Latitudinal biodiversity gradients at three levels: Linking species richness, population richness and genetic diversity
Abstract
Motivation
Theory describing biodiversity gradients has focused on species richness with less conceptual synthesis outlining expectations for intraspecific diversity gradients, that is, broad-scale population richness and genetic diversity. Consequently, there is a need for a diversity–gradient synthesis that complements species richness with population richness and genetic diversity.
Review methods
Species and population richness are the number of different species or populations in an area, respectively. Population richness can be totalled across species, within a species or averaged across species. Genetic diversity within populations can be summed or averaged across all species in an area or be averaged across an individual species. Using these definitions, we apply historical, ecological and evolutionary frameworks of species richness gradients to formulate predictions for intraspecific diversity gradients.
Review conclusions
All frameworks suggest higher average population richness at high latitudes, but similar total population richness across latitudes. Predictions for genetic diversity patterns across species are not consistent across frameworks and latitudes.
New analysis methods
Species range size tends to increase with latitude, so we used empirical data from c. 900 vertebrate species to test hypotheses relating species range size and richness to population richness and genetic diversity.
New analysis conclusions
Species range size was positively associated with its population richness but not with species-specific genetic diversity. Furthermore, a positive linear relationship was supported between species richness and total population richness, but only weakly for average population richness.
Overall conclusion
Through the lens of species richness theories, our synthesis identifies an uncoupling between species richness, population richness and genetic diversity in many instances due to historical and contemporary factors. Range size and taxonomic differences appear to play a large role in moderating intraspecific diversity gradients. We encourage further analyses to jointly assess diversity–gradient theory at species, population and genetic levels towards better understanding Earth’s biodiversity distribution and refining biodiversity conservation.
1 INTRODUCTION
The latitudinal distribution of species richness is one of the most widely recognized and predictable patterns of biodiversity (Brown, 2014; Fine, 2015; Costello, May, & Stork, 2013; Mittelbach et al., 2007; Pianka, 1966; Roll et al., 2017; Stork, 1993; Warman et al., 2004; Willig, Kaufman, & Stevens, 2003). To date, however, the extensive theoretical and empirical attention directed to species richness gradients has not been extended to understand the broad-scale distribution of other important components of biodiversity, such as intraspecific diversity. Levels of intraspecific diversity—whether characterized as functional diversity, phylogenetic diversity, population richness and/or genetic diversity within and among populations—can influence species geographic distributions, species responses to environmental change (Barrett & Schluter, 2008; Bernatchez, 2016; Jump, Marchant, & Peñuelas, 2009; Willoughby, Harder, Tennessen, Scribner, & Christie, 2018), community structure, and ecosystem functioning (Des Roches et al., 2018; Raffard, Santoul, Cucherousset, & Blanchet, 2019). This influence of intraspecific diversity on species themselves could suggest that the distribution of species richness is affected by intraspecific diversity patterns, or vice versa. Notably lacking in the literature is a foundation for theoretical expectations of intraspecific diversity and its distribution, a gap we aim to resolve in this review.
Large quantities of data on intraspecific diversity have recently become available for broad-scale analyses due to technological advances and the accumulation of smaller-scale empirical works (DeWoody & Avise, 2000; Hughes, Daily, & Ehrlich, 1997; Lawrence et al., 2019; Martinez, Willoughby, & Christie, 2018; Medina, Cooke, & Ord, 2018; Miraldo et al., 2016; Willoughby et al., 2015). When collated, such data allow for the extension of species-centric latitudinal concepts towards understanding how broad-scale intraspecific diversity patterns may better inform, for example, the speciation process (Adams & Hadly, 2013; Schluter & Pennell, 2017; Smith, Seeholzer, Harvey, Cuervo, & Brumfield, 2017) and biodiversity conservation by revealing hot/cold spots of intraspecific diversity (Marchesini, Vernesi, Battisti, & Ficetola, 2018; Paz-Vinas et al., 2018). Herein, we focus our discussion specifically on broad-scale patterns of two metrics of intraspecific diversity: population richness within species and genetic diversity, and how these metrics relate to species richness gradients. Related topics on other components of intraspecific diversity, such as functional and phylogenetic diversity, are discussed in Economo, Narula, Friedman, Weiser, and Guénard (2018), Marske, Rahbek, and Nogués-Bravo (2013), and Martinez et al. (2018).
Dynamics between and within populations are the stepping stone linking genetic diversity with species richness (Fine, 2015; Marchesini et al., 2018; Marske et al., 2013; Paz-Vinas et al., 2018; Singhal et al., 2018). Increasing genetic differentiation leads to population divergence and eventually speciation due to isolation and/or selection (Schluter, 2016; Schluter & Pennell, 2017; Taylor, 1999; Wiens, 2004). Therefore, characterizing population richness relative to species richness and genetic diversity is fundamental to refine our understanding of the interrelationships between species richness and genetic diversity. For example, an area’s species and population richness could be one indicator of the age and speciation potential of that community: high species richness but low population richness can indicate older communities with lower rates of speciation as all niches may be filled (Kennedy et al., 2018; Schluter, 2016; Schluter & Pannell, 2017). Furthermore, the joint investigation of the distribution of species richness, population richness and genetic diversity may allow more accurate inferences about ecological history, including glacial refugia, recolonization, and founder effects (Bernatchez & Wilson, 1998; Blanchet, Prunier, & De Kort, 2017; Fedorov & Stenseth, 2002; Galbreath & Cook, 2004; Marske et al., 2013; Tamkee, Parkinson, & Taylor, 2010). Some research has bridged how aspects of genetic diversity may relate to and have consequences for communities and species richness (Antonovics, 1976, 2003; Hughes, Inouye, Johnson, Underwood, & Vellend, 2008; Lamy, Laroche, David, Massol, & Jarne, 2017; Laroche, Jarne, Lamy, David, & Massol, 2014; Marchesini et al., 2018; Marske et al., 2013; Pfeiffer et al., 2018; Vellend, 2005, 2010; Vellend & Geber, 2005; Vellend et al., 2014). Still lacking, however, is a strong conceptual foundation linking species richness, population richness and genetic diversity within the framework of the latitudinal gradient. To build this foundation we draw from theories presented in the species richness literature.
Many of the non-mutually exclusive theories and hypotheses proposed to explain the latitudinal gradient in species richness (Fine, 2015; Willig et al., 2003) can be structured into historical, ecological and evolutionary frameworks (Brown, 2014; Mittelbach et al., 2007; Schemske & Mittelbach, 2017). We begin by elaborating on each of these three broad frameworks and how they relate to species richness, population richness and genetic diversity. We focus on vertebrate groups across the American continents, as they tend to be more mobile and have a large body of focal research (Bazin, Glemin, & Galtier, 2006). The Americas offer a unique opportunity for discussing latitudinal gradients because the continents are largely arranged in a north to south fashion. To ensure use of standardized terms throughout the review as well as to clarify distinctions among past works, we have broken down population richness and genetic diversity into five categories (see Glossary): (a) total number of populations across species in an area (TotPopR); (b) the number of populations for a given species in an area (PopPerSpp); (c) the average number of populations per species in an area (AvgPopSpp); (d) total genetic diversity in an area, as the sum of genetic diversity across all species at a population level (TotGenDiv); and (e) average genetic diversity across populations for a given species in an area (GenPerSpp). Note that our definition of genetic diversity refers largely to neutral genetic diversity, not adaptive genetic diversity, as it allows us to make usage of a comprehensive population genetics database that we recently compiled from c. 900 vertebrate species spanning the American continents (Lawrence et al., 2019). While data on adaptive genetic diversity are increasing, to date they are insufficiently rich for similar, standardized collation and would likely have different expectations that should be explored in future works.
