Geographical coincidence and mimicry between harmless snakes (Colubridae: Oxyrhopus) and harmful models (Elapidae: Micrurus)
Abstract
Aim
In the New World coral-snake complex, Micrurus species presumably serve as Batesian models for some species of Colubridae, including Oxyrhopus. To better understand the evolutionary role of mimicry, we explored the distributional overlap of Micrurus (models) and Oxyrhopus (mimics), the association between species and pattern richness of both taxa, and the relationship between mimetic fidelity and model diversity.
Location
The Neotropical region and southern-most Nearctic region.
Methods
We classified colour patterns and delineated the geographical distributions of species in a 1° grid, spanning the distributions of both genera. We used generalized linear models to test for significant associations of species and colour patterns. We used guided regularized random forests to identify, among Micrurus colour patterns, the best predictors of the presence and richness of Oxyrhopus colour patterns. To account for spatial autocorrelation, we implemented Bayesian hierarchical models using the integral nested Laplace approximation with a conditional autoregressive prior.
Results
With few exceptions, the presence and richness of species of Micrurus bearing a given colour pattern in grid cells were good predictors of the presence and richness of Oxyrhopus species bearing the same colour pattern. However, these relationships did not hold when we accounted for species of Micrurus bearing different colour patterns, except in a few cases. The presence of perfect (i.e. those that have a matching Micrurus model) and imperfect (those that do not) Oxyrhopus colour patterns in grid cells was positively correlated with the richness of Micrurus colour patterns.
Main conclusions
Mimicry complexes may not rely upon perfect signal matching and distributional overlap as previously supposed. Tests of mimetic relationships based on geographical distributions must account for alternative hypotheses including organisms that do not appear to be close mimics. Mimicry complexes may capitalize upon model signal diversity, allowing for the diversification of lineages with warning coloration in the face of stabilizing selection.
Introduction
Mimicry is an ecological interaction, recognized in taxa such as plants, birds and insects, with three agents: the model, the mimic and the organism that interacts with both of them (Ruxton et al., 2004). The model usually has some advantageous characteristic with high adaptive value (e.g. predator deterrence), offering some benefit to the mimic that reproduces it. The organism that interacts with both mimic and model is usually a predator that exhibits aversion to the model, promoting selection of mimics that resemble models. Model signals may include advertisement colours, behaviour and/or morphology (Komárek, 1998). Systems in which the mimic replicates the model's signal without also possessing the trait driving avoidance were first described by Bates (1862) in his work with South American Lepidoptera.
Many studies have concluded the existence of mimicry by simply observing similarity of external traits (e.g. morphology, behaviour) between the supposed model and mimic (Huey & Pianka, 1977). Conclusive demonstrations (rather than suppositions based on observed similarity) of mimetic systems have adopted a number of different approaches, though most have employed laboratory experiments in which ‘predators’ are trained to avoid a particular signal and demonstrate protection afforded to mimics (Ruxton et al., 2004). However, few studies have investigated mimicry systems within an explicit macroevolutionary framework, i.e. at large geographical scales and involving multiple species (e.g. Williams, 2007).
Mimicry of coral snakes is a classical example of this ecological–evolutionary phenomenon among vertebrates (Wallace, 1867). The coral snake colour pattern is defined as any dorsal pattern found in venomous coral snakes or any dorsal pattern containing a significant amount of red, orange or pink, distributed in a way that resembles a venomous coral snake (Savage & Slowinski, 1992). Such patterns are found in venomous snakes of the family Elapidae, particularly in the New World Micrurus and their putative mimics of Aniliidae and Colubridae (Savage & Slowinski, 1990, 1992; Greene, 1997). Many studies have employed snake replicas to demonstrate that the coral colour pattern grants protection against visually oriented predators by signalling something unpleasant or dangerous (e.g. Smith, 1975). Other work with replicas has demonstrated that coral patterns suffer fewer attacks than alternative colour patterns (Brodie, 1993). However, depending on mimic fidelity (Hinman et al., 1997), the degree of sympatry with the model (Pfennig et al., 2000, 2001, 2007) and habitat structure (França, 2008), mimics may be attacked more or less than models.
