Strengthening confidence in climate change impact science
Abstract
Aim
To assess confidence in conclusions about climate-driven biological change through time, and identify approaches for strengthening confidence scientific conclusions about ecological impacts of climate change.
Location
Global.
Methods
We outlined a framework for strengthening confidence in inferences drawn from biological climate impact studies through the systematic integration of prior expectations, long-term data and quantitative statistical procedures. We then developed a numerical confidence index (Cindex) and used it to evaluate current practices in 208 studies of marine climate impacts comprising 1735 biological time series.
Results
Confidence scores for inferred climate impacts varied widely from 1 to 16 (very low to high confidence). Approximately 35% of analyses were not associated with clearly stated prior expectations and 65% of analyses did not test putative non-climate drivers of biological change. Among the highest-scoring studies, 91% tested prior expectations, 86% formulated expectations for alternative drivers but only 63% statistically tested them. Higher confidence scores observed in studies that did not detect a change or tracked multiple species suggest publication bias favouring impact studies that are consistent with climate change. The number of time series showing climate impacts was a poor predictor of average confidence scores for a given group, reinforcing that vote-counting methodology is not appropriate for determining overall confidence in inferences.
Main conclusions
Climate impacts research is expected to attribute biological change to climate change with measurable confidence. Studies with long-term, high-resolution data, appropriate statistics and tests of alternative drivers earn higher Cindex scores, suggesting these should be given greater weight in impact assessments. Together with our proposed framework, the results of our Cindex analysis indicate how the science of detecting and attributing biological impacts to climate change can be strengthened through the use of evidence-based prior expectations and thorough statistical analyses, even when data are limited, maximizing the impact of the diverse and growing climate change ecology literature.
Introduction
Increasing evidence that climate change has altered biological systems has spurred social, political and scientific concern (Parmesan, 2006; Rosenzweig et al., 2008; Garcia et al., 2014). Management decisions, future projections and scientific advancement require clear attribution of observed biological change to a suite of natural and anthropogenic pressures, including climate change (Hegerl et al., 2010). Yet assessing confidence in the attribution of responses to climate change is difficult without generally accepted procedures for inferring climate impacts. As demand for reliable impact assessments grows and empirical research proliferates, a framework for assessing confidence in the purported attribution of biological change to climate change is urgently needed.
Attribution is the process of detecting biological change and inferring which pressures are the most likely causes of the change (Hegerl et al., 2007, 2010). Inference about specific impacts of climate change as reported in individual studies often requires expert knowledge and a mechanistic understanding of how the system in question operates, which must then be conveyed to non-expert and non-specialist readers alike. There are a variety of approaches for causal attribution and inference in observational and time-series studies, and some convey stronger support for conclusions than others (Hegerl et al., 2007, 2010; Morgan et al., 2009). Some attribution methods allow for statistical confidence assessments (Hegerl et al., 2010); however, simpler attribution methods are left with informal confidence assessments that are often based on ‘expert opinion’ and likelihood statements accompanied by probability assignments (sensu Hegerl et al., 2007, 2010; Rosenzweig et al., 2008). Even with estimated probabilities, expert opinions involve substantial subjectivity (Morgan et al., 2009).
Clear guidelines for the presentation and interpretation of impacts research can strengthen confidence in conclusions about the attribution of biological change to climate change. Guidelines must be sufficiently broad to accommodate the wide variety of research approaches and philosophies represented in the literature on climate impacts, including classic Popperian hypothesis testing and Bayesian methods of inference (Platt, 1964; McCarthy, 2007). Marine climate change impact research occurs within diverse disciplines, including biological oceanography, organismal physiology, community ecology and biogeography, joining biological responses at local, regional and even global scales with a range of climate metrics (Garcia et al., 2014). Though each discipline recognizes principles of the scientific method for drawing inference, these principles take various forms when applied to the particular problem of attributing biological change. Comparing the strengths and limitations of inference across this diverse literature is not straightforward, and is further complicated by the wide range of approaches used to communicate the logic of inference. Here, we outline a framework to guide the communication and assessment of inferences in climate change impact studies with the goal of strengthening conclusions about how climate affects biological systems. Using this framework, we develop a specific index, the confidence index (Cindex), to assess confidence in conclusions concerning attribution in a sample of marine climate impact studies and to identify areas of strength and those that need improvement in future studies. We analyse current practices, finding that researchers can maximize confidence in conclusions concerning attribution by using prior expectations based on scientific evidence from theory, models, experiments and historical data, along with quantitative analyses of appropriate time-series data.
