Polytolerance to abiotic stresses: how universal is the shade–drought tolerance trade-off in woody species?
Abstract
Aims
According to traditional ecophysiological theories stress tolerance of plants is predominately determined by universal physiochemical constraints. Plant acclimation to environmental stress therefore compromises plant performance under a different stress, hindering successful toleration of several abiotic stress factors simultaneously. Yet recent studies have shown that these trade-offs are less exclusive than postulated so far, leaving more wiggle room for gaining polytolerance through adaptations. We tested whether polytolerance to shade and drought depends on cold and waterlogging tolerances – hypothesizing that polytolerance patterns in different species groups (angiosperms versus gymnosperms; deciduous versus evergreen; species originating from North America, Europe and East Asia) depend on the length of the vegetation period and species dormancy through limiting the duration of favourable growing season.
Location
Northern Hemisphere.
Methods
Our study analysed four main abiotic stress factors – shade, drought, cold and waterlogging stress – for 806 Northern Hemisphere woody species using cross-calibrated tolerance rankings. The importance of trade-offs among species ecological potentials was evaluated using species-specific estimates of polytolerance to chosen factors.
Results
We found that both cold and waterlogging tolerance are negatively related to species capabilities of simultaneously tolerating low-light and low-water conditions. While this pattern was different in angiosperms and gymnosperms, species region of origin and leaf type had no effect on this relationship.
Main conclusions
Our results demonstrate that adaptation to different abiotic stress factors in woody plants is highly complex. The length of the vegetation period and dormancy are the key factors explaining why woody plants are less capable of tolerating both shade and drought in habitats where vegetation period is relatively short and the water table high. While dormancy enables angiosperms to more successfully face additional stress factors besides shade and drought, gymnosperms have lower polytolerance, but can better tolerate of shade and drought when other environmental factors are favourable.
Introduction
Tolerance to different abiotic stresses is a key shaper of species distribution patterns (Hawkins et al., 2014), in particular for sessile organisms like plants (Bartlett et al., 2012). According to traditional ecological knowledge, basic physical and chemical laws, like thermodynamics and process stoichiometry, largely determine the presence and strength of biological trade-offs (Smith & Huston 1989; Sack, 2004). Temperature extremes, water and light availability are considered to be the main abiotic stress factors limiting world-wide plant distribution as well as affecting the competitive relations of plants within any specific community (Valladares & Niinemets, 2008; Martinez-Tilleria et al., 2012). Trade-offs between these factors have mainly been deemed to be rather universal, predominantly depending on physiochemical constraints, whereas the relative effects of biological factors like adaptations are regarded as less important. Thus, the degree of polytolerance – the capability of tolerating multiple stress factors simultaneously – is thought to be rather low among plants (Niinemets & Valladares, 2006).
Indeed, a comprehensive meta-analysis on tolerance to key abiotic stresses demonstrated that there are significant trade-offs between all combinations of shade, drought and waterlogging tolerance, irrespective of species leaf longevity (evergreen versus deciduous), phylogeny (angiosperm versus gymnosperm) and origin (North America, Europe or Asia) (Niinemets & Valladares, 2006). Yet, for these 806 woody species from the temperate Northern Hemisphere, the explained variance in the negative relationships between stress tolerances was only 2–53%. In fact, an analogously low and variable explanatory power has been observed in several other studies looking at trade-offs between species ecological potentials from temperate to tropical biomes (Sack, 2004; Markesteijn & Poorter, 2009; Markesteijn et al., 2011a).
Trade-off patterns with low explanatory power have pushed research towards implementing new theories and mechanisms to explain the polytolerance of plants to abiotic stresses, or the lack of it. Adaptations via phenology (Martinez-Palle & Aronne, 1999), higher biomass allocation to roots (Sack et al., 2003) and concurrent physiological and morphological adaptations (Pivovaroff et al., 2014) and leaf-level functional traits (Hallik et al., 2009) have been proposed as alternative determinants of the shape and strength of trade-offs enabling polytolerance in some species. Various trait-based approaches have become the main alternative way to explain the variability of these patterns (e.g. Hallik et al., 2009; Markesteijn & Poorter, 2009; Niinemets et al., 2009; Ouedraogo et al., 2013).