To gain protection from predators, mimics are expected to have geographical distributions that match those of models (Ruxton et al., 2004). Concordant geographical variation in colour pattern between Micrurus and their putative mimics has been viewed as evidence that they are involved in mimicry systems (Greene & McDiarmid, 1981). However, spatially explicit tests of concordant geographical variation between models and mimics are wanting (Cox & Davis Rabosky, 2013). Still, this range overlap may not be exact for a number of reasons. Incongruence between the geographical distributions of model and mimic is known in many Batesian systems, and may result from dispersion of models and mimics to other areas, local extinctions, or ecophysiological differences between models and mimics (Pfennig & Mullen, 2010). In such systems, a mimic out of the model's geographical distribution may retain the model's characteristics if predation is low; conversely, the mimic may become extinct or return to an ancestral, non-mimetic phenotype under high predation pressure (Pfennig et al., 2001, 2007; Prudic & Oliver, 2008; but see Savage & Mullen, 2009).
Though mimics are only expected to gain protection in regions of sympatry with models (Pfennig et al., 2000, 2001), some mimics have a geographical distribution that extends beyond that of their putative model. In areas where the model is rare or on the edges of the model's geographical range, selection has been shown to reinforce high mimetic fidelity (Harper & Pfennig, 2007), though imperfect mimics in allopatry may persist via gene flow from areas of overlap with models (Harper & Pfennig, 2008; Kikuchi & Pfennig, 2013). Furthermore, in areas where conspicuous species of many different colour patterns overlap in their distributions, mimics may be ‘imperfect’ in that they do not closely resemble any one model (Edmunds, 2000; Sherratt, 2002).
Mimicry can play an important role in speciation because reproductive isolation may be reached as a by-product of adaptive differentiation of mimetic traits (Bates, 1862; Naisbit et al., 2003). The interaction among mimics and models can lead to a mimetic radiation (Yeager et al., 2012) resulting from the congruence between mimic and model traits that vary along the geographical distribution of models and mimics. Jiggins et al. (2006) have investigated the role of vicariant versus ecological speciation in Ithomia butterflies and found that changes in mimetic relationships are indeed associated with speciation events. Thus, the number of mimetic species within a lineage is expected to be positively correlated with model species and pattern diversity.
The South and Central American genus Oxyrhopus contains 14 species (Zaher & Caramaschi, 1992), all of which have a colour pattern presumed to mimic Micrurus (Wallace, 1867; Campbell & Lamar, 1989; Greene, 1997). Due to its wide geographical and ecological distribution, broad sympatry with Micrurus and great variation in colour patterns, Oxyrhopus is an excellent subject for an investigation of the evolution and maintenance of mimicry. Here, we investigate the existence of a mimetic relationship among species of Oxyrhopus and Micrurus and the effect of this relationship on the evolution of Oxyrhopus. Given the assumption that Micrurus is a model for Oxyrhopus, we tested the prediction that the geographical distribution of Oxyrhopus species is best explained by the distribution of Micrurus species with corresponding colour patterns. We also investigated whether the geographical distribution of Oxyrhopus species and colour pattern richness are best explained by the distribution of Micrurus species and colour pattern richness. Finally we explored the possibility that the geographical distribution of imperfect mimics is correlated with local richness of Micrurus colour patterns.
Materials and Methods
Data
We classified the colour patterns of all described species of Micrurus (77) and Oxyrhopus (14) following Savage & Slowinski (1992), with some modification. In many cases Savage & Slowinski (1992) did not differentiate white from yellow colours, which we did in this study. Additionally, we included 24 species of Micrurus not treated by Savage & Slowinski (1992). We classified all species of Oxyrhopus as either imperfect (species with colour patterns that do not match an existing specific Micrurus pattern) or perfect (those matching known Micrurus species) mimics (Dittrich et al., 1993). We are aware that fine differences within ‘perfectly’ matched patterns may permit differentiation by natural predators (Hinman et al., 1997), but it has been demonstrated that predators such as birds may rank mimics (good or bad) in a similar way to humans (Penney et al., 2012). We also classified species of Micrurus into three more general, non-exclusive patterns groups: ringed (species with rings), bicolour (species with two colours) and tricolour (species with three colours).