We structure this paper into two parts. First, we synthesize the general expectations for latitudinal patterns in species richness, population richness and genetic diversity under historical, ecological and evolutionary frameworks (Table 1). Second, using the aforementioned database, we conducted new analyses to test the following hypotheses for population richness and genetic diversity, specifically considering the role of a species’ range size: (a) larger range sizes are associated with greater numbers of genetically distinct populations per species (PopPerSpp); (b) areas with more overlapping species ranges, that is, higher species richness, have lower PopPerSpp but higher TotPopR; and (c) larger range sizes have higher levels of genetic diversity (GenPerSpp).
| Framework | Theory description and explanation | Predictions | ||
|---|---|---|---|---|
| Species | Population | Genetic | ||
| Hist | Time and area hypothesis: Tropics are older, historically larger geographically and climatically stable, allowing for more diversification to occur over time | Low latitudes: higher | Low latitudes: lower PopPerSpp; similar TotPopR | Low latitudes: higher |
| Explanation: Older low latitude communities have had more time and area for mutations to accumulate as well as populations within species to differentiate into new species, causing fewer populations per species, but perhaps retaining a similar TotPopR to high latitudes (barring nuances as discussed in text) | High latitudes: lower | High latitudes: higher PopPerSpp; similar TotPopR | High latitudes: lower | |
| References: Mittelbach et al. (2007), Pianka (1966), Wallace (1878) | ||||
| Hist | Tropical/phylogenetic niche conservatism: Species that originate in a region, whether tropical or temperate, are more likely to stay within that climate, but older clades may diversify outwards through niche evolution | Low latitudes: higher | Low latitudes: lower PopPerSpp | Low latitudes: higher |
| Explanation: The typically older age of low latitude species indicates they will have fewer populations but more GD (see time and area hypothesis) as they have remained in tropical environments longer, diversifying over time | High latitudes: lower | High latitudes: higher PopPerSpp | High latitudes: lower | |
| References: Wiens and Donoghue (2004) | ||||
| Hist Ecol | Heterogeneous area: Increased ecological heterogeneity in large areas leads to fragmentation and speciation across species’ ranges. Related to time and area hypotheses but more focused on the notion of larger areas having more heterogeneous habitat | Low latitudes: higher | Low latitudes: lower PopPerSpp | Low latitudes: higher |
| Explanation: Increased fragmentation at high latitudes in large-ranged species allows for populations to differentiate, but not fully enough to lead to new species; larger areas maintain GD within a species due to gene flow between populations | High latitudes: lower | High latitudes: higher PopPerSpp | High latitudes: lower | |
| References: Mittelbach et al. (2007), Rosenzweig (1995), Terborgh (1973) | ||||
| Ecol | Species range size (Rapoport’s rule): Low latitude species experience smaller ranges in climatic variation, and therefore more specialization and smaller range sizes; the opposite phenomenon occurs at high latitudes | Low latitudes: higher | Low latitudes: lower PopPerSpp | Low latitudes: higher TotGenDiv |
| lower GenPerSpp | ||||
| Explanation: More specialization leads to more species with smaller range sizes, fewer populations per species and more GD across many species, although may result in lower GD within a species due to specialization | High latitudes: lower | High latitudes: higher PopPerSpp |
High latitudes: lower TotGenDiv higher GenPerSpp |
|
| References: Mittelbach et al. (2007), Janzen (1967), Stevens (1989) | ||||
| Ecol | Genetic drift: Low latitude populations are smaller and tend to experience more genetic drift that differentiates populations and species | Low latitudes: higher | Low latitudes: lower PopPerSpp | Low latitudes: higher TotGenDiv |
| lower GenPerSpp | ||||
| Explanation: More genetic drift at low latitudes leads to speciation, more species, and fewer PopPerSpp, but in high latitudes with less genetic drift there are more, less distinct populations. Thus PopPerSpp is maintained at high latitudes relative to low latitudes. GD is higher across species at low latitudes due to different populations accumulating different alleles, but perhaps lower GenPerSpp if alleles are lost through drift | High latitudes: lower | High latitudes: higher PopPerSpp |
High latitudes: lower TotGenDiv higher GenPerSpp |
|
| References: Dynesius and Jansson (2000), Mittelbach et al. (2007) | ||||
| Ecol | Energy-diversity hypothesis: Regions of high primary productivity should support more individuals, and therefore there is an increased likelihood of more species | Low latitudes: higher | Low latitudes: lower PopPerSpp | Low latitudes: higher |
| Explanation: Higher productivity at low latitudes leads to more individuals and more species; but more individuals in general leads to smaller population sizes per species and increased risk of inbreeding for areas with high species richness, affecting both metrics of GD | High latitudes: lower | High latitudes: higher PopPerSpp | High latitudes: lower | |
| References: Currie et al. (2004), Pianka (1966), Storch et al. (2005) | ||||
| Ecol | Biotic interactions: Biotic interactions are stronger and represent a greater fraction of natural selection for low latitude species; abiotic interactions exert stronger evolutionary forces for higher latitude species | Low latitudes: higher | Low latitudes: lower PopPerSpp | Low latitudes: higher TotGenDiv |
| similar TotPopR | lower GenPerSpp | |||
| Explanation: More speciation at low latitudes as biotic interactions drive specialization; general adaptations at high latitudes from abiotic factors maintain gene flow among populations within species, elevating PopPerSpp; may lead to similar levels of TotPopR as different factors drive population richness; specialized adaptations at low latitudes decrease within-species GD, but increase GD across many species | High latitudes: lower |
High latitudes: higher PopPerSpp similar TotPopR |
High latitudes: lower TotGenDiv higher GenPerSpp |
|
| References: Currie et al. (2004), Pianka (1966), Mittelbach et al. (2007) | ||||
| Evol | Diversification rates: Diversification rates were historically faster at low latitudes, now are becoming faster at higher latitudes, but there are still elevated extinction rates at high latitudes relative to lower latitudes | Low latitudes: higher | Low latitudes: lower PopPerSpp | Low latitudes: higher |
| similar TotPopR | ||||
| Explanation: Low latitudes had a head start with higher diversification rates and lower extinction rates so there is higher species richness and GD at low latitudes; as high latitudes are experiencing increasing diversification rates, populations are still undergoing differentiation so higher PopPerSpp at high latitudes, but more similar TotPopR across latitudes since many populations already established among species at low latitudes, while many are still differentiating at high latitudes | High latitudes: lower |
High latitudes: higher PopPerSpp similar TotPopR |
High latitudes: lower | |
| References: Schluter (2016), Weir and Schluter (2007) | ||||
| Evol | Evolutionary speed: Higher temperatures lead to higher mutation rates, therefore increasing genetic divergence (may only apply to ectotherms) | Low latitudes: higher | Low latitudes: lower PopPerSpp | Low latitudes: higher TotGenDiv |
| Explanation: Higher temperatures at low latitudes result in more mutations leading to speciation, and therefore fewer populations per species, but more genetic diversity across species due to accelerated mutation rates across different populations | High latitudes: lower | High latitudes: higher PopPerSpp | High latitudes: lower TotGenDiv | |
| References: Mittelbach et al. (2007), Rohde (1992), Schluter (2016) | ||||
| Evol | Climate change: Milankovitch cycles stronger at high latitudes, thus high latitude species have better dispersal and less speciation than low latitude species | Low latitudes: higher | Low latitudes: lower PopPerSpp | Low latitudes: lower GenPerSpp |
| Explanation: Less dispersal and mixing at low latitudes leads to populations differentiating more in tropics, leading to fewer populations at low latitudes. GD maintained within species at high latitudes due to more gene flow | High latitudes: lower | High latitudes: higher PopPerSpp | High latitudes: higher GenPerSpp | |
| References: Dynesius and Jansson (2000), Mittelbach et al. (2007), Pianka (1966) | ||||
| Evol | Ecological opportunity hypothesis: Higher speciation rates due to more ecological niches stemming from higher solar energy and annual productivity, reduced temperature seasonality or stronger biotic interactions at low latitudes | Low latitudes: higher | Low latitudes: lower | Low latitudes: higher TotGenDiv |
| lower GenPerSpp | ||||
| Explanation: Many niches already filled at low latitudes from speciation, whereas in higher latitudes more niches are becoming open, thus populations have begun to differentiate—but not fully; lower latitudes have accumulated more GD across many niches, but less within a given species | High latitudes: lower | High latitudes: higher | High latitudes: lower TotGenDiv | |
| References: Schluter (2016) | ||||
- Abbreviations: Ecol, ecological; Evol, evolutionary; GD, genetic diversity; GenPerSpp, genetic diversity per species; Hist, historical; PopPerSpp, populations per species; TotGenDiv, total genetic diversity across species; TotPopR, total population richness for a given area.