Due to the scarcity of data, polytolerance analyses including a representative species pool do not usually consider trade-offs between more than two stress factors (i.e. polytolerance), and do not explore how other simultaneously acting factors affect these trade-offs (Valladares & Niinemets, 2008). In addition to multiple, and often species-specific ways of how polytolerance has been achieved during evolution, mapping general patterns of trade-off dynamics of abiotic stresses demands larger sample sizes and analysis on a wider spatial range (Hallik et al., 2009; Stahl et al., 2013). The present study attempts to examine broad-scale polytolerance patterns considering the main abiotic stress factors – temperature, light and conditions of limited and excess water – in a large number of species. We focused primarily on the polytolerance or polyintolerance of shade and drought stress (henceforth SADTO, for ‘shade and drought tolerance’) as the restricted co-tolerance of these two key stress factors is considered the main constraint limiting the combinations of species in forests across gradients of soil water availability and through stand development from early successional open communities to dense late-successional stands (Niinemets & Valladares, 2006).
Because the adaptations that allow plants to grow in shade can compromise their ability to grow in dry conditions, SADTO has often been considered to be physically inevitable (see premise 3 in Smith & Huston 1989). However, experimental studies have indicated that there might be a certain capacity to tolerate both stresses in species from warmer climates (Markesteijn & Poorter, 2009; Markesteijn et al., 2011a). Likewise, it has been proposed that the strength of SADTO might vary in dependence on other climatic factors that ultimately determine the effective length of the growing season. Valladares & Niinemets (2008) suggested that the length of the growing season and co-occurrence of other stress factors can dramatically affect SADTO by altering a plant's capability to tolerate low light.
In addition, the SADTO dynamics can differ depending on species background – for example, a recent study by Stahl et al. (2013) confirmed the findings of Niinemets & Valladares (2006) that gymnosperms and angiosperms have different strategies for coping with abiotic stress factors. Leaf physiognomy could also affect stress polytolerance patterns, as the onset of dormancy at the end and breaking dormancy in the beginning of the growing season and the strategy of dealing with limiting factors depends largely on whether the plants are evergreen or deciduous (Press, 1999; Kozlowski & Pallardy, 2002). The occurrence of dormancy can have a similar impact on species polytolerance as the length of the vegetation period – limiting the available favourable time for growing. Thus the effect of dormancy on polytolerance steepens concurrently with the length of the vegetation period.
As a follow-up of the meta-study of Niinemets & Valladares (2006) we added cold tolerance data to data on shade, drought and waterlogging tolerance, and analysed the shade–drought trade-off in relation to cold and waterlogging tolerance to gain insight into the determinants of polytolerance to principal abiotic stresses in Northern Hemisphere woody species. Our main hypothesis was to test how the polytolerance of shade and drought depends on the cold and waterlogging tolerances of species – whether the polytolerance patterns in different species groups (angiosperms versus gymnosperms; deciduous versus evergreen; species originating from North America, Europe and East Asia) depend on the length of the vegetation period and species dormancy due to limiting the duration of the favourable growing season.
Methods
Species shade, drought and waterlogging tolerance data with the region of origin of the species and plant functional type were taken from Niinemets & Valladares (2006). The aim of that study was to analyse tolerance to three crucially important stresses for almost half of the species pool of self-supporting tree and shrub species in the temperate zone of the Northern Hemisphere (Niinemets & Valladares, 2006). In order to have comparable data for each stress factor, available data from the literature were synchronized by cross-calibrating different scales using multiple measurements of the same species available across the scales. Ultimately, uniform stress tolerance scales ranging from 1 (very intolerant) to 5 (very tolerant) were derived for each stress factor.
The final dataset of Niinemets & Valladares (2006) contained 806 species, of which 566 were winter deciduous and 240 evergreen. There were 118 gymnosperms, including 11 winter-deciduous species. Nearly half of the species (364) were native to North America, 262 to Europe/West Asia, and 211 to East Asia. Overall, the data set covered about 40% of extant native Northern Hemisphere woody vegetation (about 73% of North American, 69% of European/West Asian and 23% of East-Asian woody species; Qian & Ricklefs, 1999, 2000; Ricklefs et al., 2004).