We digitized and georeferenced polygons and points of Micrurus distributions with ArcGIS v.9.3.1, primarily from Roze (1996), but also supplemented by additional literature records (e.g. Campbell & Lamar, 2004), especially for taxa not present in Roze's work. Distribution records of Oxyrhopus species were similarly obtained from the literature and the following herpetological collections: Coleção de Répteis do Museu de Zoologia da Universidade Estadual de Campinas (ZUEC-REP), Coleção Herpetológica da Universidade de Brasília (CHUNB), Fundación Puerto Rastrojo (FPR-Colombia), Instituto Butantan (IB), Instituto Nacional de Pesquisas da Amazônia (INPA), Museu de Biologia Professor Mello Leitão (MBML-Répteis), Museu de Zoologia da Universidade de São Paulo (MZUSP), Museu de Zoologia da Universidade Estadual de Londrina (MZUEL), Museu Nacional – Universidade Federal do Rio de Janeiro (MNRJ), Museu Paraense Emílio Goeldi (MPEG), Pontifícia Universidade Católica de Minas Gerais (PUCMG), Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Sistema de Informação do programa Biota/Fapesp (SIN Biota), Universidade Federal de Goiás (UFG), Universidade Federal de Viçosa (UFV), Universidade Federal de Mato Grosso (UFMT), Universidade Federal do Rio Grande do Sul (UFRGS), Zoneamento Ecológico Econômico do Acre – Herpetofauna (ZEE HERP).
To build distribution polygons of Oxyrhopus and Micrurus from distribution records we obtained environmental layers from http://www.worldclim.org/ (BIOCLIM) and http://glcf.umd.edu/data/vcf/ (MODIS Vegetation Continuous Fields). From these, we extracted 1000 random points within the latitudinal and longitudinal limits of the geographical distribution of each genus. To assess the correlation between environmental variables, we used the program Multiple Raster-Value Extractor (Pérez, 2007) to obtain values of environmental variables for each random point. For each pair of variables with a correlation of 0.9 or more, we retained the variable we regarded as more biologically relevant for the studied genera. Thus, we retained the following variables: elevation, aspect, annual mean temperature, mean diurnal range, isothermality, temperature seasonality, minimum temperature of the coldest month, mean temperature of the wettest quarter, mean temperature of the driest quarter, mean temperature of the warmest quarter, mean temperature of the coldest quarter, precipitation of the wettest month, precipitation of the driest month, precipitation seasonality, precipitation of the warmest quarter, precipitation of the coldest quarter, slope and MODIS Vegetation Continuous Fields. With these variables, we built species distribution models (SDMs) for each species of Oxyrhopus and Micrurus with Maxent 3.3.2 (Phillips & Dudik, 2008).
Due to possible database georeferencing errors, we organized the occurrence points of each species in order of probability of occurrence and excluded 5% of the observations with lower probabilities (Costa et al., 2010). We regarded each species as present in all areas of the SDM with a probability above this threshold. After this process, we calculated the overlap between the SDM of each species and a minimum convex polygon based on the distribution points, adding a 0.5° buffer to obtain each species' distribution polygon. Hence, we used Maxent essentially as a way of smoothing the minimum convex polygons around distributional records. For species with just one or two records (Micrurus camilae, Micrurus oligoanellatus, Micrurus pacaraimae), we built a 50-km buffer around each point. After building distribution polygons, we constructed a grid with 1° cells across the distribution of both genera using Hawth's tools (Beyer, 2004) and, for each cell, attributed 0 for absence or 1 for presence of each species or colour pattern to perform statistical analyses.
Statistical analyses
