Factor VIII activity of BAY 94-9027 is accurately measured with most commonly used assays: Results from an international laboratory study
Abstract
Introduction
Discrepancies in the measurement of modified factor VIII (FVIII) products have been recognized, highlighting the need for adjustments in clinical laboratory practices to ensure effective monitoring of patients treated with these products, particularly using the one-stage (activated partial thromboplastin time [aPTT]) assay.
Aim
To assess the ability of clinical laboratories to measure the activity of BAY 94-9027, a PEGylated extended half-life FVIII product, using routine (predominantly one-stage) assays in clinical laboratories
Methods
Blinded samples of FVIII-deficient plasma spiked with defined levels of BAY 94-9027 and a recombinant FVIII product comparator were provided to 52 clinical laboratories that routinely conduct FVIII testing. Samples were provided at 3 concentrations (low, medium and high), and laboratories analysed the samples using routine in-house one-stage and, when available, chromogenic assays. Acceptable spiked recovery (accuracy) of the local laboratory methods to measure BAY 94-9027 was the primary endpoint of the study.
Results
Accurate FVIII measurements were obtained at all concentrations for both products using the chromogenic assay and most of the commonly used one-stage reagents, both ellagic acid and silica based. Two specific silica-based reagents, APTT-SP and PTT-A, underestimated BAY 94-9027 levels at all concentrations, consistent with previous findings.
Conclusions
FVIII activity of BAY 94-9027 was accurately measured with most commonly used one-stage assays used in routine clinical practice. The chromogenic assay was also accurate. It is recommended that clinical laboratories identify and avoid specific inappropriate reagents, such as the APTT-SP and PTT-A, in their one-stage assays for FVIII monitoring.
1 INTRODUCTION
Accurate measurement of factor VIII (FVIII) activity is important for guiding treatment decisions.1, 2 FVIII activity is typically measured using the one-stage and, in some cases, the chromogenic assays.3 Although the one-stage assay is used in most laboratories worldwide for postinfusion monitoring, results are highly variable and may be influenced by reagents and different laboratory practices.3, 4 Because discrepancies in the measurement of modified FVIII products using the one-stage assays have been recognized, studies specifically addressing the ability of clinical laboratories to accurately measure these products have been undertaken.5-7
BAY 94-9027 is an extended half-life (EHL) FVIII molecule. Safety and efficacy have been demonstrated in multiple studies in adults, adolescents and children using tailored regimens with dosing intervals up to every 7 days.8, 9 BAY 94-9027 is an engineered B-domain-deleted recombinant FVIII (rFVIII) that is site-specifically conjugated to a single 60-kD polyethylene glycol (PEG) molecule (2 × 30 kDa branched) at a cysteine variant to reduce the clearance of FVIII and prolong its activity in circulation.10, 11 Because BAY 94-9027 is a modified molecule, there is the potential for differences in performance when it is measured in coagulation assays established for standard FVIII products. Thus, this study was designed to assess the ability of clinical laboratories to accurately measure FVIII activity after BAY 94-9027 administration using their routine assays and methods.
2 METHODS
2.1 Study design
The study consisted of 2 parts. In part 1, laboratories analysed test samples using their in-house (one-stage or chromogenic) assays, reagents and standards (calibrators), which referenced back to the World Health Organization (WHO) International Standard used by the laboratory and controls; the WHO 8th International Standard was provided to laboratories if requested. Laboratories that had the capacity to measure samples using both the one-stage and chromogenic assays were given an additional sample set to test. Part 1 focused predominantly on testing in-house one-stage reagents because the chromogenic assay was previously shown to accurately measure BAY 94-9027 activity.12 In part 1, information regarding assay type, instrument, reagents (activator kits, calibrators, and factor-deficient plasma) and dilutions were also collected from individual laboratories; differences in laboratory practices have been shown to affect FVIII measurements.3, 4 To ensure that part 1 captured both the prevalence and heterogeneity of one-stage assay kits used in the geographic region studied, laboratories that had ≥2 routine one-stage assays were sometimes asked to perform sample testing with one of the less commonly used kits in the laboratory. In part 2 of the study, all participating laboratories received additional sample sets and 2 silica-based one-stage assay reagents—Pathromtin® SL (Siemens, Marburg, Germany) and HemosIL® SynthASil (Instrumentation Laboratory, Bedford, MA, USA)—both provided by Bayer; these reagents have previously been shown to accurately measure BAY 94-9027 in one-stage assays.13 Part 2 of the study was intended to assess the effect on accuracy of results when guidance regarding specific one-stage reagents was provided to the laboratories. In addition, part 2 results would further define the effect of one-stage kits on BAY 94-9027 measurement accuracy because the other reagents (ie, calibrators, FVIII-deficient or -depleted plasma) used for both parts 1 and 2 would be the same.