Shade tolerance in Niinemets & Valladares (2006) was defined as the capacity for growth in conditions of low light availability. The five-point scale used for shade tolerance roughly correspond to given proportions of light availability compared with full sunlight: 1, > 50%; 2, 25–50%; 3, 10–25%; 4, 5–10%; 5, 2–5% (Niinemets & Valladares, 2006). However, as Niinemets & Valladares (2006) note, the shade tolerance characterizes a species' potential to tolerate shaded conditions relative to other competing species. The minimum relative light availability that any given species can tolerate will depend on the species' ontogeny and other environmental drivers, but the key assumption is that these factors alter minimum light requirement similarly in all species such that the relative species ranking is maintained. Drought tolerance estimations were based on site characteristics of species dispersal and physiological potentials. The relevant site features considered were total annual precipitation, ratio of precipitation to potential evapotranspiration (P:PET ratio) and duration of the dry period. As the drought tolerance rankings used by Niinemets & Valladares (2006) were based on a wide array of structural and physiological traits, drought tolerance rankings were determined as rather abstract estimations: 1, very intolerant; 2, intolerant; 3, moderately tolerant; 4, tolerant; 5, very tolerant. Ranking of waterlogging tolerance relied on the qualitative scale of Whitlow & Harris (1979) based on the duration and severity of waterlogging during the growing season: 5, very tolerant (survives deep, prolonged waterlogging for more than a year); 4, tolerant (survives deep waterlogging for one growing season); 3, moderately tolerant (survives waterlogging or saturated soils for 30 consecutive days during the growing season); 2, intolerant (tolerates 1–2 weeks of waterlogging during the growing season); 1, very intolerant (does not tolerate water-saturated soils for more than a few days during the growing season) (Niinemets & Valladares, 2006). A separate study (Stahl et al., 2013) analysing the shade and drought tolerance of 305 species of North American woody plants and independently working out continuous tolerance scales observed significant correlations of these independent estimates with the data from Niinemets & Valladares (2006).
To these three tolerance rankings, we added cold tolerance, which is based on the United States Department of Agriculture (USDA) Plant Hardiness Index (http://planthardiness.ars.usda.gov/phzmweb/default.aspx). USDA plant hardiness mapping is based on average minimum yearly temperatures in species habitats (Daly et al., 2012). The hardiness map is divided into 13 zones ranging from a long-term annual minimum temperature of below −53.9 °C (zone 0) to above 12.8 °C (zone 12), and thus covering arctic to tropical ecosystems. Although the USDA Plant Hardiness Index has been specifically developed for the USA, including the continental USA as well as Pacific islands and Puerto Rico (Daly et al., 2012), it covers an extremely broad gradient from arctic to tropics and has been successfully fitted to other parts of the world, especially in global scale meta-studies (e.g. Tonitto et al., 2006) and various floristic databases (e.g. http://www.chileflora.com).
Various botanical and gardening web pages (mainly http://davesgarden.com/; http://www.hgtvgardens.com/; http://plantlust.com/) that provide USDA plant hardiness data were used for extracting species-specific data of the coldest zone where given species is capable of growing (i.e. cold tolerance). To be fully comparable with other studied tolerance estimates, cold tolerance data were inverted and transformed to a five-point scale, with 5 indicating the highest cold tolerance (capability of tolerating the coldest temperatures) and 1 the lowest.
The polytolerance between any two stress factors was evaluated by pairwise summing the scale tolerance indices for every species. For example, while the SADTO scores of Acer platanoides are 4.2 and 2.73, respectively, SADTO scores of Acer pensylvanicum are 3.56 and 2. The SADTO of Acer platanoides is therefore 6.93 (out of maximum 10), while Acer pensylvanicum has a SADTO of 5.56. Lower sums indicate polyintolerance – the species is not capable of tolerating both stresses concurrently – while higher sums indicate polytolerance. Simple adding up of different tolerance factors was used for calculating the trade-off values based on following reasoning: (1) a tolerance scale ranging from 1 to 5 does not allow for elaboration of more complicated quantifications, i.e. there is currently no objective means to ‘weight’ tolerances to different abiotic stresses; and (2) as the tolerance to a certain factor can vary between 1 and 5 independently of other tolerance factors, the sum of tolerances (that can range between 2 and 10) comprises a species' overall capability of tolerating multiple factors at the same time. The differences in polytolerance emerge similarly to Liebig's law of the minimum. If a given species has a low tolerance to either factor, its overall polytolerance index cannot be much above the average, even when the tolerance to the other factor is high.
Statistical analyses were carried out with Statistica 8.0 (StatSoft Inc., 2007).