Using generalized linear models (GLMs) with binomially distributed errors in R (R Development Core Team, 2010), we evaluated: (1) whether the presence of Oxyrhopus in grid cells (dependent variable, coded as binary) is significantly explained by the presence of Micrurus (independent variable, coded as binary); (2) whether the presence of each colour pattern of Oxyrhopus in grid cells (dependent variable, coded as binary) is significantly explained by the presence of the corresponding pattern of Micrurus (independent variable, coded as binary); and (3) if the presence of perfect and imperfect mimics (dependent variable, coded as binary) in grid cells is significantly explained by the richness of Micrurus colour patterns (independent variable). We used the same procedures to relate the species richness of Oxyrhopus and Micrurus, for each colour pattern, but with Poisson distributed errors.
Using a different approach for statistical inference that avoids criticisms of the significance testing approach (Burnham & Anderson, 2002), we determined whether the presence of each colour pattern of Oxyrhopus (dependent variable, coded as binary) in grid cells is better explained by the presence of the corresponding colour pattern of Micrurus than by the presence of non-corresponding colour patterns of Micrurus. Here, we used the guided regularized random forest (GRRF) method, with the R packages randomForest (Liaw & Wiener, 2002) and RRF (Deng, 2013). Random forests (RF) is an ensemble classification method based on decision trees, i.e. it combines the predictions of several decision trees to improve prediction accuracy and reduce prediction variability (Breiman, 2001). Each decision tree is built from a bootstrap sample of the original data and, at each split of each decision tree, a random set of n predictors (n is often set to the square root of the number of predictors) is chosen for binary partitioning of the data based on the maximum decrease in the Gini impurity (Ceriani & Verme, 2012). Decision trees are grown fully, without pruning, and each is used to predict the out-of-bag (OOB) observations (each bootstrap sample leaves out c. 37% of the original observations, called OOB data), producing a running unbiased estimate of the classification error as trees are added to the forest. The predicted class of each observation is calculated by majority vote of the OOB predictions for that observation, with ties split randomly. When building decision trees in RF, regularization penalizes the selection of new features for splitting when the gain (e.g. a decrease in the Gini impurity or an increase in information gain) is similar to that of features used in previous splits, a method known as regularized random forest (RRF). A GRRF is an enhanced RRF in which the importance scores from an ordinary RF are used to guide the feature selection process of RRF (Deng, 2013). We used the mean decrease in the Gini impurity to select the colour patterns of Micrurus that were the best predictors of the occurrence of each colour pattern of Oxyrhopus in grid cells.
Species distributional data often display spatial autocorrelation, i.e. when values of a variable measured at adjacent locations are not independent of each other, and this can lead to violation of the assumption of independence of residuals and inflated type I error rates (Griffith, 2003). To test for the presence of spatial autocorrelation in our data, we assessed the significance of the Moran's I statistic with 10,000 random permutations, using package spdep (Bivand et al., 2013) in R. Since we detected significant spatial autocorrelation in the residuals of each model (results not shown), we implemented Bayesian hierarchical models using the integral nested Laplace approximation (INLA) with a conditional autoregressive (CAR) prior in R-INLA (Blangiardo et al., 2013) when modelling the occurrence or richness of Oxyrhopus as a function of the occurrence or richness of Micrurus. We conducted all analyses in R (R Development Core Team, 2010) and report means plus or minus one standard deviation.
Results
We identified a total of 20 colour patterns: 10 in Oxyrhopus and 16 in Micrurus (Table 1). Among them, six patterns occurred in Oxyrhopus and Micrurus, four only in Oxyrhopus (imperfect mimics) and ten only in Micrurus (Table 1, Fig. S1 in Appendix S1 in Supporting Information). Micrurus has a broader distribution, from 40° S to 35° N, and occupied 1692 cells, whereas Oxyrhopus ranged between 44° S to 16° N and occupied 1494 cells (Fig. 1, Table S1 in Appendix S2). Of the 1770 grid cells comprising the distribution of both genera, Oxyrhopus were present and Micrurus absent in only 78 (4.4%) (Fig. 1). The mean species richness of Micrurus per cell was higher than that of Oxyrhopus (Micrurus, 3.08 ± 2.23; Oxyrhopus, 2.83 ± 2.03; t1769 = 5.20, P < 0.001). A maximum of 11 Micrurus species occupied a single cell, whereas a maximum of 8 species of Oxyrhopus occurred in a single cell. In both genera, species richness was highest in western Amazonia (Fig. 1).
| Colour pattern | Code | Micrurus | Oxyrhopus |
|---|---|---|---|