To our knowledge, this is the first conceptual review to link core concepts of population genetics, population ecology, and macroecology to help move towards a better understanding of biodiversity gradients at species and below species levels. We hope the review encourages further interdisciplinary collaboration and continuous integration of such broad-scale concepts.
2 REVIEW: UNDERSTANDING THE LATITUDINAL GRADIENT OF BIODIVERSITY VIA THREE LEVELS
2.1 Historical framework
Historical hypotheses examine Earth’s history and how the duration and extent of environments in the past structure current species richness patterns (Brown, 2014; Mittelbach et al., 2007; Miller & Román-Palacios, 2019; Sandel et al., 2011). Two of the most encompassing historical hypotheses are the time and area hypotheses (Table 1; Fine, 2015; Li & Wiens, 2019; Mittelbach et al., 2007; Pianka, 1966; Schluter, 2016; Willig et al., 2003). These hypotheses are based largely on the historical extent of the tropical region being larger and older than that of temperate regions (e.g. not covered with glaciers, greater latitudinal extent due to warming periods in the early Tertiary; Fine, 2015; Mittelbach et al., 2007; Pianka, 1966; Sluijs et al., 2006). Namely, the tropics have had more time and historically more space for organisms to speciate, leading to many species radiating from tropical environments towards temperate ones (Fine, 2015; Stephens & Wiens, 2003; Wiens & Donoghue, 2004). Of course, these expectations may be context-dependent. For example, they may apply less in the Americas since temperate North America is larger than more tropical Central/South America. The area available for speciation in the tropics would then be considerably less than in the relatively species-poor Northern Hemisphere, with consequences for broad-scale patterns of intraspecific diversity.
2.1.1 Historical framework: Population richness
Extended to the population level, the greater time and area for species to live in the tropics may have also provided ample time for populations to differentiate across a heterogeneous tropical habitat (Mittelbach et al., 2007; Rosenzweig, 1995; Terborgh, 1973). Therefore, across the very large number of species at low latitudes, we might expect high numbers of populations overall (TotPopR; Table 1). This high TotPopR might still apply to tropical environments in the Western Hemisphere even though contemporarily they have a smaller geographic area relative to North America, because the low latitude environments have been open and inhabited longer by species than habitable area at higher latitudes. On the other hand, the smaller contemporary area of tropical environments also means that there is now less opportunity for new populations to diverge within species (PopPerSpp) compared to within temperate environments (see also related concepts on species range sizes and diversification rates in the ecological and evolutionary frameworks below, respectively). Moreover, the lower number of temperate species in North America have had less time residing in open (connected) habitats, resulting in incomplete speciation but greater population structure across a species’ range. Such incomplete speciation in turn would likely lead to higher PopPerSpp than found at low latitudes and reduce or perhaps even eliminate tropical versus temperate differences in TotPopR. Aside from the ample time that species have had occupying low latitudes in the past, we next consider how historical adaptations can structure future evolution, and the implications for population richness.
The ancestral niche of a species clade determines future regions and habitat that the clade can disperse to and persist in, a phenomenon known as phylogenetic niche conservatism (Ackerly, 2003; Peterson, Soberon, & Sanchez-Cordero, 1999; Ricklefs & Latham, 1992; Wiens, 2004; Wiens & Donoghue, 2004). A species can then only broaden its niche through niche evolution and dispersal (Sandel et al., 2011; Wiens & Donoghue, 2004), which would influence population differentiation across a species’ range. For example, niche evolution may result in an increase in genetic differentiation among populations at range edges due to strong directional selection, similar to expectations from the central-marginal hypothesis (Eckert, Samis, & Lougheed, 2008; Guo, 2012; Hargreaves & Eckert, 2019; Willi, Fracassetti, Zoller, & Buskirk, 2018). With more differentiated populations at range edges, we might expect high latitudes to have higher PopPerSpp, as species that have expanded their ranges outwards from the tropics would likely have more populations in these high latitude areas. Conversely, low latitude clades would again be expected to have lower PopPerSpp. These clades are typically older, having had more time for speciation of the edge populations to occur, thus resulting in fewer populations in the remaining range.
Overall, when considering historical influences, we would expect higher PopPerSpp at high latitudes relative to low latitudes. Predictions about TotPopR are less clear in part because they are dependent on the magnitude of the difference in species richness and PopPerSpp between tropical and temperate regions. Nonetheless, more similar levels of TotPopR across latitudes seem plausible. For example, while the sheer number of species in the tropics may result in a high TotPopR at low latitudes, multiple factors discussed here may also generate high or higher TotPopR at high latitudes; or at least TotPopR might not be proportional to species richness or PopPerSpp. In short, these points illustrate how species richness and population richness may be uncoupled in many instances.
2.1.2 Historical framework: Genetic diversity
The historical extent of habitat and its availability can also influence current patterns of genetic diversity, but we would expect an opposite pattern from population richness—higher genetic diversity at low to intermediate latitudes. For example, since more time has passed for evolution to occur at lower latitudes, genetic diversity would accumulate across species (TotGenDiv) (Adams & Hadly, 2013). Average genetic diversity across populations within a tropical species (GenPerSpp) could also follow the same pattern as TotGenDiv, since older clades tend to have more genetic diversity (Willi et al., 2018). Conversely, habitat has been available for less time at high latitudes so organisms may not have had sufficient time to accumulate as much genetic diversity.
In addition to purely just having had more time or not, clade age and history would play a large role in structuring patterns of genetic diversity. For example, many North American clades have experienced glacial fragmentation across their ranges, followed by founder effects after glacial retreat. Founder effects like these can lead to an overall decrease of genetic diversity within a species (GenPerSpp) (Galbreath & Cook, 2004; Green, Sharbel, Kearsley, & Kaiser, 1996; Hewitt, 2000; Provan & Bennett, 2008; Stewart, Lister, Barnes, & Dalen, 2010; Willi et al., 2018). Additionally, adaptation to colder or new environments (i.e. niche evolution, Wiens & Donoghue, 2004) tends to elicit strong directional selection, often leading to losses in GenPerSpp (Eckert et al., 2008; Hargreaves & Eckert, 2019; Pierce, Gutierrez, Rice, & Pfennig, 2017).