In both parts of the study, plasma samples spiked with antihaemophilic factor (recombinant) plasma/albumin-free method (rAHF-PFM; Advate®, Shire, Westlake Village, CA, USA) were used as comparators. Normal control plasma (Siemens) and unspiked pooled haemophilic plasma served as high and low controls, respectively. Laboratories were blinded with respect to the identity of the spiked product (BAY 94-9027 or rAHF-PFM) or plasma control and target levels. The comparator product and plasma controls were used to ensure that laboratories were able to test FVIII activity accurately using their routine practices. FVIII activity results of the analysed samples as well as information regarding laboratory methodology were documented on case report forms, which were verified for completeness. FVIII spiked recovery, defined as 100× measured FVIII level divided by nominal FVIII level, was the primary endpoint, with measured FVIII levels reported as a secondary endpoint. Accuracy was assessed in terms of spiked recoveries falling within the acceptance criteria (geometric mean of 80%-125% of the target value).
2.2 Field study assays
Part 1 of the study assessed the accuracy of in-house assays, both one-stage and chromogenic (if available), to measure the activity of BAY 94-9027 and rAHF-PFM at these different concentration levels. Different concentrations of BAY 94-9027 and rAHF-PFM concentrates were added to pooled plasma from patients with severe haemophilia A with normal levels of von Willebrand factor (HRF Inc., Raleigh, NC, USA) to generate pools of the different nominal FVIII concentrations used in the study. Each laboratory received 3 sets of 26 blinded and randomized samples with unique identifiers for analysis (Table 1); an additional sample set was provided to the laboratory if it had the capacity to measure FVIII using the chromogenic assay. Each sample set consisted of triplicate BAY 94-9027 or rAHF-PFM spiked at low (<10 IU/dL), medium (10-50 IU/dL), or high (50-100 IU/dL) concentrations in pooled haemophilic plasma. Additional samples (3 normal control plasma, 3 unspiked pooled haemophilic plasma, and 2 extra samples from the other 24 BAY 94-9027 or rAHF-PFM samples in the set) were included to prevent inadvertent observer bias in testing and outcome. Nominal spiked target levels were low (4.3 IU/dL), medium (37.5 IU/dL) and high (86.5 IU/dL) for BAY 94-9027 and rAHF-PFM based on the labelled chromogenic potency. The high control plasma contained 87.8 IU/dL FVIII based on the one-stage assay, in line with standard laboratory calibration practice.
| Sample or spiked product | Quantity in sample set | FVIII levela |
|---|---|---|
| BAY 94-9027 | 3 | Low |
| 3 | Medium | |
| 3 | High | |
| Comparator product (rAHF-PFM) | 3 | Low |
| 3 | Medium | |
| 3 | High | |
| Unspiked pooled haemophilic plasma (low control) | 3 | Negative |
| Normal plasma (high control) | 3 | High |
| Additional random samples | 2 | Negative, low, medium, or high |
- FVIII, factor VIII.
- a Low < 10 IU/dL, medium = 10-50 IU/dL, high = 50-100 IU/dL.
For part 2, all sites received 1 kit each of Pathromtin SL and SynthASil aPTT, with instructions to verify that each laboratory's test definition for the FVIII assay matched those provided in the manufacturer's instructions. Aside from these minimal but essential modifications to the test definitions to allow FVIII detection of the provided samples, no further modifications were recommended, and no information was collected on how individual laboratories might have modified the tests in part 2.
2.3 Statistical analysis
All laboratories that provided data for ≥1 sample were included in the full analysis set. The comparator product and plasma controls were used to assess the ability of laboratories to measure FVIII based on their in-house assays and laboratory methods. To minimize variability resulting from general testing issues, laboratories that achieved 80% to 125% of the target value in ≥66% of the high control samples (ie, 2 of 3 or 3 of 4 tests) in part 1 were included in the valid assessments in high control (VAHC) analysis set. Statistical analyses were performed using SAS® 9.2 software (SAS Institute Inc., Cary, NC, USA). Results were analysed for intralaboratory and interlaboratory variability. Sample statistics (mean, SD, median, quartiles, ranges) were calculated for numeric data, and frequencies were calculated for categorical data. Because of separate endpoints, results were analysed separately for in-house reagents in part 1 and Bayer-provided reagents (Pathromtin SL and SynthASil) in part 2.
