Global Tiger Density Linked With Forest Carbon Stock, Top-Down and Bottom-Up
Funding: This research was funded by the National Key R&D Program of China (2023YFF1305000) and Fundamental Research Funds for the Central Universities (2572022DS04). N.J.R. was supported by a Chinese government scholarship.
ABSTRACT
enTiger (Panthera tigris) survival, as apex predators in forest ecosystems, largely depends on abundant prey in healthy, intact forests. Because large herbivore prey are drivers of plant biomass, we reasoned that tiger distribution and density are probably also closely linked with forest carbon (C) stock, the management of which is critical for mitigating climate change. However, whether tigers exert top-down control of forest C stocks or are passive surrogate C indicators bottom-up is a salient unanswered question in conservation and management, particularly in trophic rewilding. Here, we compiled estimates of global tiger presence and density to test the top-down effects of tigers on forest C stocks and tiger-carbon relationships along a gradient from “empty forests” without tigers to “target state” ecosystems with tigers living at different abundances. Our results showed that tiger presence was associated with higher forest vegetation C stocks, lower C emissions, and higher C inputs globally. Top-down effects via ungulate biomass were stronger in less established forests. Furthermore, forest vegetation or soil C stocks increased with tiger density or reached tiger-carbon peaks in four forest habitat types covering most of the tiger range. Our findings reveal that tigers, represented by their presence and density, are both an indicator and a driver of forest ecosystem C stocks, depending on underlying ecological conditions, and could safeguard forests against future C emissions and improve our understanding of climate-C cycle feedback.
摘要
zh作为森林生态系统中的顶级捕食者,虎(Panthera tigris)的生存高度依赖于健康、完整森林中丰富的猎物资源。由于大型食草猎物是植物生物量的驱动因素,我们认为虎的分布和密度很可能也与森林碳储量密切相关——而森林碳储量的管理对缓解气候变化至关重要。然而,虎是否通过下行效应控制森林碳储量,还是仅仅作为上行效应过程中的被动碳指标?这一问题在保护和管理中,尤其是营养级再野化方面,仍然是一个悬而未决的突出问题。本研究通过全球老虎的分布和密度数据,沿着从“空森林”(无虎)到“目标状态”生态系统(具有不同虎密度)的梯度,检验了虎对森林碳储量的“下行效应”及“虎-碳”关系研究。研究结果表明,在全球范围内,虎的存在与更高的森林植被碳储量、更低的碳排放和更高的碳输入有关。有蹄类生物量介导的下行效应在较不成熟的森林中更强。在涵盖虎主要分布区的四类森林栖息地中,森林植被或土壤碳储量随虎密度增加而增加,或达到“虎-碳”峰值。我们的研究还发现,虎的存在和种群密度既是森林生态系统碳储量的指标,也是其驱动因素,这取决于潜在的生态条件。虎能够保护森林免受未来碳排放的影响,并提升我们对气候-碳循环反馈的理解
1 Introduction
As top predators, tigers (Panthera tigris) are assumed to be an indicator of forest ecosystem health, dependent on bottom-up ecosystem integrity and exerting top-down effects on lower trophic levels, regulating ecosystem stability (She et al. 2023). Range-wide across Asia, however, there exist over 700,000 km2 of forests that have been effectively emptied of tigers by targeted poaching (Gray et al. 2023; Sanderson et al. 2023). Losing tigers could lead to ecological cascade effects, including a hyperabundance of large herbivores and overexploitation of vegetation (Estes et al. 2011), or, where large herbivores are also lost, a downgrading and simplification of biodiversity and vegetation structure heterogeneity (Trepel et al. 2024). The bi-directionality of the interactions and interdependence between top predators, large herbivores, and vegetation is especially important for natural resource managers tasked with forest landscape restoration, trophic rewilding (Carver et al. 2021), and nature-based solutions to climate change (UNEP and IUCN 2021) because it is likely that large carnivore conservation promotes vegetation recovery (Villar 2023; Xu et al. 2023), just as forest protection and restoration support top predators (Jiang et al. 2017; She et al. 2023; Villar 2023; Xu et al. 2023).
Among conservation scientists and decision-makers, fervent interest exists in identifying and quantifying top-down ecosystem service values of wildlife (e.g., Dirzo et al. 2014; Hughes et al. 2024; Schmitz et al. 2023), especially in the context of ecosystem carbon because of its role in climate change mitigation. In forest ecosystems, the animal-carbon concept of “animating the carbon cycle” (Schmitz et al. 2018) is typified by studies and simulations of African forest elephants (Loxodonta cyclotis), demonstrating that browsing and seed dispersal affect carbon stocks (Berzaghi et al. 2019, 2023). Animal presence, whether herbivore or predator, can have cascading effects that increase ecosystem carbon stocks in vegetation and soil compared with their absence (Rizzuto et al. 2024). More broadly, global carbon hotspots, to some extent, overlap with biodiversity hotspots, but this largely depends on the scale of analysis and the metric used to measure biodiversity (Jung et al. 2021; Soto-Navarro et al. 2020; Zhu et al. 2021). For example, in Asia, carbon hotspots more closely match biodiversity hotspots at lower latitudes, such as rainforests in Southeast Asia, than at higher latitudes (Zhu et al. 2021), and carbon-biodiversity overlap has a temporal dimension as the dynamics change with forest age (Capellesso et al. 2020). Still, recognizing the likelihood of synergies, co-benefits, and cost-effectiveness of unified approaches to tackle challenges together, the United Nations Framework Convention on Climate Change (UNFCCC) and Convention on Biological Diversity (CBD) demand the conservation and restoration of natural or near-natural ecosystems to achieve respective climate and biodiversity goals (Dinerstein et al. 2020; IPCC 2022; Soto-Navarro et al. 2020), delivering benefits immediately (conservation) or within decades (restoration) (UNEP and IUCN 2021).