GenPerSpp and TotGenDiv therefore may be lowest at high latitudes due to the strong influence of historical events and the recent establishment of populations [e.g. after the Last Glacial Maximum 23,000–18,000 yr bp (Hewitt, 2004)], leading to less time for alleles to accumulate in a given population from mutations. Miraldo et al. (2016) may support this expectation in finding higher mitochondrial genetic diversity (TotGenDiv) at low latitudes. However, re-analyses of their data (Gratton et al., 2017; Schluter & Pennell, 2017) showed a systematic northward bias in spatial autocorrelation and that the pattern was not consistent across species (i.e. GenPerSpp). Despite this, Schluter and Pennell (2017) demonstrated that mammalian and amphibian mitochondrial genetic diversity, equivalent to GenPerSpp here, has a slightly negative slope with latitude. Another study found some evidence for scale- and taxa-dependent latitudinal gradients in genetic diversity (Millette et al., 2019). These results are mostly consistent with the expectations from a historical perspective, wherein species at low latitudes have experienced more time for genetic diversity to accumulate, but some nuances appear to blur gradient patterns.
2.1.3 Historical framework: Conclusion
In general, historical hypotheses tend to predict higher PopPerSpp at high latitudes, similar levels of TotPopR across latitudes and more genetic diversity at low latitudes. While the historical time and area available for diversification may form the foundation from which species evolve, current patterns of intraspecific diversity may be the product of both historical and contemporary processes. Changes in population differentiation and genetic diversity can occur relatively quickly throughout time (e.g. tens to hundreds of years instead of thousands or millions) due to stochastic processes as well as increasing anthropogenic impacts (Goossens et al., 2006; Riley et al., 2006; Weider, Lampert, Wessels, Colbourne, & Limburg, 1997). Human impacts can cause species range barriers more quickly than otherwise expected, greatly reducing connectivity and thus increasing the likelihood of population differentiation (Ascensão et al., 2016; Cheptou, Hargreaves, Bonte, & Jacquemyn, 2017; Meyer, Kalko, & Kerth, 2009; Riley et al., 2006). Alternatively, human influences can homogenize populations by the movement of individuals through introductions, translocations, stocking and supplementation (Johnson et al., 2010; Tringali & Bert, 1998). The roles and effects of more contemporary ecological hypotheses are discussed in more depth below.
2.2 Ecological framework
Ecological hypotheses focus on the mechanisms underlying species coexistence, maintenance, and responses to abiotic elements on Earth (Brown, 2014; Mittelbach et al., 2007), which are more relevant to contemporary timeframes. Most hypotheses falling under this umbrella have been reviewed (Fine, 2015; Willig et al., 2003), including population dynamics (e.g. species range sizes or population sizes), resource availability, local dispersal, spatial heterogeneity and biotic interactions. Due to the wide range of these hypotheses, we will not review each of them here, although many are presented in Table 1. Instead, we consider one of the more relevant hypotheses that applies to population richness and genetic diversity: population dynamics.
2.2.1 Ecological framework: Population richness
A prominent hypothesis of population dynamics is Rapoport’s rule (Ruggiero & Werenkraut, 2007; Stevens, 1989), the positive correlation of geographic range size with latitude, focusing on the climatic variation that organisms are exposed to and adapted for. Temperate species tend to populate large geographic ranges, so they experience a wide range of climatic variation; tropical species, conversely, are limited to small geographic ranges due to specialization, with some exceptions (Ruggiero & Werenkraut, 2007; Stevens, 1989). If a species has a small range, there is less area for populations to become isolated and differentiated, and the species will likely have fewer populations. Additionally, each population may have smaller population sizes as Currie et al. (2004) noted that both population size and density of individuals decrease towards low latitudes. This could interfere with the ability of populations to become established in a new area if they have small suitable ranges within which to disperse. If consistent across taxa, species with large range sizes may, in general, harbour more PopPerSpp, leading to a reverse latitudinal trend for population richness relative to species richness.
The size of a species’ range would also influence gene flow and subsequent population differentiation. Greater distances between populations in large-ranged species would make gene flow more difficult, all else being equal. Thus, according to Rapoport’s rule, we would expect populations at or near the edge of a species’ range to experience lower gene flow. Range-edge populations are more likely to be geographically isolated and more differentiated from neighbouring populations, particularly for large-ranged species (Eckert et al., 2008; Hargreaves & Eckert, 2019; Pelletier & Carstens, 2018; Stevens, 1989; Willi et al., 2018). A likely consequence across a species’ range would be more distinct populations at or near range edges, and fewer within the ‘core’ due to increased gene flow (Pelletier & Carstens, 2018). This could result in higher TotPopR in areas where many species range edges overlap extensively, that is, at low latitudes. Overall, larger range sizes tend to be associated with increased distances between populations, leading to the expectation of increasing PopPerSpp with range size.
2.2.2 Ecological framework: Genetic diversity
A species’ range size could also reflect levels of genetic diversity, where small ranges may have lower genetic diversity per species (GenPerSpp) (Fine, 2015). Small population size or density, typical of tropical species, can lead to increased levels of ecological or genetic drift (Fine, 2015; Mittelbach et al., 2007; Siqueira et al., 2019). While genetic drift has not received much empirical support as an explanation for the species richness gradient, it could be more relevant for intraspecific diversity. As patch size or a species’ range size is correlated with population size (Bernos & Fraser, 2016; Currie et al., 2004), it is a reasonable assumption that species with small ranges have, on average, smaller population sizes. This would lead to increased levels of genetic drift and an increased possibility of inbreeding, and hence lower GenPerSpp. Alternatively, for species that are population rich, different populations could drift in different directions, and show an inflated GenPerSpp collectively across the species. Herein the combined analysis of genetic diversity metrics such as observed heterozygosity and mean number of alleles (MNA) within species (see GenPerSpp in Glossary) could provide a better indication of genetic diversity within a species or taxonomic group. MNA responds to inbreeding, population bottlenecks and genetic drift more quickly than heterozygosity (Allendorf, 1986; Allendorf & Luikart, 2007; Nei, Maruyama, & Chakraborty, 1975) and could indicate which populations are at risk of low genetic diversity. Additionally, heterozygosity can relate to long-term effective population size (see Glossary; Bazin et al., 2006; Hansson & Westerberg, 2002) and in some instances, adaptation to environmental change (Fraser et al., 2019; Saccheri et al., 1995). Assessing either MNA or heterozygosity alone might mask some of these potential patterns and thus it is important to distinguish between the two metrics. However, larger geographic ranges may not necessarily correspond to higher levels of GenPerSpp. As previously discussed, many high latitude organisms have also experienced glacial fragmentation that has resulted in a history of small population size and thus lower overall GenPerSpp and TotGenDiv (Galbreath & Cook, 2004; Green et al., 1996; Hewitt, 2000; Provan & Bennett, 2008; Stewart et al., 2010).