Results
Mean values for any tolerance score exhibited only limited variation between regional or leaf physiognomic groupings of species (Table 1). Nearly half of the species (46%) in our sample tolerated long-term average temperature minima between −40 °C and −29 °C (Fig. A in Appendix S1 in Supporting Information). One-third of species exhibited shade–drought polytolerance scores between 5 and 6, while 79% of species had SADTO scores between 4 and 7 out of possible range of 2 to 10 (Fig. B in Appendix S1). Thus, within any given species grouping there was a large interspecific variability, but the proportions of species with given tolerance rankings were similar among the groups. This indicates that the results demonstrated here are not driven by different species ecological potentials in different continents or by performance differences specific to plant functional type.
| Species group | No. | Cold tolerance | Shade tolerance | Drought tolerance | Waterlogging tolerance | Shade-Drought tolerance | Cold-Waterlogging tolerance | Cumulative stress tolerance |
|---|---|---|---|---|---|---|---|---|
| Gymnosperms | 118 | 3.17 ± 0.06 | 2.69 ± 0.12 | 3.08 ± 0.11 | 1.46 ± 0.07 | 5.77 ± 0.09 | 4.62 ± 0.09 | 10.39 ± 0.12 |
| Angiosperms | 688 | 3.24 ± 0.02 | 2.40 ± 0.04 | 2.78 ± 0.03 | 1.83 ± 0.03 | 5.18 ± 0.04 | 5.07 ± 0.03 | 10.25 ± 0.05 |
| Evergreen | 240 | 3.45 ± 0.05 | 2.61 ± 0.07 | 3.10 ± 0.07 | 1.68 ± 0.06 | 5.70 ± 0.08 | 5.13 ± 0.07 | 10.83 ± 0.09 |
| Deciduous | 566 | 3.22 ± 0.02 | 2.38 ± 0.04 | 2.75 ± 0.04 | 1.83 ± 0.03 | 5.13 ± 0.05 | 5.05 ± 0.04 | 10.18 ± 0.05 |
| East Asia | 211 | 3.43 ± 0.05 | 2.58 ± 0.07 | 2.67 ± 0.05 | 1.77 ± 0.05 | 5.25 ± 0.08 | 5.20 ± 0.06 | 10.45 ± 0.10 |
| Europe | 262 | 3.09 ± 0.04 | 2.19 ± 0.06 | 3.04 ± 0.07 | 1.95 ± 0.07 | 5.23 ± 0.09 | 5.04 ± 0.07 | 10.27 ± 0.07 |
| North America | 333 | 3.30 ± 0.03 | 2.69 ± 0.06 | 2.86 ± 0.06 | 1.93 ± 0.06 | 5.56 ± 0.06 | 5.23 ± 0.07 | 10.79 ± 0.08 |
| Total | 806 | 3.28 ± 0.09 | 2.49 ± 0.09 | 2.88 ± 0.09 | 1.89 ± 0.09 | 5.37 ± 0.09 | 5.17 ± 0.09 | 10.54 ± 0.09 |
Our analyses demonstrate that shade–drought polytolerance is significantly related to other primary abiotic stress factors, indicating that the length of the vegetation period plays a crucial role in the quantitative importance of polytolerance across different climates. SADTO was negatively related to species cold tolerance (Fig. 1). In particular, species that were more cold tolerant tended to have a stronger trade-off between shade and drought tolerances. A similar trend was also found between SADTO and waterlogging tolerance (Fig. 2). Species that were more cold tolerant were slightly inclined to have higher waterlogging tolerance as well (Fig. 2); indeed, cold tolerance and waterlogging tolerance were positively correlated (y = 6.73 + 0.2766x; r = 0.16; P = 0.00). Therefore, the regression between cold and waterlogging tolerance trade-off and shade and drought trade-off was even more pronounced (Fig. 3).
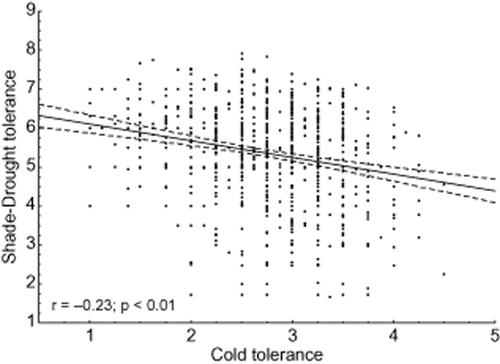
Relationship between species shade–drought tolerance and species cold tolerance. A species' shade–drought tolerance is defined as the sum of the species' potential to tolerate shade and drought on a ten-point scale (1 is low and 10 is high polytolerance), and the cold tolerance is the species' potential to tolerate low temperatures on a five-point scale (1 is low and 5 is high). Both the regression line and its 95% confidence intervals are demonstrated. Each dot corresponds to one species.
