., L.S.G. and O.R.Z. contributed to data collection. All authors have access to the primary clinical trial data. All authors reviewed and approved the final version of this manuscript. S.L., C.K., F.D’S., L.S.G., O.R.Z. and F.P. have all received investigator fees from Octapharma. I.K., B.S., C.S. and S.K. are all employees of Octapharma. The authors thank all the study teams and patients that participated in this study, as well as the members of the IDMEAC (Roger Lewis [Department of Emergency Medicine, Harbor-UCLA Medical Center, CA, USA], Craig Kessler [Georgetown University Medical Center, Lombardi Cancer Center, Washington, DC, USA] and Wolfgang Miesbach [Hämophiliezentrum, Med. Klinik III / Institut für Transfusionsmedizin Klinikum der Johann Wolfgang Goethe-Universität, Frankfurt am Main, Germany]). Editorial assistance was provided by Ben McDermott (Portland Medical Communications Ltd), funded by Octapharma, in accordance with GPP3.
FUNDING INFORMATION
This study was funded by Octapharma.