A species’ range size and dispersal abilities also influence the extent of gene flow between core and edge populations, affecting the maintenance of genetic diversity (Bohonak, 1999; Martinez et al., 2018; Pelletier & Carstens, 2018; Willoughby et al., 2017). For example, having a large geographic range but being mobility-limited, such as in small rodents, reduces the likelihood of gene flow between northern and southern populations strictly because these would be so far apart. Conversely, species with small geographic ranges may have comparatively more gene flow across their range and have fewer genetically distinct populations. Fishes show interesting patterns: large ranges typical of marine or anadromous fishes tend to have higher genetic diversity and lower population differentiation, whereas freshwater fishes with typically limited dispersal capabilities tend to have lower genetic diversity and higher population differentiation (DeWoody & Avise, 2000; Martinez et al., 2018; Medina et al., 2018). Thus, species that are not as capable of dispersing large distances may show stronger latitudinal patterns for both population and genetic diversity, regardless of range size, due to differences in population differentiation across their range (Bohonak, 1999). Populations may become more easily differentiated in large ranges, and as a result, local adaptation may inflate total genetic diversity across all populations (TotGenDiv) within a species’ range. Interestingly, however, non-migratory vertebrate species tend to have more genetic diversity than their migratory counterparts, except for birds, which show the opposite pattern (Willoughby et al., 2017). This could be an indication that migratory species more frequently encounter fragmentation that causes reductions in genetic diversity, potentially blurring the otherwise expected pattern of increasing genetic diversity with range size.
2.2.3 Ecological framework: Conclusion
Overall, biological differences between taxonomic groups can play a large role in determining population richness and genetic diversity gradients. In general, we expect large-ranged, limited dispersers to show more intraspecific diversity, and small-ranged, capable dispersers to show lower intraspecific diversity. If intraspecific diversity generally increases with range size, we would expect both higher population richness and genetic diversity at higher latitudes. However, dynamics within particular taxonomic groups could cause population richness and genetic diversity gradients to be much more idiosyncratic than species gradients.
2.3 Evolutionary framework
To further clarify the expectations for diversity gradients, we next consider hypotheses taking an evolutionary approach that focuses on rates of diversification and how these are affected by abiotic and biotic environmental factors (Mittelbach et al., 2007). The premise is simply that the tropics are older, warmer and have had historically higher diversification rates along with lower extinction rates than temperate latitudes (Mittelbach et al., 2007; Pianka, 1966; Schluter, 2016; Schluter & Pennell, 2017; Weir & Schluter, 2007). Proposed explanations for higher diversification rates in the tropics include: enhanced tropical genetic drift (Fedorov, 1966; Mittelbach et al., 2007); stronger high latitude climate change cycles (Dynesius & Jansson, 2000; Mittelbach et al., 2007); greater geographic extent allowing for diversification across space (Mittelbach et al., 2007; Terborgh, 1973); narrow physiological tolerances in the tropics (Ghalambor, 2006; Janzen, 1967; Mittelbach et al., 2007; Stevens, 1989); temperature effects on evolutionary speed (Mittelbach et al., 2007; Orton, May, Ly, Lee, & Adamowicz, 2019; Rohde, 1992); a stronger influence of biotic over abiotic interactions in the tropics; and greater ecological opportunities (Schluter, 2016). Many of these explanations are outlined in Table 1 and overlap with discussions under the other two frameworks. The extent of support for these proposed explanations is variable, but whichever factor(s) caused increased tropical speciation rates in the past appear to be shifting in current times (Orton et al., 2019; Schluter, 2016; Schluter & Pennell, 2017; Weir & Schluter, 2007). This shift has consequences for current patterns of biodiversity; as speciation slows at low latitudes and increases at high latitudes the latitudinal gradient in species richness may dissolve as temperate regions ‘catch up’ in species richness. It is also important to consider rates of extinction along with speciation—low latitude extinction rates could increase if climate changes so drastically that species struggle to keep within suitable habitat (Sandel et al., 2011), potentially changing the gradient more quickly.
2.3.1 Evolutionary framework: Population richness
Temperate clades are seeing an increase in speciation rates, and this is at least in part due to the opening of available habitat (Schluter, 2016; Schluter & Pennell, 2017). Schluter (2016) describes this as the ecological opportunity hypothesis, wherein areas having more open niches tend to correspond with faster diversification rates. Low latitudes have historically had a wider range of niches and higher rates of speciation than higher latitudes, giving the tropics a ‘head start’ to accumulate species. If this is the case, the tropics could have ‘maxed out’ on speciation rates and population richness; species are now restricted to small ranges due to specialization of niches and have on average fewer populations (PopPerSpp). Note again it is possible that if every species has at least one population, the tropics could have higher TotPopR than temperate regions simply due to having much higher species richness, although this effect could be mediated if high latitude species have much higher PopPerSpp. This is where comparing PopPerSpp and TotPopR is useful. The tropics might have a higher or similar absolute number of populations (TotPopR), but higher latitudes—which tend to have larger species ranges (Stevens, 1989) and now increasing speciation rates—would have a higher PopPerSpp (Figure 1).

Increasing diversification rates at high latitudes has consequences for PopPerSpp. As climate shifts open historically inhospitable regions in temperate areas, more diversification is facilitated (Schluter, 2016; Schluter & Pennell, 2017; Weir & Schluter, 2007). This diversification process leads to higher PopPerSpp as species begin moving into novel habitats, without completing speciation due to insufficient time. Faster diversification rates should lead to more populations diverging, thus leading to an increase in PopPerSpp, and TotPopR for a given area over time.
2.3.2 Evolutionary framework: Genetic diversity
Diversification rates can also play a role in a species’ ability to adapt and maintain genetic diversity. For example, if a species experiences faster diversification rates at the edge of its range due to strong directional selection pressures, a given population could become locally adapted and may see a drop in genetic diversity relative to other populations (Eckert et al., 2008; Ellegren & Galtier, 2016; Guo, 2012; Hargreaves & Eckert, 2019; Willi et al., 2018). Higher diversification rates could lead to more rapid population differentiation, leading to decreases in fitness should ongoing gene flow occur (Schluter, 2016; Seidel, Rockman, & Kruglyak, 2008) and encouraging further speciation. Because this process of diversification has likely already occurred at low latitudes, they may remain a hotspot for genetic diversity across species (TotGenDiv). However, we expect patterns of within-species genetic diversity (GenPerSpp) to be sensitive to taxonomy, particularly for older clades, which may have accumulated more genetic diversity, whether such clades originated at high or low latitudes.
2.3.3 Evolutionary framework: Conclusion
Evolutionary processes such as diversification rates influence intraspecific diversity expectations by affecting the trajectory of populations within species. While PopPerSpp is expected to be highest at high latitudes due to incomplete diversification within species, TotPopR may have similar levels across latitudes regardless due to many populations across a much larger number of species having already diverged or partially diverged over time. Genetic diversity is predicted to be typically higher at low latitudes, especially for TotGenDiv where diversification across species has led to the accumulation of genetic diversity. On the other hand, trends for GenPerSpp are much more variable across taxonomic groups due to different diversification rates and decreases in genetic diversity from niche specialization and/or novel adaptations.
3 REVIEW SUMMARY: LATITUDINAL PREDICTIONS
- Species richness is highest at low latitudes.
- Within-species population richness (PopPerSpp) is highest at high latitudes, but among-species population richness (TotPopR) may be more similar across latitudes.
- Genetic diversity patterns are more variable and have no clear latitudinal gradient across species.