3 RESULTS
3.1 Participating laboratories
Fifty-two laboratories from North America (n = 25), Europe (n = 26) and Israel (n = 1) participated in the study. All 52 laboratories were included in the full analysis set, and 48 laboratories qualified for the VAHC analysis set, indicating that most laboratories could accurately measure FVIII. In part 1, 36 laboratories tested samples using only the one-stage assay, 3 tested samples using only the chromogenic assay and 13 used both assays. Assay reagents, including kit and use of FVIII-deficient or depleted plasma, varied in part 1 of the study; differences in the analyzer and calibration practices were also observed in part 1 (Table 2). In part 2, SynthASil and Pathromtin SL were used to test samples in 52 and 51 laboratories, respectively, which included some laboratories that used only the chromogenic assay in part 1.
| One-stage assay (n = 49) | Chromogenic assay (n = 16) | |
|---|---|---|
| Kit, n (%) | ||
| Actin FS (Siemens) | 8 (16.3) | 0 |
| Actin FSL (Siemens) | 8 (16.3) | 0 |
| Pathromtin SL (Siemens) | 6 (12.2) | 0 |
| C.K. Prest (Stago) | 3 (6.1) | 0 |
| PTT-A (Stago) | 6 (12.2) | 0 |
| HemosIL SynthASil (IL) | 15 (30.6) | 0 |
| HemosIL APTT-SP (IL) | 2 (4.1) | 0 |
| FVIII chromogenic (Siemens) | 0 | 6 (37.5) |
| HemosIL ELECTRACHOME (Siemens) | 0 | 3 (18.8) |
| Chromogenix Coamatic (IL) | 0 | 7 (43.8) |
| Missing | 1 (2.0) | 0 |
| Analyzer, n (%) | ||
| ACL (IL) | 13 (26.5) | 5 (31.2) |
| Stago | 7 (14.3) | 1 (6.3) |
| BSC XP (Siemens) | 8 (16.3) | 2 (12.5) |
| CS2100i (Sysmex) | 3 (6.1) | 0 |
| CA-7000 (Sysmex) | 1 (2.0) | 0 |
| CS-5100 (Sysmex) | 1 (2.0) | 0 |
| MC10 plus (Merlin) | 1 (2.0) | 0 |
| Plate reader (Dynex) | 0 | 1 (6.3) |
| Not specified | 15 (30.6) | 7 (43.8) |
| Deficient plasma, n (%) | ||
| FVIII depleted | 39 (79.6) | N/A |
| Congenital FVIII deficient | 6 (12.2) | N/A |
| Not specified | 4 (8.2) | N/A |
| Calibration frequency, n (%) | ||
| Daily | 9 (18.4) | 2 (12.5) |
| Weekly | 1 (2.0) | 0 |
| According to individual laboratory practice | 38 (77.6) | 14 (87.5) |
| Missing | 1 (2.0) | 0 |
| Calibrators, n (%) | ||
| Freshly prepared | 44 (89.8) | 15 (93.8) |
| Frozen | 4 (8.2) | 1 (6.3) |
| Not specified | 1 (2.0) | 0 |
| FVIII activity of calibrator assigned to WHO IS, n (%) | ||
| Yes | 46 (93.9) | 14 (82.4) |
| Not specified | 3 (6.1) | 2 (17.6) |
- FVIII, factor VIII; IL, Instrumentation Laboratory; N/A, not applicable; WHO IS, World Health Organization International Standard.
3.2 Chromogenic assay results
Geometric mean FVIII recovery ranged from 104.4% to 117.1% for BAY 94-9027, which was similar to results for rAHF-PFM (87.7%-107.8%) using the chromogenic assay (Figure 1A). Accurate results were obtained for all 3 chromogenic assay reagents used in part 1 (Figure 1B). Interlaboratory and intralaboratory variability (percentage coefficient of variation [CV]) was very low for high control (pooled normal plasma), indicating that most laboratories could accurately measure FVIII (Figure 1C and D). For all chromogenic assay reagents, variability in results across different laboratories was low (Figure 1C and D).