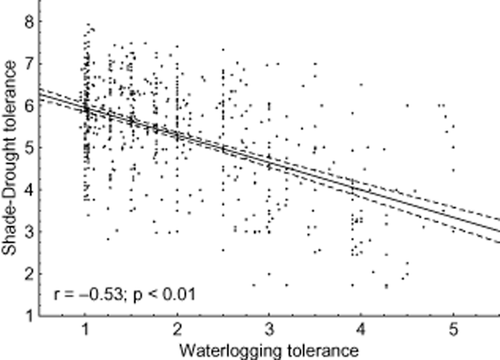
Relationship between species shade–drought tolerance and species waterlogging tolerance. Data fitting and categorization as in Fig. 1.
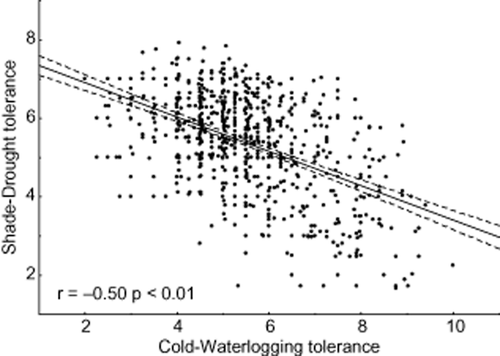
Species shade–drought tolerance in relation to species cold-waterlogging tolerance. The cold-waterlogging tolerance is defined as the sum of a species' capability to tolerate both cold and waterlogging on a ten-point scale (1 is low and 10 high polytolerance). Data fitting and categorization as in Fig. 1.
Our hypothesis that the dynamics of SADTO depends on both the cold and waterlogging tolerance of species was also proven; species that are capable of tolerating colder climates and more flooding were less capable of simultaneously tolerating shady and dry conditions (Fig. 3 & Fig. C in Appendix S1). Analysis involving additional variables (phylogenetic distance, genus diversification, cold niche breadth, evolutionary age) showed that interactions among different abiotic stress factors are indeed the main influence on SADTO (see additional methods and tables in Appendix S2).
The most pronounced dissimilarity in shade–drought polytolerance was between angiosperms and gymnosperms. SADTO of angiosperms was negatively related to cold and waterlogging tolerance in both evergreen and deciduous species, but gymnosperms exhibited no relationship whatsoever (Figs 4 & 5). Leaf longevity did not play a significant role in shaping the tolerances that were basically the same in evergreen and deciduous species (Fig. 4). The geographic origin of species did not affect the tolerances qualitatively – all regional species pools (East Asia, Europe, North America) showed equally significant negative regression between shade–drought and cold–waterlogging tolerance (Fig. 5).
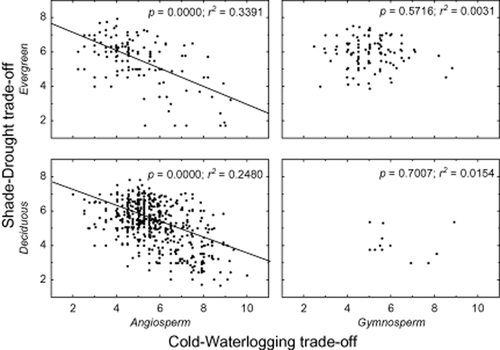
Results of linear regression analyses between shade–-drought tolerance and cold–waterlogging tolerance (both expressed on a ten-point scale, 1 being polyintolerant and 10 polytolerant) separately fitted for evergreen versus deciduous (upper and lower graphs, respectively) and angiosperms versus gymnosperms (left and right graphs, respectively). Each dot stands for one species.
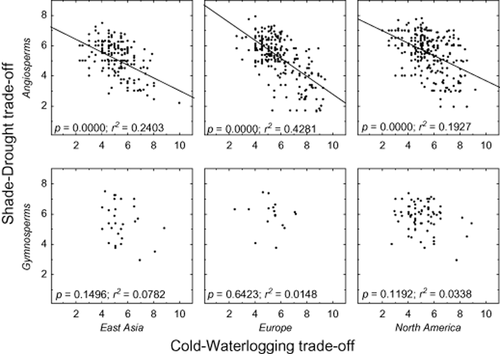
Results of linear regression between shade–drought tolerance and cold–waterlogging tolerance separately demonstrated according to species origin (which exhibited no difference between continents) and for angiosperms and gymnosperms. Data fitting and categorization as in Fig. 4.