The predictions for latitudinal genetic diversity patterns are more difficult to untangle due to the combined effects of history and current population size/distribution, and perhaps even the limited range/variability of genetic diversity levels (see Leffler et al., 2012). On one hand, because tropical species tend towards smaller geographic ranges, one could envision lower genetic diversity in these groups. On the other hand, tropical species tend to be older and inhabit more stable environments, and so some authors have suggested that genetic diversity could be maintained/accumulated throughout time (Adams & Hadly, 2013; Smith et al., 2017). Complicating expectations further, temperate species have a longer history of fragmentation, bottlenecks and founder effects, which all may contribute to a sharp decline in genetic diversity at high latitudes. This glacial history at high latitudes likely plays a large role in structuring genetic diversity patterns, with greater TotGenDiv at low latitudes but perhaps the highest GenPerSpp at intermediate latitudes. For example, species at intermediate latitudes are likely to have more variable clade ages (Schluter, 2016), to have experienced fewer genetic bottlenecks, to have larger range sizes than tropical species (Stevens, 1989) and to have intermediate levels of gene flow across their range. Complicating expectations even further, anthropogenic impacts are highest at intermediate latitudes where most land conversion for agriculture and human population density exist (Cincotta, Wisnewski, & Engelman, 2000; Ellis & Ramankutty, 2008; Gibbs et al., 2010; Matthews, 1983). While the broad-scale impacts of humans on species genetic diversity are unclear (Millette et al., 2019), they could blur latitudinal patterns if human activities causing habitat loss reduce genetic diversity in regions where high levels of genetic diversity might be otherwise expected (see Ascensão et al., 2016; Cardillo et al., 2004; Cincotta et al., 2000). Collectively, a number of factors operating differently along the latitudinal gradient appear to have varying consequences for genetic diversity both among and within species. Thus, genetic diversity is not expected to have a clear latitudinal gradient relative to species or population richness.
4 NEW ANALYSES DRAWING FROM REVIEW
4.1 Intraspecific diversity and range size: Hypotheses and predictions
Hypotheses describing latitudinal species richness have direct links to both population richness and genetic diversity. These links form the foundation upon which we further elaborate on population richness and genetic diversity expectations relative to a species’ range size. We outline and test three novel hypotheses to explain latitudinal trends in intraspecific diversity. Data used to test the hypotheses below were obtained from the MacroPopGen database (Lawrence et al., 2019), a georeferenced dataset of microsatellite genetic diversity for almost 900 vertebrate species and over 9,000 genetically distinct populations across the Americas (see Supporting Information Appendix S1 for details). Populations were designated as genetically distinct within MacroPopGen using a commonly applied, operational definition of a population (reviewed in Waples & Gaggiotti, 2006); population richness in the database (PopPerSpp or TotPopR) represented only populations that had been sampled with microsatellite loci. As such, some observed patterns may not be as strong as otherwise expected perhaps due to sampling bias of populations. We strive to acknowledge this in our discussion of results below. Range size data came from International Union for Consevation of Nature (IUCN), BirdLife International (IUCN, 2016, BirdLife International, 2017), and Meiri et al. (2017). As an indication of sampling intensity across the Americas, we mapped sampled species richness and population richness as well (Figure 2).

4.1.1 H1: Geographic distribution hypothesis
We term the first hypothesis the geographic distribution hypothesis, which posits that a positive relationship exists between a species’ geographic range size and its population richness. PopPerSpp should therefore increase with increasing latitude because temperate species ranges are typically larger than in tropical species. Broadly speaking, we also expect different vertebrate groups to show different strengths for this pattern because of inherent differences between dispersal capabilities and environments inhabited (Sandel et al., 2011). For example, relative to other vertebrates, freshwater and anadromous fish species may show greater TotPopR and/or greater PopPerSpp across their ranges due to the easily fragmented nature of aquatic freshwater habitats through natural barriers (Tatarenkov, Healey, & Avise, 2010; Wofford, Gresswell, & Banks, 2005; Underwood, Mandeville, & Walters, 2016), dams (Roberts, Angermeier, & Hallerman, 2013; Wofford et al., 2005; Underwood et al., 2016), and the connectivity between fluvial environments and lakes (Hébert, Danzman, Jones, & Bernatchez, 2000; Underwood et al., 2016). Amphibians and reptiles (collectively, herptiles) may also show strong patterns between range size and PopPerSpp due to their generally limited ability for dispersal (Araújo, Pearson, & Rahbek, 2005; Green et al., 1996; Medina et al., 2018; Sandel et al., 2011) that leads to high subpopulation differentiation across a given species’ range. Birds and some mammals, conversely, tend to have greater dispersal capabilities than herptiles and some freshwater fishes (Araújo et al., 2005; Medina et al., 2018; Munguía, Townsend Peterson, & Sánchez-Cordero, 2008; Servín, Sánchez-cordero, & Gallina, 2003; Sutherland, Harestad, Price, & Lertzman, 2000). Thus, we expect these groups will have a lower TotPopR than fishes and herptiles due to homogenization of population structure, but more variable PopPerSpp depending on the specific species’ dispersal ability.
To test the geographic distribution hypothesis, we used a generalized linear model fitted with a gamma distribution where the number of populations for a given species (i.e. PopPerSpp, Supporting Information Table S1) was our dependent variable (n = 625 species, 5,172 populations; see Supporting Information Appendix S1: H1), while the natural logarithm of range size (km2), latitudinal extent (decimal degrees), and taxonomic class (amphibian, bird, anadromous or freshwater fish, mammal, reptile) were fixed effects. PopPerSpp and range size for each species can be found in Supporting Information Table S1. We also tested the linear relationships between range size and PopPerSpp for each taxonomic group (Figure 3a,b). These linear relationships were significant for all taxonomic groups combined (p .001, R2 = .03; Figure 3a), and fish separately (p = .003, R2 = .07; Figure 3b), although they did not explain much variation in the data. There were no significant relationships between range size and PopPerSpp within amphibians, birds, mammals or reptiles (Figure 3b). For the generalized linear model, both the natural logarithm of range size and the latitudinal extent were significant (p = .022, < .001 respectively, Figure 3a, Supporting Information Table S2). The discrepancy across taxonomic groups could be due to a lack of thorough sampling across species ranges. When assessing taxonomic groups separately, amphibians, reptiles and birds tended to have data that were sparsely sampled across species ranges compared to other species, especially fishes. To account for this, we recommend future studies estimate the area represented by each population so that the percent of the species’ range that has been sampled can be included.

4.1.2 H2: Overlapping range hypothesis
Areas that have extensive species range overlap may have lower PopPerSpp due to higher competition, smaller range sizes, etc. (Kennedy et al., 2018; Pelletier & Carstens, 2018). For instance, lower latitudes are more likely to have high species richness, moderate TotPopR and lower PopPerSpp. Species richness is to the point of oversaturation at low latitudes (Schluter, 2016), and tropical species are generally restricted to smaller ranges (Currie et al., 2004; Stevens, 1989). The combination of small range sizes and fewer open niches would lower the number of intraspecific populations able to differentiate (or speciate with time), because fewer opportunities for local adaptation or population differentiation are available to occur across a species’ range (Schluter & Pennell, 2017; Weir & Schluter, 2007). Collectively, one might expect TotPopR to increase as species richness increases, but PopPerSpp to decrease with increasing species richness (overlapping range hypothesis).
To test the overlapping range hypothesis, we calculated the species richness in 500-km2 equal area grid cells generated in the Behrmann projection across the American continents and correlated it using a linear model with both the absolute population richness (TotPopR) and the number of sampled populations of each species (PopPerSpp) (Figure 3c, Supporting Information Table S3; see Supporting Information Appendix S1: H2). The number of species in an area was positively correlated with TotPopR (p < .001, R2 = .75; Figure 3c, Supporting Information Table S3), and PopPerSpp (p < .001, R2 = .22, Figure 3d). While our analysis does not show the expected trend for PopPerSpp, this may again be due to incomplete population sampling for each species in the dataset. We note that the slopes of the two relationships (4.74 and 0.08 for TotPopR and PopPerSpp, respectively; Supporting Information Table S3) do provide some indication that the trends between the two population richness metrics are different and that different mechanisms may underpin them. Perhaps as species richness increases, PopPerSpp does not increase at a corresponding rate, indicating that species richness has some impact on the capacity for evolution of population richness within a species. If the actual number of populations within a species’ range is known, we expect this positive relationship between PopPerSpp and species richness to break down further, showing the negative relationship as predicted, or a very weak relationship.