A Framework to Guide Communication of Inference in Climate Impact Studies
Strong inference that climate change has caused a biological response rests on three pillars of the scientific method (Fig. 1). First is a statement of an evidence-based prior expectation for how climate factors affect biological patterns. Second are data: appropriate climate and biological data must be available for testing expectations. Third is quantitative analysis to detect change through time and facilitate the inference of causality. An example consistent with this framework is the conclusion that in the Northern Hemisphere warming associated with climate change has contributed to the reduction of glass eel populations to small fractions of their historical abundances (Bonhommeau et al., 2008). Bonhommeau et al. (2008) formulated the expectation that ocean warming has reduced the availability of food for eel larvae and has thus reduced recruitment rates of young eels. Their expectation is based on independent historical evidence of correlated declines in food availability and eel recruitment. An alternative causal pressure – overfishing – was considered and discounted as the only driver of synchronized global declines in eel recruitment. The authors analysed data on sea surface temperature and marine primary production at a spatial (gridded ocean basin) and temporal scale (four decades) sufficient to detect the signal of ocean warming associated with climate change (Henson et al., 2010), and they tested for responses in eel recruitment data at multiple sites within the range of each species. They then used appropriate statistical analyses to detect a temperature-driven signal in eel recruitment throughout their large geographic ranges. Together, these pillars of the framework allowed Bonhommeau et al. (2008) to provide strong support for the conclusion that ocean warming, and not confounding factors such as autocorrelation or overfishing, is the primary cause of the globally declining abundance of glass eels.
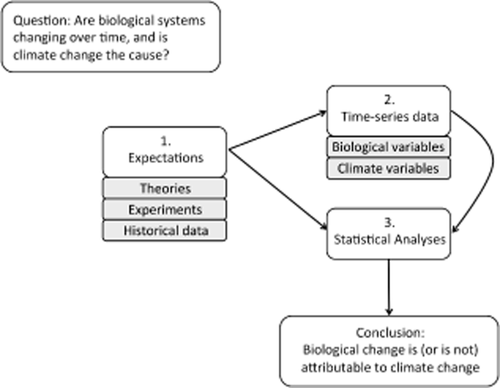
A framework outlining the basic scientific process through which a question about attribution of biological change to climate change can be answered, drawing on evidence from theories, experiments and historical data. These evidence-based expectations should guide the choice and use of appropriate data to test the expectation, as well as statistical analyses that allow researchers to distinguish a change through time due to climate from no change, autocorrelation, non-stationarity or response to an alternative driver. Successive iteration of this process reduces the set of plausible expectations and/or builds confidence in a single expected change.
Climate impacts research should begin with prior expectations, or statements about a biological response to climate change that can be evaluated against data. Expectations take different forms in different philosophies of inference. In a Popperian hypothesis testing approach, an expectation could be a falsifiable hypothesis (Platt, 1964). In Bayesian inference, expectations take the form of prior distributions (prior knowledge) that are used in combination with observed data to explore a hypothesis (McCarthy, 2007). Predictions are specific deductions from hypotheses or conceptual frameworks. We use the term ‘expectation’ here inclusively, and emphasize that the important step is to formally invoke evidence in the statement of a logical relationship between climate and biological change, but the particular expression of that statement may vary among statistical approaches or scientific disciplines. Importantly, our use of ‘expectation’ is not meant to convey predisposition toward a particular outcome such as a signal of climate change in biological data.
Informative expectations draw upon multiple, independent lines of evidence, and specify a relationship between climate change, the biological response and natural variability in the climate factor and biological variable that might be independent of climate change (Table 1, Appendix 3 in Supporting Information, Fig. 1). Expectations may specify the direction of response (i.e. in Bonhommeau et al. (2008) warming will decrease the availability of food for eel larvae) or may more generally specify a climate effect, in contrast to no climate effect (e.g. Hsieh et al. (2005) state that ocean climate change should shift the geographic ranges of fish species). Prior expectations are articulated early in the research process, prior to data analysis and ideally when the study is designed. Explanations of climate impacts often appear as post hoc interpretations without reference to prior expectations, and post hoc interpretations do not provide an equivalent level of support for conclusions (Crisp et al., 2011).
| Expectation to be tested against time-series data | Theory | Experiment | Palaeo |
|---|---|---|---|
| General expectation 1: Ocean warming shifts species geographic ranges to higher latitudes and deeper water | Cheung et al. (2010) | ||
| (i) Physiological temperature dependence constrains species range | Stillman & Somero (2000), Kuo & Sanford (2009) | Greenstein & Pandolfi (2008) | |
| (ii) Range limits are more dependent on ocean currents than temperature | Gaylord & Gaines (2000) | ||
| (iii) Species geographic ranges change as species interactions at the range borders change | Poloczanska et al. (2008) | Harley (2003) | |
| General expectation 2: OA reduces the abundance of calcifiers | |||
| (i) OA reduces calcification causing dissolution of CaCO3 shells in marine calcifiers | Orr et al. (2005) | Gazeau et al. (2007) | Moy et al. (2009) |
| (ii) OA has a greater effect on calcifiers that produce aragonite and high magnesium–calcite forms of CaCO3 than those that produce low magnesium-calcite forms of CaCO3 | Ries et al. (2009) | Kiessling & Simpson (2011) | |
| (iii) Calcifiers that regulate pH levels at the site of calcification were able to maintain calcification rates | de Beer & Larkum (2001), Al-Horani et al. (2003) | Knoll et al. (2007) | |
| General expectation 3: Climate change reduces population connectivity | |||
| (i) Larval development times are shorter in warmer water, reducing potential distance dispersed | O'Connor et al. (2007) | Houde (1989), Pepin (1991) | |
| (ii) OA reduces larval size for many marine invertebrates, reducing potential survival and larval duration | Kurihara (2008) | ||
| (iii) Warming and OA do not reduce connectivity because ocean currents control dispersal distance | Gaylord & Gaines (2000) |
- OA, ocean acidification.