|
BIRbr | 11 | 3 |

|
BIRbw | 5 | 1 |

|
BIRby | 2 | 1 |

|
BIBbr | 1 | 1 |

|
BIFr | 2 | 0 |

|
BIFw | 1 | 0 |

|
TMw | 28 | 2 |

|
TMy | 18 | 0 |

|
TTw | 26 | 4 |

|
TTy | 6 | 0 |

|
TZbyr | 1 | 0 |

|
TZbwr | 0 | 2 |

|
TPw | 1 | 0 |

|
TPy | 1 | 0 |

|
TMiw | 1 | 0 |

|
NBR | 1 | 0 |

|
BILbr | 1 | 0 |

|
BISbr | 0 | 1 |

|
U | 0 | 3 |

|
Tsb | 0 | 1 |
- BIRbr, bicolour with black rings alternating with red rings; BIRbw, bicolour with black rings alternating with white rings; BIRby, bicolour with black rings alternating with yellow; BIBbr, bicolour with black bands alternating with red bands; BIFr, bicolour with black rings fused dorsally and with red blotches in venter that can extend to lateral portions; BIFw, bicolour with black rings fused dorsally and with white blotches in venter that can extend to lateral portions; TMw, tricolour with one black ring between two white rings followed by red rings; TMy, tricolour with one black ring between two yellow rings followed by red rings; TTw, tricolour with three black rings alternating with two white rings followed by red rings; TTy, tricolour with three black rings alternating with two yellow rings followed by red rings; TZbyr, tricolour with black and yellow rings interrupted by red dorsal saddles; TZbwr, tricolour with black and white rings interrupted by red dorsal saddles; TPw, tricolour with five black rings alternating with three white rings followed by red rings; TPy, tricolour with five black rings alternating with three yellow rings followed by red rings; TMiw, tricolour with one dorsally incomplete black ring between two white rings followed by red rings; NBR, tricolour with one red ring between two white rings followed by yellow rings; BILbr, bicolour with black blotches on a red dorsum; BISbr, bicolour with black dots on a red dorsum; U, unicolour red; Tsb, tricolour with black saddles that are bordered with white on a red dorsum.
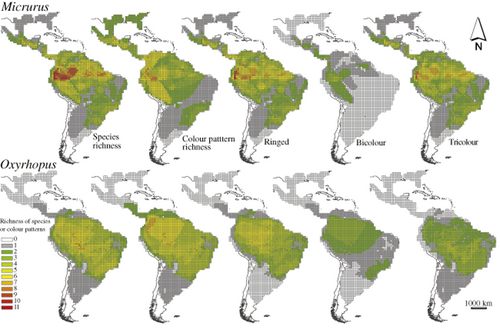
Richness of species and colour patterns of Micrurus and Oxyrhopus, across their geographical distributions. Each grid cell measures 1° × 1°.
The overall distribution of Micrurus significantly explained the overall distribution of Oxyrhopus (Z = 8.40, P < 0.001). Taking into account just the pattern-specific cases, the only colour pattern of Micrurus that did not explain the distribution of the corresponding colour pattern of Oxyrhopus was the bicolour pattern BIBbr (Table 1, Fig. S2 in Appendix S1, Table S2 in Appendix S2). With the exception of TTw, the presence of most Oxyrhopus colour patterns was not explained by the presence of the corresponding (i.e. equivalent) Micrurus colour pattern when we included other colour patterns in the model and accounted for spatial autocorrelation (Table S3 in Appendix S2).
Species richness of Micrurus was positively correlated with Oxyrhopus species richness (Z = 29.0, P < 0.001). Only the species richness of Micrurus with BIRbr and BIBbr patterns did not significantly explain the richness of Oxyrhopus with the corresponding colour patterns (Fig. S3 in Appendix S1, Table S4 in Appendix S2). With the exception of TTw, species richness of each Oxyrhopus colour pattern was not explained by species richness of the corresponding Micrurus colour pattern when we included other colour patterns in the model and accounted for spatial autocorrelation (Table S5 in Appendix S2).
Imperfect colour patterns of Oxyrhopus (i.e. those that do not match a local Micrurus model) were highly correlated with the richness of Micrurus colour patterns (Table S6 in Appendix S2). Only the distribution of the Tsb colour pattern was negatively correlated with the richness of Micrurus colour patterns. Similarly, the distributions of all perfect colour patterns of Oxyrhopus were positively associated with the richness of Micrurus colour patterns (Table S6 in Appendix S2).
Discussion
Distribution of colour patterns