4.1.3 H3: Range-restricted gene hypothesis
If species range size influences population size and gene flow between populations (Currie et al., 2004; Fine, 2015), and range size is also correlated with PopPerSpp (H2), then genetic diversity will be more strongly associated with range size than with latitude (range-restricted gene hypothesis)—although some latitudinal patterns may occur as a result of this association. Previous studies have found latitudinal trends for genetic diversity, where higher alpha and beta genetic diversity (equivalent to GenPerSpp and TotGenDiv, respectively) were observed at low latitudes (Adams & Hadly, 2013; Miraldo et al., 2016; Schluter & Pennell, 2017). Even when spatial autocorrelation (Gratton et al., 2017), number of DNA sequences and species identity (GenPerSpp) (Schluter & Pennell, 2017) were accounted for, authors found a latitudinal gradient in genetic diversity—although the slope of the relationship was very small (e.g. −0.002, Supplementary Methods in Schluter & Pennell, 2017). However, these data were based on mitochondrial genetic diversity (mitochondrial DNA, mtDNA) rather than nuclear DNA. mtDNA may not be selectively neutral (Bazin et al., 2006), which is important for standardized comparisons across species and populations. Moreover, mtDNA may not reflect genetic variation in the nuclear genome, which is integral for adaptation to environmental change (Ballard & Whitlock, 2004; Bazin et al., 2006; Ghalambor, McKay, Carrols, & Reznick, 2007; Hurst & Jiggins, 2005; Sgrò, Lowe, & Hoffmann, 2011). Conversely, microsatellite nuclear DNA variation can be a reasonable metric of genome-wide variation, and the polymorphic nature of microsatellite loci is able to better resolve population structure at fine scales (Angers & Bernatchez, 1998; Jarne & Lagoda, 1996; Väli, Einarsson, Waits, & Ellegren, 2008). Microsatellite-based estimates of GenPerSpp may show a weaker latitudinal pattern than the TotGenDiv metric adopted in past mtDNA studies (e.g. Miraldo et al., 2016). Although non-neutrality has been observed in some studies involving nuclear microsatellite loci (Ranathunge et al., 2018; Selkoe & Toonen, 2006; Wiehe, 1998), this does not appear to be widespread in MacroPopGen (Lawrence et al., 2019; see also Selkoe & Toonen, 2006).

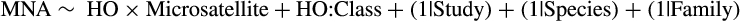
The retention of HO, MNA and the number of microsatellite loci in the models is not entirely surprising and indicates that these factors are more associated with genetic diversity than range size or latitudinal extent, although this effect varies according to taxonomic grouping (Figure 3f). While these measures of genetic variation are sometimes (weakly) correlated (Comps, Gömöry, Letouzey, Thiébaut, & Petit, 2001), the two metrics still indicate differences in population processes, as we discussed in the ecological framework, where decreases in MNA do not always correspond with decreases in HO (Allendorf, 1986). Additionally, we used variance inflation factors to test for collinearity between variables and found no evidence for any statistically significant collinearity (see Supporting Information Appendix S1: H3). Thus, we wanted to include both metrics in model selection to test how the effects of range size would compare to the effect of each metric on each other. Indeed, when we tested a model that only included range size and latitudinal extent, only latitudinal extent (not range size) was significant. Figure 3e demonstrates this lack of significance for range size, while a positive relationship is found in Figure 3f (note only MNA is shown but results were similar for HO). Varying relationships among taxa were also supported by the different slopes of linear relationships shown in Figure 3f. The inclusion of HO, MNA and number of microsatellite loci could indicate that genetic diversity metrics are sensitive to the number of alleles present within a population, where more alleles and loci being present increases the likelihood of being heterozygous and vice versa (Figure 3f). Together, the results of these models suggest that genetic diversity is not particularly influenced by range size or the latitudinal breadth of a species’ range.
5 NEW ANALYSIS SUMMARY
We proposed three hypotheses relating range size to population richness and genetic diversity, taking inspiration from a synthesis of species richness theories. However, we found minimal support for our hypotheses, highlighting the idiosyncrasies in intraspecific diversity patterns previously found between taxonomic groups (DeWoody & Avise, 2000; Martinez et al., 2018; Medina et al., 2018; Millette et al., 2019; Willoughby et al., 2017). While we have not explicitly considered taxa-specific traits (e.g. migratory behaviour, age at maturity, body size), the differences found between taxonomic groups may indicate that such data could further explain trends in intraspecific diversity.
Overall, we found marginal support for two of our three hypotheses. This is likely due to a number of factors, one being that accurate data for population richness are under-developed, as many populations are under-sampled. Additionally, large range sizes may not necessarily correspond with more genetic diversity. For example, animals with larger body sizes may have large range sizes but relatively lower population sizes simply because they need more space per individual or per population. This could mean that a large-ranged animal may still have fewer individuals per population, resulting in fewer populations overall and potentially lower genetic diversity. Future analyses should consider factors such as body size in conjunction with range size to better explain variation in genetic diversity.
Of the taxa examined in our analyses, fishes had the strongest, most significant positive relationships between range size, population richness (Figure 3b) and the genetic diversity metrics (Figure 3e,f). This latter relationship was particularly steep for anadromous fish, consistent with previous works that have found that anadromous fishes tend to have higher genetic diversity than freshwater fishes (DeWoody & Avise, 2000; Martinez et al., 2018). All other taxonomic groups did not show significant relationships between range size and population richness. This is likely due to incomplete sampling across species ranges relative to many of the fish species in this database, leading to an underrepresentation of population richness (e.g. average PopPerSpp for anadromous fish = 109, amphibians = 20, Supporting Information Table S4). This underrepresentation could also be affecting our results for range size with genetic diversity—perhaps the populations that were sampled from species with large ranges happened to be lower (or higher) in genetic diversity then otherwise expected. This is a sort of sampling bias that could be corrected if we had complete data on populations for a few large- and small-ranged species to investigate further.
Our results contribute to the idea that disentangling intraspecific diversity patterns can be much more complicated than species richness as many factors require simultaneous consideration (see Blanchet et al., 2017; Marchesini et al., 2018; Martinez et al., 2018; Medina et al., 2018; Millette et al., 2019; Paz-Vinas et al., 2018; Willoughby et al., 2017). The limited scope in the scale of genetic diversity, and perhaps the minimum and maximum degree of genetic diversity required for viable populations (e.g. 0 to 1 for heterozygosity; Ellegren & Galtier, 2016; Leffler et al., 2012) could also have a major impact on the detection of broad-scale patterns. The magnitude of differences in genetic diversity across a latitudinal gradient would additionally not be as large as seen in the species richness gradient. For example, there are at least c. 143% more species in tropical relative to temperate countries (e.g. Brazil: c. 170,000–210,000 known species, Canada: c. 70,000 known species; Canadian Endangered Species Conservation Council, 2001; Lewinsohn & Prado, 2005). In contrast, Miraldo et al. (2016) only found 27% more total mitochondrial genetic diversity in the tropics, summed across terrestrial mammals and amphibians (i.e. TotGenR). The influence of these factors could explain why our analyses of intraspecific diversity do not show as clear a pattern as species richness, warranting further exploration in tandem with environmental properties, anthropogenic factors and species- or population-specific functional/life history traits.