- A longer list of expectations derived from the marine climate literature is presented in Appendix S3.
Despite the importance of prior expectations in scientific inference (Platt, 1964; Crisp et al., 2011), surprisingly little attention has been paid to procedures for generating and testing prior expectations in climate change ecology (Rijnsdorp et al., 2009). The first step is the development of prior expectations based on theoretical, empirical or historical evidence (Fig. 1, Table 1). Theoretical evidence takes the form of predictions derived from logical frameworks, sometimes formalized mathematically. Theories can guide the generation of expectations, with the advantage that theoretical frameworks mechanistically link biological change to climate change in a series of logical, deductive steps, allowing each step to be identified, evaluated and tested. For example, metabolic theory relates biological rates to temperature and produces predictions for non-intuitive responses to warming such as stable abundance, despite effects of warming on productivity (O'Connor et al., 2011).
Expectations can be based on experimental evidence, especially when theory is limited, as in the case of ocean acidification (Table 1). Experiments may provide a quantitative estimate of the magnitude and direction of how climatic factors affect biological processes, while controlling for other variables. Experimental manipulations of multiple factors can also inform expectations about synergistic or antagonistic effects (Crain et al., 2008), and may provide insight into the shape of functional responses (Zavaleta et al., 2003). For example, in marine systems, numerous experiments have shown that lower pH negatively affects calcification rates in corals (Ries et al., 2009). These findings suggest that ocean acidification may have reduced the abundance of coral, though the time-series data on pH are not yet long enough to test for an acidification effect over time. When generating expectations for changes through time, experimental evidence should be used with care due to the spatial and temporal constraints of experimental conditions.
Finally, expectations for how modern climate change affects species distributions and relative abundances can be based on historical evidence, including palaeontological and archaeological evidence, encompassing centuries to hundreds of millions of years. For example, an expectation that tropical coral reefs will shift their geographic range poleward to track ocean warming is based on evidence that warmer sea surface temperatures during the last interglacial period (125 ka) resulted in Pleistocene coral reefs in what is now the temperate zone of Western Australia (Table 1; Greenstein & Pandolfi, 2008). Expectations based on historical data do not, however, always provide mechanistic understanding nor do they consider synergies between concurrent anthropogenic pressures that may not have co-occurred in the past. Palaeontological studies have the advantage of long time series where palaeobiological and palaeoclimatic proxy data can be assessed simultaneously, though challenges include dealing with gaps in data, variability in preservation of different groups of organisms and often resolution of data that differs from the temporal resolution of the biological response to environmental change.
Importantly, evidence for generating expectations must be independent of the data used to test the expectation. For example, Bonhommeau et al. (2008) cite historical evidence that eels eat plankton, together with historical evidence that the abundance of plankton has changed with temperature. These datasets are distinct from those used to test for a recent decline in eel recruitment. Similarly, Nye et al. (2009) cite historical evidence of shifts in the abundance and distribution of fish species off the Canadian east coast to support a set of directional expectations for how sea surface temperatures should affect fish distributions off the coast of the north-eastern United States. In each case, the expectation for biological change is not based on the recent historical trend in the same system.
Carefully matching the expectation with available data allows inferences to build on the first and third pillars (Fig. 1). For any expected relationship between climate and a biological response, the spatial and temporal scale of the underlying process prescribes the scale and resolution of data appropriate for testing the expectation (Henson et al., 2010). Expectations can also guide the selection of climate metrics, an important step in analysing combined local and regional effects (Garcia et al., 2014). Sufficient data are often not available to properly test an evidence-based expectation. In these cases, the expectation could be restated, perhaps more generally (Table 1), to be testable with the data available. For example, in marine systems, ecological time series are often shorter in duration and coarser in resolution than required to test most expectations concerning ecological impacts of climate change (Henson et al., 2010). Henson et al. (2010), a rare example of an impact study that determined data requirements for detecting a climate change response prior to testing, calculated that attribution of observed changes in primary production in oceanic gyres to anthropogenic climate change requires a minimum of 30 years of ocean colour data, whereas the principal source of ocean colour data (a satellite) had only been operating for 14 years. Many marine biological responses have been attributed to climate change with datasets that are too short to deliver conclusive tests. When long-term, continuous datasets are absent, several datasets of shorter duration may be concatenated to create a longer time series (Poloczanska et al., 2008).