Discussion
The decisive role of physiochemical limitations in determining how well plants can tolerate different abiotic stress factors has been questioned in recent literature (Sack, 2004; Bartlett et al., 2012; Pivovaroff et al., 2014). According to classical ecological models, fundamental physiological and physical constraints regulate the capability of plants to use resources and essentially preclude the possibility of polytolerance (Smith & Huston 1989). While these constraints indeed limit plant growth and survival, high variability in abiotic polytolerance in existent species indicates that the comprehensiveness of these fundamental physiochemical constraints is debatable (Sack, 2004; Pivovaroff et al., 2014). Our results suggest that the role of adaptations in tolerating abiotic factors in woody species is much higher than expected on the basis of classical models (Figs 1-3). While some woody species exhibit high polytolerance, other species have strong trade-offs between different abiotic stress factors. The gradient of polytolerance follows species cold tolerance – species distributed in colder climates are more prone to lower SADTO due to a shorter vegetation period and species-specific adaptations preventing growth during unsuitable seasons (i.e. dormancy). Additionally, polytolerance seems to depend on the broad evolutionary classification of species (unlike gymnosperms, angiosperms exhibit strong interrelated patterns between abiotic stress factors), but not on their region of origin (Figs 4 & 5).
Shade–drought and cold–waterlogging polytolerance: general patterns
The key premise in studying such trade-offs is that any stress factor temporarily reduces the plant carbon gain, and the strength of the overall effect depends on the cumulative duration of the stressed period relative to the total length of the growing season (Valladares & Niinemets, 2008). The duration of cold period is particularly relevant as it determines the overall length of the growing season. Thus, a drought period of moderate duration under shade has a much less severe effect on whole-canopy carbon gain in warmer climates than an equally long period in cooler climates, where it constitutes a greater proportion of the overall length of the growing season. Furthermore, the higher a species' cold tolerance, the deeper the dormancy it develops to cope with the frost period, the earlier it starts to develop dormancy in the autumn and the later it breaks it in the following spring (Cox & Stushnoff, 2001; Hänninen, 2006; Beaubien & Hamann, 2011). This would effectively reduce the growing season length at a given cumulative heat sum in species with higher cold tolerance, further reducing the effective length of the growing season.
In fact, dormancy is not only relevant in the context of winter survival, but it can be the principal way in which plants avoid unfavourable periods other than frost (Poorter & Markesteijn 2008). Dormancy could be the key adaptation shaping SADTO in places where unfavourable seasons are long and regular, as it enables plants to effectively reduce the adverse length of the growing season. Both low temperatures and occasional waterlogging can elongate the period of dormancy (Butt et al., 2008); flooding can also alternate with extremely dry periods, creating different dynamics in open and understorey habitats, therefore affecting the strength of SADTO even if the communities in both habitats consist of the same species (García-Sánchez et al., 2007; Bailey-Serres & Voesenek, 2008).
Hence, in places with a longer growing period, the shade–drought tolerance trade-off could be weak or even non-existent (Markesteijn & Poorter, 2009), while at higher latitudes, a short growing season can reinforce species adaptations to the dominant stress source (Sack, 2004). From the species point of view, plants that exhibit higher tolerance to cold temperatures and waterlogging are expected to have lower tolerance to shade and drought (Pearson et al., 2003; Valladares & Niinemets, 2008). The frequency of dormancy presence in a species pool more or less correlates with climate extremes (Kollas et al., 2014), therefore the cold tolerance of a species should be a rather good proxy for indicating the dynamics of SADTO.
The situation is similar with waterlogging tolerance. High waterlogging tolerance requires a continuous input of carbon to meristematic tissues in roots and stems that are needed to develop adventitious roots (Rich et al., 2012; Cardoso et al., 2013). Such higher respiratory activity, which can decrease leaf photosynthesis rate (Romina et al., 2014), could strongly curb a plant's capacity to resist severe co-occurring stresses (D'Odorico et al., 2013). In addition, several waterlogging-tolerant species have unique structural adjustments such as aerial roots that inherently require greater carbon inputs and compromise the plant's capacity to form a large leaf area (Kozlowski, 1997). Therefore adaptations to both cold and waterlogging tolerance are expected, through the prolongation of dormancy period, to reduce the capability of plants to avoid damage from simultaneous shade and drought conditions.