Despite being harmless, all species of Oxyrhopus exhibit some kind of conspicuous coloration. This may have a high energy cost, and when the selective pressure that maintains these characteristics is relaxed a return to a less energetically costly coloration is expected (Ohsaki, 2005). With the exception of the BIBbr pattern, the distribution of Micrurus colour patterns largely explained the distribution of the corresponding patterns of Oxyrhopus. The BIBbr pattern only occurs in the Central American Micrurus bernardi and the South American Oxyrhopus petolarius. It is possible that predators cannot or do not discriminate between BIBbr and BIRbr. Notably O. petolarius is polytypic, exhibiting both (BIBbr, BIRbr) colour patterns, suggesting just such a generalization or lack of discrimination by predators as has been reported in other systems (Cuthill & Bennett, 1993). However, the distribution of the Micrurus BIRbr pattern does explain the distribution of the BIBbr pattern in Oxyrhopus (Z = 5.08, intercept = 0.61, slope = 0.88, P < 0.0001; Fig. S2 in Appendix S1).
Contrary to our expectations, there was a negative association between the distributions of the tricolour pattern in the two genera (Fig. 1). This pattern is present in many species of Micrurus and Oxyrhopus and has a broad geographical distribution. As the two genera have distinct latitudinal distributions, with Micrurus reaching further north and Oxyrhopus further south, there are some regions where their ranges do not overlap. This may explain the small negative coefficient in the GLM for this colour pattern. Nevertheless, when a predator that lives in sympatry with a model experiences selection to avoid the model, this rejection may be maintained via predator migration to areas where mimics are not sympatric with models. Non-overlapping model/mimic distributions have been shown to result from migration of mimics from areas of sympatry to areas of allopatry (Harper & Pfennig, 2008; Ries & Mullen, 2008).
Richness of colour patterns
We found a positive relationship between the species richness of corresponding patterns for Micrurus and Oxyrhopus. In general, the more model species there are with a given colour pattern, the more mimics will benefit from the protection given by resemblance to the model. Hence, it is possible that the diversification of the two genera is due in part to coevolution. Nevertheless, the highest richness of Micrurus and Oxyrhopus is concentrated in western Amazonia and this pattern is paralleled by several other taxa, being attributed to high productivity, climatic differentiation and habitat diversity, among other factors (Haffer, 1969; Bini et al., 2004; França, 2008).
Perfect and imperfect mimics
The richness of Micrurus colour patterns explained the distribution of imperfect colour patterns in Oxyrhopus, supporting the hypothesis that imperfect mimics are found in areas with higher model richness. One might expect that an imperfect mimic that partially resembles multiple model species will potentially gain protection from a greater number of predators (Edmunds, 2000; Sherratt, 2002). Furthermore, the species of Micrurus have various levels of toxicity (Jorge da Silva & Aird, 2001) and predators tend to have a generalized aversion to conspicuous patterns in the presence of models with varying toxicities (Darst & Cummings, 2006), contributing to the maintenance of imperfect colour patterns.
Contrary to our expectations, the distribution of the Tsb colour pattern was negatively correlated with the richness of Micrurus colour patterns. The Tsb pattern is only found in Oxyrhopus rhombifer, a widely distributed species in south-central South America (Table 1, Fig. S1 in Appendix S1), and no species of Micrurus bears this or a similar colour pattern. One possible explanation for the origin of this colour pattern is relaxed selection (Sherratt, 2002; Penney et al., 2012): this may be true as bird richness decreases towards meridional South America. The lower number of species can lead to low predation pressure that may have allowed the emergence of the Tsb pattern. Additionally, predation on coral snakes is higher in forest habitats than in open habitats (França, 2008), allowing the appearance of this pattern in this open-habitat species. Another possible explanation for the emergence of the Tsb pattern is the