6 OVERALL CONCLUSION
Although there has been some recent support for latitudinal gradients in intraspecific diversity (Adams & Hadly, 2013; Gratton et al., 2017; Martin & McKay, 2004; Millette et al., 2019; Miraldo et al., 2016; Schluter & Pennell, 2017), no study has generated latitudinal expectations for both population richness and genetic diversity by drawing from species-level literature—indeed there is an admitted lack of theoretical foundation (Millette et al., 2019). We demonstrate that the distinct latitudinal patterns found in species richness are much more complicated at the intraspecific level. Our synthesis suggests that species richness, population richness and genetic diversity within species will be uncoupled in many instances due to a combination of historical and contemporary factors. Factors such as range size (i.e. Rapoport’s rule) and biological differences between and within taxonomic groups appear to play a larger role in moderating population richness and genetic diversity gradients. These inferences have implications for the fundamental understanding of the species richness gradient and for biodiversity conservation, as they shed light on what may drive changes to species distributions and species adaptability at different latitudes in the future.
Our focus on population richness and genetic diversity was complemented by the usage of microsatellite data obtained from the MacroPopGen database (Lawrence et al., 2019). This database does not include adaptive, functional or phylogenetic diversity, as standardized phylogenies below the species level, for example, do not exist for most populations studied with nuclear DNA. We expect future analyses that include these other aspects of intraspecific diversity will only clarify the patterns described here further and perhaps account for some of the noise in the data. As mentioned, the increased sampling of populations within species would also be useful to test latitudinal gradient theories with more certainty. While the relationships presented here may not be very strong, the results are likely to be strongly affected by lack of full sampling within species ranges. As technology advances, results collated from genome-wide assessments will also help refine our hypotheses further and more fully represent genetic diversity and population richness.
While we have largely focused our discussion on the theories for latitudinal patterns in biodiversity, our results also have conservation implications. As larger range sizes are typically associated with greater population richness and genetic diversity, species with small ranges are likely to be at greater risk (Fine, 2015), whereas population rich species are likely to be less at risk from changing conditions. This is reminiscent of the theory of island biogeography where just as smaller areas are associated with fewer species, so are small areas generally associated with fewer genetically distinct populations. Our conclusion may not seem novel, but our study is the first to fully discuss this with respect to populations as a quantifiable unit. These results may have consequences for conservation management where only assessing an area’s species richness may not capture the extent of biodiversity in that area. Assessing population richness for each species and its genetic diversity may give a better indication of ecosystem health and the species’ ability to remain intact (Martinez et al., 2018; Paz-Vinas et al., 2018).
We argue for a more holistic approach in biodiversity science and conservation where all aspects of biodiversity are considered together (ecosystem diversity, species diversity, functional diversity, intraspecific diversity), especially as future technology refines and improves our understanding of intraspecific diversity even further.
GLOSSARY
Genetic diversity: Defined in this review as neutral genetic diversity within a population or species. Often assessed with microsatellite data as observed heterozygosity or allelic diversity/mean number of alleles per locus (MNA).
Observed heterozygosity: A measure of genetic diversity representing the percentage of heterozygous loci of individuals within a population. Declines in isolated populations as effective population size decreases (Coltman & Slate, 2003; Frankham, 1996; Frankham, Ballou, & Briscoe, 2002).
MNA: Mean number of alleles—a measure of genetic diversity where the number of alleles is counted for each locus and averaged across individuals in a population. Declines more rapidly than heterozygosity when effective population size decreases (Coltman & Slate, 2003; Frankham, 1996; Frankham et al., 2002).
Population richness: In general, the number of genetically distinct populations—either across all species (TotPopR), within a species (PopPerSpp) or averaged across many species in an area (AvgPopSpp).
TotPopR: Total population richness—the total number of populations within a given area across species, for example Hughes et al. (1997).
PopPerSpp: Populations per species—refers to how many distinct populations one species has across its range or within an area. For example, an area with many populations would be considered ‘population rich’ according to TotPopR but might be classified as ‘population poor’ by PopPerSpp if each species is represented by only a small number of populations (Figure 1). TotPopR and PopPerSpp have different implications. Analysing both TotPopR and PopPerSpp outlines more clearly which species or taxonomic groups may have more populations, and gains an understanding of the genetic history, along with the vulnerability or level of endemism characterizing a certain species or taxonomic group.
AvgPopSpp: Average number of populations per species within a given area. Calculated by first determining the PopPerSpp for each species in an area, and then averaging these values for all species in the area.
TotGenDiv: Total genetic diversity—reported in previous large-scale syntheses as a sum or mean of genetic diversity across all species and their populations within a given area (Gratton et al., 2017; Miraldo et al., 2016; Willoughby et al., 2015). Does not reflect the genetic diversity between species, and masks idiosyncrasies between lower levels of taxonomic groups, identifiable when assessed in individual species, as in GenPerSpp (Adams & Hadly, 2013; Martin & McKay, 2004). For simplicity in our discussions, we define TotGenDiv as the sum of neutral genetic diversity across all species and their populations. Note that an additional measure to analyse TotGenDiv patterns would be to assess the variance of genetic diversity across species within an area. This would identify regions with abnormal levels of variability in genetic diversity, indicating that the TotGenDiv of the area may be skewed by a certain species. Alternatively, taking the weighted average of genetic diversity across species (e.g. Millette et al., 2019) and populations in an area would account for differences among sample size and/or number of populations in the area (Schluter & Pennell, 2017). Then, assessing sum, variance, and mean genetic diversity together for broad scale analyses yields more refined insights than simply totalling across species.
GenPerSpp: Refers to the sum of neutral genetic diversity within a single species across all its populations in an area—that is, species-specific genetic diversity. Provides a more realistic representation of genetic diversity, allows for idiosyncrasies between groups to be identified, and avoids oversimplification at large scales.
Effective population size: Represents the number of individuals in a population that are contributing to the next generation (Wright, 1931); also gives an indication of how quickly loss of genetic diversity occurs in a finite-sized population through random genetic drift (Belmar-Lucero et al., 2012; Frankham et al., 2002).
ACKNOWLEDGMENTS
We would like to thank members of three labs at Concordia University (Fraser, Peres-Neto, Lessard) for their comments and feedback on the manuscript. In particular, we would like to thank J-M. Matte for consultations on the statistical approaches used here. We would also like to thank the International Union for Conservation of Nature as well as BirdLife International for the usage of their data on vertebrate and bird species range maps, respectively. This work was funded by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant, a Concordia University Research Chair and a Quebec Centre for Biodiversity Science Seed Grant.
Open Research
DATA AVAILABILITY STATEMENT
The data used in analyses are available on figshare: https://doi.org/10.6084/m9.figshare.7207514.v2 (Lawrence et al., 2019). Code and assoociated analysis files available from Dryad https://doi.org/10.5061/dryad.xgxd254ck.
REFERENCES
BIOSKETCHES
Elizabeth Lawrence (https://erlawrence.weebly.com/) and Dylan Fraser (www.dylanfraser.com) are biologists interested in integrating ecology, evolution and genetics/genomics towards more effective conservation and management. They are particularly interested in the integration and consideration of intraspecific diversity data into larger, macroecological frameworks.