Significant relationships between SADTO and all combinations of cold and waterlogging tolerance suggest that the endurance of abiotic stress factors, even on the macroecological or macrophysiological scale, is substantially related to specific adaptations that create niche differences (as suggested by Sack, 2004). Physiochemical constraints that do not allow plants to exhibit a high growth rate in low-resource and otherwise stressful conditions (see Smith & Huston 1989) are not as strict as traditional theories state, and there is much more evolutionary wiggle room than previously suggested. When taking into account all the species analysed, our results show that polytolerance in woody plants tends to depend more on interspecific variability than rather strict and ubiquitous physiochemical constraints, the effective strength of which can actually be significantly ameliorated by dormancy.
Differences between angiosperms and gymnosperms: phylogeny versus leaf longevity
Differences between angiosperms and gymnosperms (Figs 4 & 5) suggest that, in some species groups, basic physiochemical constraints have a bigger impact on how species tolerate abiotic stress, while other species groups there is more adaptive diversity. Thus we can assume that phylogeny plays an important role in blazing the trail for new adaptations, and indeed, several recent macroecological studies (Stahl et al., 2013; Zanne et al., 2013; Hawkins et al., 2014) have reached very similar conclusions. Therefore we also carried out a species-level phylogenetic analysis; although phylogeny turned out to be a significant factor in univariate analysis of SADTO (Table A in Appendix S2; see Appendix S2 also for methods and additional results regarding phylogenetic analysis), the importance of species-level phylogeny was marginal compared with cold and waterlogging tolerance.
A crucial phylogenetic signal seems to run at a taxonomically very high level – between gymnosperms and angiosperms. Similar niche conservatism patterns on a higher taxonomic level have been found to affect how well woody species tolerate low temperatures (Hawkins et al., 2014), and how adaptation to colder climates is predominately induced by developing small hydraulic conduits (Zanne et al., 2013). Unlike dormancy, which evolves under straightforward environmental pressure(s), adjusting the size of hydraulic conduits is claimed to have already happened before angiosperm woody species moved from tropical conditions to colder climates (Zanne et al., 2013). In contrast to angiosperms, neither evergreen nor deciduous gymnosperms showed SADTO differences at different levels of cold and waterlogging tolerance (Fig. 4). Gymnosperms are predominately evergreen (91.7% of gymnosperm species in our sample were evergreen; compared with 20.1% evergreen species among angiosperms). In the case of evergreens, development of winter dormancy is often delayed or may even be absent in warmer climates (Olsen, 2010). Therefore, the length of vegetation period in most gymnosperms in their specific habitat is often longer compared than in most co-occurring angiosperms.
According to the whole-plant trait spectra analysis made for 305 North American woody plant species (Stahl et al., 2013), angiosperms have much higher functional diversity regarding tolerance to various abiotic stress factors than gymnosperms. Stahl et al. (2013) also found that the shade and drought tolerance of gymnosperm species were inversely correlated on a principal components analysis axis, while angiosperms had inverse correlation between drought and waterlogging tolerance (Stahl et al., 2013). When studying 339 woody species from the Northern Hemisphere, Hallik et al. (2009) also found that leaf functional traits showed significant trade-off patterns between shade and drought tolerance in all functional groups except deciduous broad-leaved species.
While the gymnosperms in our sample tolerated, on average, both shade and drought better than angiosperms, they also had a weaker trade-off between shade and drought. In contrast, angiosperms exhibited much higher waterlogging tolerance and also cold-waterlogging polytolerance (Table 1). Because of these differences, we suggest that gymnosperms are actually better able to tolerate shade–drought than angiosperms even if the functional traits for shade and drought tolerance are more polarized than in angiosperms (Stahl et al., 2013). However, this higher polytolerance capacity is only realized if the climatic and water conditions do not add too much extra abiotic stress. On the other hand, prolonged dormancy favours deciduous angiosperms in places where, in addition to shade and drought stress, there are also spring floods or other events causing waterlogging.
Species ecological site occupancy, tolerances and growing season length
It is important to distinguish between a species' ecological potentials per se and the species' occurrence across different habitats. Clearly, having a greater tolerance to a given environmental factor provides an important basis for colonizing habitats where this particular factor is limiting. On the other hand, due to the unique physiological, morphological and allocational modifications that lead to enhanced tolerance to the given factor, the species might become less competitive in other environments (Niinemets, 2010).