greater number of alternative prey, allowing the presence of imperfect mimics (Lindstrom et al., 2004). This explanation may be plausible as richness of Dipsadinae, possible alternative prey, increases in meridional South America (França, 2008). In a view based on economy of energy, Speed & Ruxton (2007) postulated that the diversity of warning signals results from physiological costs in maintaining conspicuousness, giving an alternative explanation for the appearance of the Tsb pattern in this area with lower energy availability (greater distance from Equator). This result indicates that evolutionary forces other than mimicry may be driving the distribution of the Tsb pattern.
We did find a close geographical association between Micrurus and Oxyrhopus with corresponding colour patterns (Table S2 in Appendix S2), supporting the hypothesis of a mimetic relationship between these genera. In general, the richness and presence of mimics was positively correlated with model richness, regardless of the quality of the mimic (perfect or imperfect), indicating that Micrurus richness may drive the diversification of Oxyrhopus. However, our analyses further reveal that the geographical distributions of mimetic patterns are not best explained by the distribution of their respective phenotypic models. This result does not preclude the existence of a mimetic relationship between the two genera, but rather suggests that mimicry complexes may not be as reliant upon perfect signal matching and distributional overlap as previously supposed. Our work clearly demonstrates that mimicry complexes may capitalize upon diversity of model signals and the generalization of predator avoidance to unmodelled signals. This in turn provides an explanation for the diversification of lineages with warning coloration in the face of stabilizing selection, and highlights the need for further study of the diversification of such model/mimic radiations. Previous works documenting the existence of mimetic relationships have usually focused on colour variation within the mimic and its correspondence with the model's geographical distribution, ignoring other possible models within the mimic's distributional range. Our results reinforce the idea that to test a mimetic relationship using primarily geographical distribution one must also test for alternative hypotheses with organisms that do not appear to be close mimics.
Acknowledgements
We thank the following institutions, collections and their curators for the loan of distributional data: Coleção de Répteis do Museu de Zoologia da Universidade Estadual de Campinas (ZUEC-REP), Coleção Herpetológica da Universidade de Brasília (CHUNB), Fundación Puerto Rastrojo (FPR-Colombia), Instituto Butantan (IB), Instituto Nacional de Pesquisas da Amazônia (INPA), Museu de Biologia Professor Mello Leitão (MBML-Répteis), Museu de Zoologia da Universidade de São Paulo (MZUSP), Museu de Zoologia da Universidade Estadual de Londrina (MZUEL), Museu Nacional – Universidade Federal do Rio de Janeiro (MNRJ), Museu Paraense Emílio Goeldi (MPEG), Pontifícia Universidade Católica de Minas Gerais (PUCMG), Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Sistema de Informação do programa Biota/Fapesp (SIN Biota), Universidade Federal de Goiás (UFG), Universidade Federal de Viçosa (UFV), Universidade Federal de Mato Grosso (UFMT), Universidade Federal do Rio Grande do Sul (UFRGS), Zoneamento Ecológico Econômico do Acre – Herpetofauna (ZEE HERP). G.R.C. thanks CAPES, CNPq and FAPDF for financial support.
References
Renan Bosque is a PhD student at the University of Mississippi under supervision of Dr Brice Noonan. His main interest is to understand the evolutionary forces that drive macroevolutionary patterns, with emphasis on herpetology.
Brice Noonan is an associate professor at the University of Mississippi whose primary interests lie in tropical diversification and the mechanisms driving this process.
Guarino Colli is an associate professor at Universidade de Brasília. His research interests include the biogeography, conservation, ecology and evolution of the Cerrado herpetofauna.