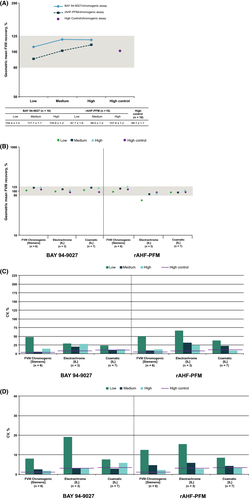
3.3 One-stage assay results (overall)
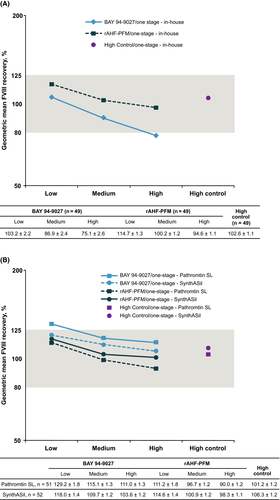
Overall recoveries for both BAY 94-9027 and rAHF-PFM were also similar for most of the one-stage reagents tested in parts 1 and 2 of the study (Figure 2A and B), and recovery was similar between the VAHC analysis set and the full analysis set (data not shown). Intralaboratory variability was comparable between BAY 94-9027, rAHF-PFM and normal plasma in both parts 1 and 2 (Tables 3 and 4). Interlaboratory variability was higher for BAY 94-9027 in part 1, but percentage CV decreased in part 2 when all participating laboratories used the Pathromtin SL and SynthASil kits, which can accurately measure BAY 94-9027 and full-length FVIII (Tables 3 and 4).
| CV, % | BAY 94-9027 | rAHF-PFM | High Controlb | ||||
|---|---|---|---|---|---|---|---|
| Lowb | Mediumb | Highb | Lowb | Mediumb | Highb | ||
| Interlaboratory | 91.96 | 108.04 | 122.23 | 23.45 | 17.46 | 13.57 | 11.41 |
| Intralaboratory | 5.35 | 3.95 | 3.11 | 6.55 | 4.85 | 2.75 | 3.38 |
- CV, coefficient of variation; FVIII, factor VIII; rAHF-PFM, antihaemophilic factor (recombinant) plasma/albumin-free method.
- a n = 49.
- b Low, medium, and high levels were 4.3, 37.5, and 86.5 IU/dL, respectively; the high control (normal plasma) contained 87.8 IU/dL FVIII.
| CV, % | BAY 94-9027 | rAHF-PFM | High controla | ||||
|---|---|---|---|---|---|---|---|
| Lowa | Mediuma | Higha | Lowa | Mediuma | Higha | ||
| Interlaboratory | |||||||
| Pathromtin SL, n = 51 | 63.43 | 26.98 | 24.50 | 61.99 | 22.36 | 15.59 | 20.52 |
| SynthASil, n = 52 | 36.60 | 19.47 | 19.73 | 32.95 | 16.76 | 13.42 | 14.31 |
| Intralaboratory | |||||||
| Pathromtin SL, n = 51 | 7.60 | 6.08 | 3.46 | 6.41 | 6.11 | 3.92 | 3.58 |
| SynthASil, n = 52 | 4.96 | 3.22 | 3.48 | 5.42 | 5.27 | 3.48 | 3.26 |
- CV, coefficient of variation; FVIII, factor VIII; rAHF-PFM, antihaemophilic factor (recombinant) plasma/albumin-free method.
- a Low, medium, and high levels were 4.3, 37.5 and 86.5 IU/dL, respectively; the high control (normal plasma) contained 87.8 IU/dL FVIII.
Because the overall one-stage assay data suggested some differences between the results obtained with in-house assays (part 1) versus specific appropriate assays (part 2), the data were further analysed. Part 1 results were analysed to determine if all one-stage assays were comparable in performance, whereas part 2 results were assessed to determine whether further improvements in assay outcomes were possible when guidance regarding appropriate one-stage reagents was given.