In the case of polytolerance, species stack traits that enhance their persistence in multiple habitats. However, enhanced stress tolerance is typically associated with a reduced capacity for carbon gain in non-stressed environments (Grime et al., 1997; Wright et al., 2004; Hallik et al., 2009). Thus, polytolerance should not mean an enhanced niche breath. Species with a greater tolerance to abiotic factors are actually more likely to experience a shorter vegetation period, whether through global patters (prolonged cold season) or local patterns (prolonged flooding season). Combinations of multiple stresses also appear in a different manner – they can occur either simultaneously or successively (Niinemets, 2010). A shorter vegetation period enhances the chance of several stress factors acting upon plants simultaneously, which in turn generates evolutionary pressure, especially on sessile organisms like plants, for adapting to habitats that exhibit a wide variety of climatic conditions. Polytolerance can therefore be interpreted as an adaptation that is not so much in the service of populations dispersal, but is more likely a way to survive in places with short vegetation period and high climatic variability (i.e. more frequent and severe abiotic stress).
Also, if a species has adapted to periodically low temperatures and conditions of excessive water, its capability to concurrently cope with low light and dry conditions is significantly reduced, as cold and waterlogging adaptations employ contingent trait solutions with shade and drought tolerance: shaded habitats tend to be moist and waterlogged habitats usually do not experience root zone drought (Niinemets & Valladares, 2006). While cold tolerance affects vegetation patterns on a larger scale, tolerance of excessive soil water or waterlogging tolerance acts more locally – in low-lying areas, places with poor drainage and/or extreme fluctuations in precipitation (Poot & Lambers, 2003). So far, relatively little is known about the broad set of mechanisms and underlying traits that make tolerance of excessive water possible in woody species (Cardoso et al., 2013), and therefore further research is needed to look for the reasons behind these differences in tolerance.
Conclusions
A trait-based approach has been the principal alternative to traditional theories for explaining the variability in species ecological potentials and trade-offs (Niinemets et al., 2009; Ouedraogo et al., 2013). According to critics of the traditional physiochemical constraint model, the high rate of interspecific variability in tolerating different stress types points to key roles of adaptive evolution and ecological filtering in making polytolerance possible (Sack, 2004; Niinemets & Valladares, 2006; Valladares & Niinemets, 2008). Although the trait-based approach has demonstrated correlative patterns among species ecological potentials and plant physiological traits (e.g. Hallik et al., 2009; Markesteijn & Poorter, 2009; Markesteijn et al., 2011b; Brenes-Arguedas et al., 2013), explanation of polytolerance patterns among stresses has been rather unsuccessful. In fact, these analyses have mainly shown that plants survive unfavourable conditions by exploiting stress-specific traits that often vary the same way in response to different stresses, for example an increase in leaf longevity both in drought- and shade-tolerant species (Hallik et al., 2009; Brenes-Arguedas et al., 2013).
The results of our study demonstrate that different abiotic stress factors in woody plants are significantly integrated with each other, and traditional physiochemical constraints do not explain these patterns. Tolerance to low temperatures and waterlogging had a clear trade-off with tolerance to shade and drought. The length of the vegetation period and dormancy are the key factors explaining why woody plants are less capable of tolerating both shade and drought in habitats where the vegetation period is relatively short and/or the water level is high. While dormancy enables angiosperms to face additional stress factors besides shade and drought, gymnosperms have lower polytolerance but are better able to tolerate shade and drought when other stress factors have weak effects. These results imply that we need to revise our understanding of global abiotic stress patterns in woodlands. More emphasis is clearly needed on the specific stress response trait data, geographic variability of environmental conditions and evolutionary history of species.
There are several promising directions for further studies. First, there is a need to have a better understanding of the exact mechanisms and traits that allow for polytolerance. As our results demonstrate, woody species polytolerance patterns depend significantly on species functional qualities, and therefore the associated mechanisms could vary in different types of species. Second, we should also strive to gain a better idea of the extent to which intraspecific variability, with its ecotypic and plastic components (Niinemets, 2015), affects trade-off patterns between different stress factors.
Acknowledgements
Financial support was provided by the Estonian Research Council (grants IUT 8-3, IUT 21-1 and PUT 607); the European Commission through the European Regional Fund (the Center of Excellence in Environmental Adaptation); and the European Research Council (advanced grant 322603, SIP-VOL+). We thank P. van Bodegom and two referees for their constructive comments.
References
Lauri Laanisto is a research scientist at the Estonian University of Life Sciences. His main interest is macroecology and currently his research focuses on global patterns of plant stress, especially abiotic stress polytolerance dynamics in woody species.
Ülo Niinemets is the professor of plant physiology at the Estonian University of Life Sciences and Member of the Estonian Academy of Sciences. His research interests extend from plant stress dynamics and acclimation, emissions of biogenic volatile organic compounds to carbon gain and trace gas exchange models.