Finally, expectations can be used to identify appropriate quantitative tests (Fig. 1). Statistical analyses are necessary to detect a change in particular climate and ecological variables, and to test relationships between them whilst considering confounding factors. Testing expectations against observational data presents many challenges. The simple expectation that climate change is causing biological change implies an expectation that internal variability, other biotic or abiotic factors, or non-stationarity (the tendency of time series to exhibit temporal trends in their statistical properties) are not the only causes of the observed change. Consequently, advanced time-series analyses are often required to detect and attribute the impacts of climate change (Pyper & Peterman, 1998; Brown et al., 2011). Correlation analysis is commonly used in climate change studies, but this method has rarely accounted for temporal or spatial autocorrelation, non-stationarity or other important time-series issues. Failure to control for these potentially confounding factors introduces problems when assessing the statistical significance of correlations between biological and climate data (Pyper & Peterman, 1998; Brown et al., 2011). Model-based methods of statistical analysis, such as generalized additive modelling and structural equation modelling, can account for multiple drivers of change and autocorrelation simultaneously, allowing researchers to distinguish the contribution of climate change and its interaction with other drivers (Brown et al., 2011). Testing prior expectations that are mechanistically meaningful is important, because neither correlation nor more sophisticated statistical tests alone can determine causation. For instance, if two variables such as fishing pressure and warming are strongly correlated, it will be impossible to distinguish their effects on a species' distribution using statistical methods, whereas it may be possible to exclude one driver from analyses on the basis of a prior expectations for the direction and strength of effect.
Methods
Application of the framework: a quantitative assessment of current practices
The framework outlined here provides guidelines for strengthening inferences drawn from studies attempting to attribute an observed ecological change to climate change (Fig. 1). These guidelines may be applied when designing, reporting or interpreting a climate change impact study, and when synthesizing studies in impact assessments or meta-analyses. To facilitate such applications, we developed a confidence index (Cindex) that applies a numerical score to individual conclusions about impacts. We used the Cindex to assess current practices in the marine biological literature, and to compare confidence in conclusions about the impact of climate change across taxonomic, geographic and response-type groups. Although application of some components of the framework, such as the minimum number of years required (Table 2, Appendix S1), may be specific to marine climate change, the Cindex could be adapted easily to other systems by changing the specific values of some scoring categories.
| Detection (D) | Understanding (U) | Score |
|---|---|---|
| (a) Expectations (SE) | Max. = 6 | |
| (i) 0 = no clear prior expectation stated | ||
| +2 = prior expectation is clearly stated | ||
| (ii) +2 = study invokes specific evidence to support prior expectation | ||
| (iii) +1 = prior expectations are based on multiple lines of evidence | ||
| (iv) +1 = prior expectations for alternative causal factors are articulated or confounding factors are discounted | ||
| (b) Data (SD) | Max. = 6 | |
| Temporal | ||
| (i) 0 = ≤ 10 years of data | ||
| +1 = 11–25 years of data | ||
| +2 = > 25 years of data | ||
| + 1 = data spans > 30 years | ||
| (ii) +1 = continuous data (annual) | ||
| Spatial | ||
| (iii) 0 = single site study | ||
| +1 = multiple site study | ||
| (iv) 0 = local (≤ 1000 km2) | ||
| +1 = regional (> 1000 km2) | ||
| +1 = broad scale (> 100,000 km2) | ||
| (c) Quantitative analysis (SQ) | Max. = 6 | |
| (i) +1 = biological trend was statistically tested to distinguish from no trend | (iv) +2 = relationship between biological variable and climatic variable was statistically tested | |
| (ii) +1 = a trend in a climatic variable was statistically tested to distinguish from no trend | (v) +1 = alternative causal factors tested | |
| (iii) +1 = temporal/spatial autocorrelations have been considered | ||
| Max. = 9 | Max. = 9 | Max. = 18 |
Development of the confidence index
The Cindex measures confidence in scientific inferences derived from climate change impact studies based on scores derived from the scientific method and the spatial and temporal scale of climate change in marine systems (Table 2). Total Cindex scores reflect confidence in the strength of relationships between climate change and biological variables (Fig. 1) based on the type of information typically available for reporting. The Cindex includes an expectation score (SE) that is based on whether a prior expectation was clearly stated for how the biological variable may respond to climate change, whether expectations invoked specific evidence for putative climate pressures and whether expectations were stated for alternative (non-climate-change) causes or confounding factors (Appendix S1). A data score (SD) is based on whether the spatial and temporal extent of the data is well-matched to the scale of relevant climate change (Henson et al., 2010). Scores reflect the number of years in the dataset, the number of sites and the spatial extent of observations (local, regional). A quantitative score (SQ) is based on the statistics used to test (or account for) change through time, autocorrelation and alternative stressors (Brown et al., 2011).
The expectation, data and statistics scores (and their components, Table 2) can be consolidated into scores for detection (D = SD + SQi–iii) of biological change and understanding (U = SE + SQiv–v) of how the biological change relates to climate change (Table 2) (Kunkel et al., 2013). Detection implies a prior expectation of change in the system, and consequently requires data and statistical procedures that are sufficient to distinguish change from a null pattern and variability inherent in the data, such as natural variation in population size (Brown et al., 2011). Understanding relationships between biological change and climate change (or an alternative causal factor) requires the testing of biological and climate data against prior expectations for how the biological system should respond to observed climate change as well as considering and testing for alternative causal factors. Together, high D and U scores convey high confidence in conclusions concerning attribution, and thus may indicate studies that are most relevant to synthetic impacts assessments.