3.4 One-stage assay results (part 1)
In part 1, accurate results for BAY 94-9027 were obtained at all levels with the ellagic acid-based reagent Actin® FSL (Siemens) and were within or close to range for medium and high levels with the silica-based reagents SynthASil and Pathromtin SL (Figure 3A); analyzers used are listed in Table 2. ACL (Instrumentation Laboratory) was used for SynthASil and BSC XP (Siemens, Erlangen, Germany) and CS2100i (Sysmex, Kobe, Japan) for Pathromtin SL in part 1 of the study. These results confirmed the previous findings13, 14 that both ellagic acid- and silica-based reagents can be used to measure FVIII activity after BAY 94-9027 administration. Increased variability was seen with the kaolin-based reagent C.K. Prest® (Stago, Asnières sur Seine, France) versus other one-stage reagents. Actin FS also overestimated activity, particularly at low concentrations (Figure 3A). The interlaboratory and intralaboratory variabilities with these kits were generally low for BAY 94-9027 and comparable to rAHF-PFM (Figure 3B and C). Two silica-based reagents, PTT-A and APTT-SP, underestimated FVIII recovery (<25% and <18%, respectively; Figure 3A) at all concentrations of BAY 94-9027, which confirmed previous in vitro study findings.12, 14 The intralaboratory and interlaboratory variations for BAY 94-9027 obtained with these 2 kits were higher versus other one-stage kits (Figure 3B and C). The one-stage assay interlaboratory variability for recoveries measured by laboratories with these 2 reagents was ≥75%, whereas the variability with the other one-stage reagents was generally <25% (Figure 3B). The results obtained from these specific reagents indicate that they likely contributed to the overall one-stage assay interlaboratory variability for BAY 94-9027 in part 1 (Table 3) and further demonstrate the importance of using appropriate one-stage assay reagents when measuring BAY 94-9027 activity.
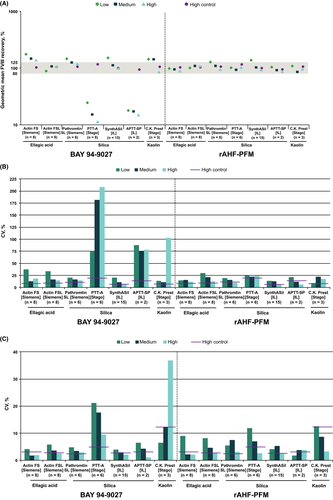
3.5 One-stage assay results with specified kits (part 2)
In part 2, the accuracy and variance in BAY 94-9027 assay performance was assessed when laboratories were given appropriate reagents, although no guidance was given regarding the analyzers to be used. In addition to the FVIII spiked recoveries falling within the expected range with both Pathromtin SL and SynthASil (Figure 2B), the interlaboratory variability was reduced for BAY 94-9027 in part 2 when using these reagents (Table 4) versus part 1 using in-house kits (Table 3); variability was comparable to rAHF-PFM and high control, demonstrating that laboratories were uniformly able to accurately measure FVIII activity (Table 4). These results also indicated that laboratories can accurately measure FVIII activity with BAY 94-9027 using one-stage assays with appropriate reagents and a variety of analyzers.
4 DISCUSSION
Discrepancies in the measurement of modified FVIII products have been recognized and highlight the need for adjustments in clinical laboratory practices to ensure effective monitoring of patients treated with these products, particularly using the one-stage assay.1, 2 This international laboratory study was designed to assess the ability of clinical laboratories to measure the activity of BAY 94-9027, a PEGylated EHL FVIII product, using routine one-stage assays in clinical laboratories. Overall, the results of the study showed that selected one-stage and chromogenic methods already established in most clinical laboratories can accurately measure BAY 94-9027. Accurate results at all BAY 94-9027 levels were observed with test sites using the ellagic acid-based reagent Actin FSL and silica-based reagents Pathromtin SL and SynthASil in part 2 of the study, when all sites used these specific reagents. Actin FS and the kaolin-based reagent C.K. Prest showed some overestimation of BAY 94-9027 activity, particularly at lower concentrations, versus the selected one-stage assay reagents tested. Two specific silica-based one-stage assays, APTT-SP and PTT-A, underestimated BAY 94-9027 activity at all concentrations; this is consistent with previous findings.12, 14
These data expand upon the existing literature on assay performance with BAY 94-9027.12-14 Earlier studies have shown that FVIII activity measurements for BAY 94-9027 spiked into FVIII-deficient plasma were similar to the WHO 8th International Standard using both chromogenic assay and ellagic acid-based one-stage assays. These studies also showed that specific silica-based one-stage assays underestimated BAY 94-9027 activity.12, 14 Another earlier study showed that other commonly used silica-based one-stage assays (Pathromtin SL and SynthASil) were able to provide accurate measurements,13 which contradicted earlier conclusions that only ellagic acid-based activators can be used to measure FVIII activity with BAY 94-9027.12, 14 These important findings have been replicated and confirmed by this international laboratory study.