The maximum possible Cindex score, 18, describes a data-rich statistical test of a clearly stated, evidence-based expectation for how climate change affects a biological response, including a concurrent test for the role of alternative causal factors. Notably, confidence in conclusions concerning the cause of a biological change is independent of a study's findings. A conclusion may earn high confidence regardless of whether a biological response is attributed to climate change, to other causal factors, or no change was detected. For example, scores do not indicate whether the prior expectation was supported by the statistical test: a high-scoring study may find that climate change is not driving biological change, and the methods used may convey high confidence in this conclusion.
Analysis of Cindex scores
We applied the Cindex to 1735 biological time series, or ‘observations’, in 208 marine climate change impact studies to evaluate overall confidence in assessments of marine biological responses to climate change (Appendix S2). Here, we consider an ‘observation’ to be a time series of a single biological variable (i.e. abundance or phenology).
For this dataset, Poloczanska et al. (2013) determined whether each observation was consistent with the original authors' stated or implied expected impact of climate change. The characterization of ‘consistent’, ‘not consistent’ or ‘no change’ is not included in the Cindex, which remains neutral on the outcome of an analysis with regard to the direction or magnitude of its concluded biological response to climate change. After calculating Cindex scores for each observation, we matched these scores with the previous classification of consistency.
Cindex total scores were not normally distributed (Shapiro–Wilks test: W = 0.98, P < 0.001), and we tested for differences in scores among ocean, taxonomic groups and response-type groups using Kruskal–Wallis nonparametric tests. Although the grouping of observations within studies represents a kind of pseudoreplication, we chose not to model the study-level variation. Our objective was to assess confidence in conclusions at the level of observation because these are typically the units used in subsequent meta-analyses and syntheses that inform a collective understanding of climate impacts, and study-level factors are likely to contribute to these patterns.
Results
Results of Cindex analysis in marine biological studies
We found a wide range of Cindex scores (from 1 to 16) across our set of biological observations (Fig. 2). Most observations scored highly in only one category (e.g. data, SD), and no observation scored highly in all three categories. The maximum possible scores for expectations (SE), data (SD) and statistics (SQ) were achieved by 210 (12%), 32 (2%) and 120 (7%) observations, respectively (Fig. 2). More than 35% of observations were not reported in association with a prior expectation, and only 35% of statistical tests involved a test of an alternative driver of the observed result.
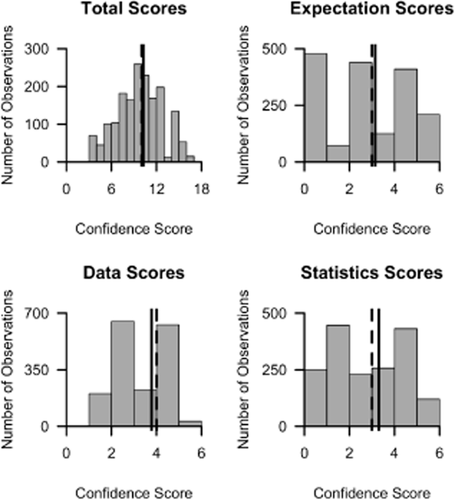
Distribution of Cindex scores for marine climate impact studies, along with distributions of subscores for prior expectations, data and quantitative scores. A solid black line indicates the mean score and a dashed line indicates the median score.
The highest scoring observations (330 observations, or 19%, across 37 studies) earned scores of six or more out of a possible nine in both detection (D) and understanding (U) (Fig. 3a, Appendix 2). Of these, 91% articulated a prior expectation and 86% articulated an expectation for an alternative causal factor; 100% statistically tested a change in climate but only 63% statistically tested for a change in an alternative causal factor; 90% used time series with more than 30 years of data; and 98% were multisite comparisons. Biological change was considered by the original authors to be consistent with expected effects of climate change in 226 (68%) of these high-scoring observations.
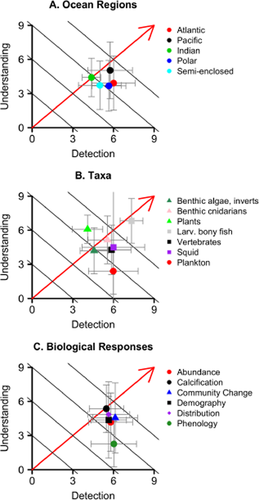
The confidence index (Cindex) quantifies confidence in attribution of an observed biological change to climate change. Attribution requires formal detection of change in a biological system, as well as formal understanding of how climate relates to the observed change (Table 1). Increasing total confidence is indicated by the red arrow. Black lines represent isoclines. Higher scores convey higher confidence in the conclusions of a a study. Cindex scores vary with available data and research approaches, and influence confidence in the current scientific attribution of biological responses to climate change as measured by the mean (± SD) Cindex score for studies grouped by ocean region (a), taxon (b) and biological response (c).