With the use of selected commonly available one-stage assay reagents, we anticipate BAY 94-9027 activity measurement to be reproducible. This is confirmed by the low interlaboratory variability in recovery measurements seen when appropriate one-stage reagents are used. Standardization of reagents and laboratory practices may further decrease variability when measuring FVIII with all products in clinical practice.
It has been shown that discrepancies and variability in results using different assays or reagents have been documented for all modified rFVIII products, including B-domain-deleted and EHL products.5-7, 15-17 Although the methods used to modify FVIII vary among manufacturers of EHL products,2 it is apparent that some modified proteins behave differently in some one-stage assays compared with unmodified FVIII products.15 The variable performance of modified rFVIII products (eg, Afstyla® [CSL Behring, Marburg, Germany], N8-GP [Novo Nordisk, Bagsværd, Denmark], and BAY 94-9027) in one-stage assays may reflect the sensitivity of the modified rFVIII product to variations in contact activation. More recently, available reagents, such as SynthASil, also appear to provide accurate results with different PEGylated molecules compared with some older reagents. This possibility is supported by work from Gu et al,14 who assessed the ability of the SynthAFax kit (which can accurately measure BAY 94-9027 activity) and the APTT-SP kit (which underestimates BAY 94-9027 activity) to accelerate turbidity development during plasma clot formation in the aPTT reaction. With the SynthAFax kit, haemophilia A plasma spiked with different doses of BAY 94-9027 or the WHO 8th International Standard had similar clot times. In contrast, use of the APTT-SP kit revealed a delayed appearance of the peak for BAY 94-9027 compared with the WHO 8th International Standard, indicating a slower acceleration of turbidity development and a prolonged clot time with the APTT-SP kit compared with the SynthAFax kit. Thus, underestimation of activity with these particular reagents may be related to PEG disturbance of silica-mediated contact activation (“possibly due to interference of contact activation on the silica surface by the 60-kDa PEG moiety of the conjugated FVIII”14).
As previously reported,3 and as confirmed in this study, there is a wide range of one-stage assay reagents and laboratory practices used worldwide to measure FVIII activity. Without global standardization of assays and methods, clinicians and clinical laboratories must be informed of the specific modified FVIII product each patient has received so that the appropriate assay and reagents can be used for optimal monitoring.2 As reported in this study and others,7, 16 the availability of assay- and reagent-specific data is vital to assist clinical laboratories in choosing the appropriate assay conditions to ensure safe and effective postinfusion monitoring. Given the number of available aPTT assays, the assays tested in field studies are by no means exhaustive, and laboratories should determine that they have suitable assays available before undertaking patient monitoring. One approach may be the one adopted by the UK Haemophilia Centres Doctors’ Organisation, which recommends that laboratories use an assay that has been validated for use with the specific EHL product being measured.18
5 CONCLUSION
This international laboratory study indicated that BAY 94-9027 activity can be accurately measured with most common silica- and ellagic acid-based one-stage and chromogenic reagents used in clinical laboratories. Two specific silica-based assays, PTT-A and APTT-SP, underestimated results and should be avoided when measuring plasma samples that contain BAY 94-9027. It remains important that clinical laboratories test the accuracy of their local procedures before monitoring patients treated with FVIII products.