Biological change through time was not detected for all observations (Poloczanska et al., 2013) (Table 3). Observations for which change was detected (consistent with climate change) earned the lowest average Cindex scores (Kruskal–Wallis test: χ2 = 23.2, d.f. = 2, P < 0.001; Table 3) due to lower expectation and statistics scores (Table 3). When multiple species responses were reported in the same study, observations tended to earn higher scores than observations reported in studies that only considered one or two species (Kruskal–Wallis test: χ2 = 8.93, d.f. = 1, P < 0.003). The difference is due to statistics scores (Kruskal–Wallis test: χ2 = 11.54, d.f. = 1, P < 0.0007) and data scores (Kruskal–Wallis test: χ2 = 10.96, d.f. = 1, P < 0.001).
| i | j | Difference in means (i – j) | Difference in median (i − j) | W | P |
|---|---|---|---|---|---|
| Cindex score | α = 0.02 | ||||
| Consistent | No change | 9.97−10.58 = −0.62 | 10−11 = −1 | 196,388 | < 0.01 |
| Opposite to expected | 9.97−10.88 = −0.91 | 10−11 = −1 | 105,389.5 | < 0.01 | |
| No change | Opposite to expected | 10.58−10.88 = −0.29 | 11−11 = 0 | 44,775.5 | 0.48 |
| Expectation score | α = 0.02 | ||||
| Consistent | No change | 2.97−3.30 = −0.33 | 3−3 = 0 | 207,683.5 | < 0.02 |
| Opposite to expected | 2.97−3.69 = −0.72 | 3−5 = −2 | 97,831 | < 0.01 | |
| No change | Opposite to expected | 3.30−3.69 = −0.39 | 3−5 = −2 | 38,808.5 | < 0.01 |
| Data score | α = 0.02 | ||||
| Consistent | No change | 3.76−3.76 = 0.01 | 4−3 = 1 | 224,198 | 0.78 |
| Opposite to expected | 3.76−3.89 = −0.13 | 4−4 = 0 | 116,087 | 0.14 | |
| No change | Opposite to expected | 3.76−3.89 = −0.12 | 3−4 = −1 | 43,901.5 | 0.24 |
| Stats score | α = 0.02 | ||||
| Consistent | No change | 3.22−3.52 = −0.30 | 3−3 = 0 | 202,323.5 | < 0.02 |
| Opposite to expected | 3.22−3.29 = −0.07 | 3−3 = 0 | 120,108.5 | 0.50 | |
| No change | Opposite to expected | 3.52−3.29 = 0.23 | 3−3 = 0 | 50,626.5 | 0.05 |
To assess patterns in confidence of inferred climate change responses, we considered only observations for biological change consistent with climate change, as determined by a study's authors (n = 1098 observations; Appendix 2). Scores differed among ocean regions (Kruskal–Wallis test: χ2 = 29.23, d.f. = 4, P < 0.001, Table 4), with the Pacific and Atlantic oceans tending to earn higher confidence scores than other regions (Fig. 3, Table 4). Taxonomic groups also differed (Kruskal–Wallis test: χ2 = 233.03, d.f. = 6, P < 0.001), and the highest confidence scores were observed for larval bony fish, followed by vertebrates and benthic cnidarians (Fig. 3c, Table 4). Phenological responses earned significantly lower Cindex scores than abundance, distribution, demography, calcification and community change (Fig. 3, Table 4) (Kruskal–Wallis test: χ2 = 53.89, d.f. = 5, P < 0.001).
| Factor (i) | n | Mean score | Median score | Contrast (j) | W | P |
|---|---|---|---|---|---|---|
| Ocean region | α = 0.005 | |||||
| Atlantic Ocean | 595 | 9.93 | 10 | Pacific Ocean | 73,079.5 | 0.014 |
| Polar seas | 37,483 | 0.140 | ||||
| Semi-enclosed seas | 21,362 | < 0.003 | ||||
| Indian Ocean | 20,083.5 | 0.005 | ||||
| Pacific Ocean | 274 | 10.79 | 10 | Indian Ocean | 4870.5 | < 0.001 |
| Polar seas | 18,805 | 0.004 | ||||
| Semi-enclosed seas | 10,380 | 0.002 | ||||
| Polar Seas | 116 | 9.34 | 10.5 | Semi-enclosed seas | 3835 | 0.128 |
| Indian Ocean | 2717.5 | 0.107 | ||||
| Semi-enclosed Seas | 58 | 8.72 | 9 | Indian Ocean | 1684.5 | 0.604 |
| Indian Ocean | 55 | 8.80 | 8 | |||
| Taxa | α = 0.002 | |||||
| Vertebrates | 543 | 10.47 | 10 | Plankton | 42,156.5 | < 0.001 |
| Benthic invertebrates | 33,362.5 | < 0.001 | ||||
| Larval bony fish | 47,790.5 | < 0.001 | ||||
| Benthic cnidarians | 10,649 | 0.130 | ||||
| Plants | 6325 | 0.920 | ||||
| Squid | 1091 | 0.990 | ||||
| Plankton | 219 | 8.35 | 9 | Benthic invertebrates | 20,556.5 | 0.176 |
| Larval bony fish | 20,526 | < 0.001 | ||||
| Benthic cnidarians | 5481 | < 0.001 | ||||
| Plants | 1809.5 | 0.026 | ||||
| Squid | 391 | 0.714 | ||||
| Benthic invertebrates | 174 | 8.77 | 9 | Larval bony fish | 1323.5 | < 0.001 |
| Benthic cnidarians | 1337 | < 0.001 | ||||
| Plants | 1414 | 0.021 | ||||
| Squid | 340 | 0.941 | ||||
| Larval bony fish | 101 | 14.44 | 15 | Benthic cnidarians | 350.5 | < 0.001 |
| Plants | 2128.5 | < 0.001 | ||||
| Squid | 199 | 0.965 | ||||
| Benthic cnidarians | 34 | 10.75 | 11 | Plants | 497.5 | 0.075 |
| Squid | 67 | 0.980 | ||||
| Plants | 23 | 9.82 | 9 | Squid | 46 | 1 |
| Squid | 4 | 10.50 | 10.5 | |||
| Observation type | α = 0.003 | |||||
| Abundance | 487 | 9.98 | 10 | Distribution | 77,951.5 | 0.003 |
| Phenology | 45,683 | < 0.001 | ||||
| Demography | 10,307 | 0.865 | ||||
| Community change | 6919.5 | 0.434 | ||||
| Calcification | 4817.5 | 0.077 | ||||
| Distribution | 363 | 10.51 | 11 | Phenology | 38,386.5 | < 0.001 |
| Demography | 1752.5 | 0.366 | ||||
| Community change | 5535 | 0.880 | ||||
| Calcification | 4830.5 | 0.586 | ||||
| Phenology | 149 | 8.29 | 9 | Demography | 4107.5 | 0.005 |
| Community change | 3010 | 0.007 | ||||
| Calcification | 2838.5 | < 0.001 | ||||
| Demography | 43 | 10.02 | 10 | Community change | 715.5 | 0.593 |
| Calcification | 6160 | 0.355 | ||||
| Community change | 31 | 10.68 | 9 | Calcification | 426.5 | 0.522 |
| Calcification | 25 | 10.84 | 11 |
Detection scores, primarily reflecting the availability of data, explain differences in confidence scores among ocean regions (Fig. 3a). In contrast, understanding scores for how climate influences different responses explain differences among biological response types (Fig. 3c): phenological observations received detection scores similar to those of other observation types, but earned lower understanding scores (Fig. 3d). In general, confidence scores for observations of benthic organisms were limited by detection scores, while confidence scores for observations of planktonic organisms scored highly for detection but weakly for understanding (Fig. 3b).
Discussion
Recommendations for increasing confidence in climate impact studies
As quantified by the Cindex, observations of marine biological change are attributed to climate change with, on average, moderate confidence. Our findings suggest high confidence in attribution to climate change for changes involving larval bony fish, Pacific and Atlantic ocean regions and distributional, calcification and community-level responses. These findings differ from a confidence ranking based only on sampling effort (number of observations) (Table 4), and suggest that estimates of confidence based on vote counting do not necessarily align with estimates based on the integrated use of data, statistics and evidence-based expectations.
Several of our findings could reflect subtle publication biases in the impacts literature. When no change was detected in a biological time series, or when observations were contrary to an expected result of climate change, these conclusions earned higher confidence scores than analyses for which change was consistent with expectations. In addition, observations from multispecies assemblages tended to earn higher confidence. Multispecies studies tend to report changes (or no change) through time for all species in a dataset, regardless of consistency with expected impacts of climate change (e.g. Hsieh et al., 2005). These patterns in Cindex scores are consistent with literature bias against studies that show no effect of climate change. Solutions to publication bias include enhancing opportunities for publishing results that are not found to be consistent with climate change (Parmesan & Yohe, 2003) and for individual researchers to determine data needs based on prior expectations and then to report all results, including any that are indeterminate or counter to expectations.
Most observations did not earn high confidence scores. In an effort to highlight priorities for future research, we explore where points were lost and suggest possible solutions to three common problems we observed in our synthesis.
Data may be insufficient to test an expectation of climate-driven change
Weak SD scores were common (Fig. 2). Studies from the Indian Ocean or focusing on benthic taxa, for example, scored low on detection (Fig. 3) because relatively few long-term datasets exist or have been analysed in the context of the impacts of climate change. One solution is to increase investment in time-series datasets so that these may be available for future analysis (Reichman et al., 2011). In addition, efforts to support public archiving of data will ensure that existing data can be used well into the future (Wolkovich et al., 2012; Vines et al., 2014).
Statistical analysis may have been inappropriate or had insufficient power to detect change and test for climate-driven causes
Fewer than 10% of analyses of time series scored the maximum possible statistics scores; correlation analyses were most commonly used (Brown et al., 2011). Brown et al. (2011) reviewed an expanded version of the database we have analysed here and provided recommendations on the most robust approaches for tackling statistical challenges. More complex methods of detecting causation such as the convergent cross mapping (CCM) approach can help to identify causation by the increased ability of independent variables to predict dependent variables – and vice versa in the case of non-external independent variables – over time (Sugihara et al., 2012). This lack of convergence can cause re-examination of time series to produce conflicting results (Myers, 1998). CCM proves useful in the case of external forcing on two non-coupled variables, as cross-mapping will show no evidence of convergence between the two biological variables thus demonstrating no causation between them, although they may appear correlated (Sugihara et al., 2012).