ACKNOWLEDGEMENTS
Nikki Church contributed to study design, wrote the protocol and assisted with development of the statistical analysis plan, reviewed and interpreted data, and oversaw preparation of the final study report. Lilley Leong proposed the initial study design; worked with Lisa A. Michaels, Nikki Church, Yvonne Katterle and Hannes-Friedrich Ulbrich to finalize the study design; and provided assay insights into inform data analysis and manuscript preparation. Yvonne Katterle was responsible for sample preparation, randomized labelling, and internal potency control of the prepared field study samples. Hannes-Friedrich Ulbrich contributed to study design, provided the randomization lists, codeveloped the statistical analysis plan and contributed to interpretation of statistical analysis results. Iris Noerenberg contributed to study management, CRF design, organization of sample management and contract negotiation. Steve Kitchen contributed to data analysis and interpretation. Lisa A. Michaels made substantial contributions to study conception and design and to data analysis and interpretation. All authors contributed to the development of the manuscript, reviewed and commented on each draft and approved the final draft. The authors thank Jorge Caicedo (Bayer) and Stefan Bruns (Winicker Norimed GmbH) for their contribution to the study. This study was funded by Bayer. Medical writing assistance was provided by Ken Wannemacher, PhD, from Complete Healthcare Communications, LLC (West Chester, PA, USA) and was funded by Bayer. We also thank the participating laboratories: Blood Center Wisconsin (Milwaukee, WI, USA); University of California Davis Health System (Sacramento, CA, USA); Cincinnati Children's Hospital and Medical Center (Cincinnati, OH, USA); Emory Medical Laboratory (Atlanta, GA, USA); Columbia University College of Physicians and Surgeons (New York, NY, USA); University of Rochester (Rochester, NY, USA); Children's Hospital of Michigan (Detroit, MI, USA); Boston Children's Hospital (Boston, MA, USA); University of Minnesota Medical School (Minneapolis, MN, USA); Pennsylvania State University (Hershey, PA, USA); University of North Carolina Hospitals (Chapel Hill, NC, USA); University of Texas Internal Medicine (Houston, TX, USA); Florida Hospital Center for Thrombosis Research (Winter Park, FL, USA); Bloodworks Northwest (Seattle, WA, USA); Mayo Comprehensive Hemophilia Center (Rochester, MN, USA); University of California-San Diego Medical Center (San Diego, CA, USA); University of Michigan (Ann Arbor, MI, USA); University of Miami Hospital Special Coagulation Laboratory (Miami, FL, USA); Johns Hopkins University Hematology and Coagulation Laboratory (Baltimore, MD, USA); Duke University Medical Center (Durham, NC, USA); Wake Forest University Baptist Medical Center (Winston-Salem, NC, USA); TriCore Research Laboratories (Albuquerque, NM, USA); MVZ Labor Leipzig Dr. Reising-Ackermann und Kollegen (Leipzig, Germany); Institut für Exp. Hämostaseologie/Gerinnungslabor (Bonn, Germany); Raphaelsklinkik Münster Hämostaseologisches Labor im Zentrallabor (Münster, Germany); CRC-Coagulation Research Center GmbH (Duisburg, Germany); Universitätsklinikum Klinikum der Universität München (München, Germany); Universitätsklinikum Freiburg (Freiburg, Germany); Universitätsklinik Erlangen (Erlangen, Germany); IPH GmbH medlab Südheide (Hohne, Germany); Medizinisches Labor Rostock (Rostock, Germany); Newcastle upon Tyne Hospitals (Newcastle upon Tyne, UK); St. Thomas Hospital (London, UK); Royal Hallamshire Hospital (Sheffield, UK); Bristol Royal Infirmary (Bristol, UK); Cambridge Haemophilia and Thrombophilia Centre (Cambridge, UK); Hospital Universitari i Politècnic La Fe (Valencia, Spain); Hospital Teresa Herrera Materno Infantil (Coruña, Spain); Hospital Universitaris Vall D'Hebron (Barcelona, Spain); Hospital Universitario La Paz (Madrid, Spain); University Hospital of Geneva (Geneva, Switzerland); Central Laboratory of Hematology CHUV (Lausanne, Switzerland); University Hospital Zürich (Zürich, Switzerland); Diagnostische Hämatologie Universitätsspital Basel (Basel, Switzerland); CHU Sainte-Justine Laboratoire d'Hemostase (Montréal, QC, Canada); Scientist Lawson Health Research Institute (London, ON, Canada); Hamilton Regional Laboratory Medicine Program (Hamilton, ON, Canada); D.A.I. di Medicina di Laboratorio A.O.U. Federico II (Napoli, Italy); Fondazione Luigi Villa Centro Studi di Patologia Molecolare Applicata alla Clinica (Milan, Italy); Sheba Medical Center at Tel Hashomer (Ramat-gan, Israel); Institutul Clinic Fundeni Centrul de Hematologie si Transplant Medular (Bucuresti, Romania); Medizinische Universität Wien (Vienna, Austria).
DISCLOSURES
S. Kitchen has received consultancy payments and speaker fees from Bayer. N. Church, L. Leong, Y. Katterle, H.-F. Ulbrich, I. Noerenberg and L. A. Michaels are employees of Bayer.