Prior expectations for climate impacts and alternative explanations may be lacking, vague or insufficient in the final manuscript
Many studies (119 of 208 in our sample) lacked a clearly stated expectation for how climate change may affect the study system. Possible explanations for not stating a prior expectation in a climate impact study include the assumption by the authors (or reviewers) that readers possess expert knowledge sufficient to independently assume an expectation, convention in certain disciplines or cultures to de-emphasize prior expectations in scientific reporting and analyses, and uncertainty in the expected biological response to climate change.
When data are limited, the statement of prior expectations can strengthen confidence in attribution. For one iconic taxon, reef-building corals, for which extensive time-series data are generally lacking, climate impacts are attributed with moderate confidence in our database (Fig. 3). Studies of benthic cnidarians, including reef-building corals, scored higher Cindex values than other taxa with moderate detection scores (Fig. 3b), reflecting the use of evidence-based expectations to support conclusions about how climate has affected corals (De'ath et al., 2009; Pandolfi et al., 2011). Furthermore, prior expectations allow for proper selection of climate metrics when the vulnerability of a species to climate change is species or system specific (Garcia et al., 2014).
Our analysis quantified confidence in attribution within primary research studies, and we used these results to identify strengths and opportunities for improvement in climate impact studies. The framework we have developed, and the associated confidence index, does not suggest that a greater number of observations confers greater confidence in the conclusions. The Cindex and the framework from which it is derived suggest that greater confidence is achieved by integrating independent evidence for climate impacts into expectations that are then statistically tested against time-series data. Other approaches for assessment and attribution, such as meta-analyses and hierarchical models, pooled observations and consolidate shared information across groups to reduce uncertainty and thereby strengthen inference (Hedges et al., 1999; Gelman, 2006). These statistical approaches are necessary to determine the strength of evidence provided by a set of observations. The Cindex can be used within synthetic statistical frameworks as a weighting factor, to give greater influence to observations with a higher confidence score, strengthening cross-study comparisons such as those made by the Intergovernmental Panel on Climate Change (IPCC) Assessment Reports and advancing the study of climate change ecology. Currently the IPCC uses ‘expert opinion’ linked to a probability rating to determine strength of attribution to climate change within meta-analyses. Ranking studies or time series using a numerical scale as we suggest here would reduce the subjectivity that may be inherently present in expert judgements (Morgan et al., 2009) such that the confidence in attribution to climate change could be averaged per species, per region or per response type as we have done here. Because observations are ranked in an ordinal manner, greater emphasis can be placed on those observations with greater confidence when defining percentage consistency with climate change, as done in many meta-analyses of climate impacts (e.g. Parmesan & Yohe, 2003; Poloczanska et al., 2013), thus better reflecting the current state of knowledge of the impacts of climate change on biological changes.
Conclusions
We have offered the Cindex as a more transparent approach to assessing confidence in climate impacts studies, given the variability in presentation and methods in this literature. Strengths of the Cindex include its synthesis of evidence from a broad pool of scientific information to formulate testable expectations consistent with the scientific method, and some degree of transparency in how confidence is assessed and compared. The approach therefore overcomes some of the limitations associated with less transparent methods based on subjective expert opinions.
Nonetheless, reproducibility remains a major challenge in climate impacts research and assessments. The challenge of reproducibility is two-fold. First, given the same data, are conclusions and confidence assessments reproducible? Second, are findings reproducible when longer or more extensive datasets become available (Myers, 1998)? Our confidence index is one attempt to develop a confidence assessment procedure that may be more reproducible than expert opinions (Morgan et al., 2009) due to the clearly defined categories and transparent assignment of scores. The Cindex only indirectly addresses a second challenge of reproducibility, by encouraging communication of logic such that research processes and conclusions might be reproducible even with more data. However, if historical data and analytical code are not made available with publications, a deeper understanding of biological change through time may be hampered, despite the availability of more data (Wolkovich et al., 2012; Vines et al., 2014).
We found that many studies present compelling, but not conclusive, evidence that climate change more than other factors has affected marine biological systems. Confidently attributing biological change to climate change is essential to the application of scientific research to decision making. To improve this process, we have outlined an approach for assessing confidence in attribution at the level of the research analysis. Future investment in long-term datasets and the basic science to support evidence-based prior expectations for climate impacts will directly strengthen confidence in impacts studies and assessments.
Acknowledgements
We are grateful to Dr Sylvain Bonhommeau and the anonymous referees for thoughtful comments and suggestions that improved this work. We acknowledge financial support from the National Center for Ecological Analysis and Synthesis (NCEAS) to A.J.R. and E.S.P.
References
Additional references to the data sources may be found in Appendices S1 & S2. [weblink]
Mary O'Connor is a marine ecologist researching the drivers of variation in community structure and function, with a particular focus on the role of temperature-dependent metabolism in determining community- and ecosystem-level processes.
Johnna Holding studies the metabolic and ecological consequences of changing ocean conditions for marine life.
Carrie Kappel's research focuses on quantifying the ways humans depend upon and affect marine species and ecosystems and developing tools and approaches to enhance ocean management.
All authors are climate change ecologists, and have collaborated on various projects related to climate impact arising from the working group Marine Biological Impacts of Climate Change, sponsored by the National Centre for Ecological Analysis and Synthesis, and led by Anthony Richardson and Elvira Poloczanska.