Many countries reference forests in their nationally determined contributions (NDCs) to climate change as nature-based solutions (UNEP and IUCN 2021; Zhu et al. 2021). After all, about 99.9% of global atmospheric CO2 removals (hereafter, carbon removals) come from nature-based climate solutions, around two-thirds related to forests (UNEP and IUCN 2021; Smith et al. 2023), removing CO2 from the atmosphere and storing it in plants, soils, and animals, building biomass. Additionally, among all climate change solutions, nature-based solutions have considerable potential for reducing CO2 emissions via “avoided emissions” by protecting ecosystems from deforestation, particularly in subtropical/tropical regions (Friedlingstein et al. 2022). Increasing carbon removals and, more so, reducing carbon emissions are the crux of climate change mitigation (IPCC 2021, 2023), but we and others (Schmitz et al. 2023) posit that there exist opportunities to better account for and utilize new knowledge about how wild animal presence and density determine carbon stock and flux. To emphasize, net carbon flux between the atmosphere and ecosystems could either be enhanced or reduced in the presence of wild animals depending on the functional composition of the wildlife species (Schmitz and Leroux 2020), and wild animals in both terrestrial and marine environments have been observed to influence carbon cycles in orders of magnitude relevant to major decision making (Schmitz and Leroux 2020; Schmitz et al. 2018, 2023), even though most populations exist at only a fraction of what they were decades to millennia before (Barnosky 2008; Estes et al. 2011; WWF 2022). Recovering population abundance, not just species richness, is a key component of the CBD goals of the global biodiversity framework (GBF), and so, population abundance should also be factored into carbon models.
In this study, we selected the tiger as our focal species because ecologically, animal-carbon research lacks representation of top predators and, as an indicator of biodiversity (Natsukawa and Sergio 2022), expands the breadth and significance of the research beyond the species level. The tiger's wide distribution geographically overlaps with areas identified in a regional analysis as spatial priorities that maximize the protection of Asia's carbon and vertebrate species richness, primarily outside protected areas (Zhu et al. 2021), and spans multiple biomes and habitat types. The region also lacks animal-carbon research (Schmitz et al. 2023). Socially, tigers attract much scientific, political, financial, and public interest (Wikramanayake et al. 2011), allowing us to collate data range-wide and for our results to appeal to diverse users. At the species level, the tiger is also a conservation-reliant endangered species with disproportionately unique phylogenetic diversity, that is, “evolutionary distinctiveness” (Gumbs et al. 2023), and having lost between 90% and 95% of its indigenous range (Sanderson et al. 2025), it provides an opportunity to compare tiger-present versus tiger-absent “empty” habitat and be highly relevant for species recovery initiatives (Gray et al. 2023). We note that tiger density varies considerably within and between different habitat types and regions (Wikramanayake et al. 2011), and forests are the most dominant habitats by total land area and are the most studied.
Our objectives were to determine (1) the difference between tiger-present and tiger-absent forests in their carbon stock and flux; (2) the degree to which tigers exert top-down control of carbon stock, and (3) direct and indirect tiger-carbon relationships, even if bottom-up. If top-down dominance is found, tiger conservation and restoration will make a critical difference to forest carbon storage; if bottom-up is dominant, tiger density becomes a passive, surrogate indicator of forest carbon, and results can be used to guide bottom-up ecosystem restoration by resolving how variation in forest carbon stock drives tiger density. Our scientific questions were (1) do carbon stock and flux differ between tiger-present and tiger-absent forests? (2) Do tigers exert top-down control of vegetation and soil carbon stocks, or are relationships bottom-up dominant, and what factors mediate tiger-carbon pathways? (3) How do tiger density-carbon stock relationships vary between forest habitat types and regions, including in response to human disturbance? (4) Is tiger density recovery linked with forest carbon neutrality? (5) Where direct tiger-carbon relationships are not found, does concomitant variation in intermediate trophic level wildlife diversity/abundance confound relationships? We hypothesized that (1) tiger-present forests have lower site-level carbon emissions (Sanderson et al. 2023), higher carbon removals (Jiang et al. 2017), and higher carbon stocks than forests without tigers (Rizzuto et al. 2024), (2) across the species' range, tigers exhibit both top-down and bottom-up relationships with carbon stock, directly and/or indirectly via ungulate biomass (She et al. 2023), and top-down controls are stronger in “emptier” forests with lower ungulate biomass (McCauley et al. 2018), (3) tiger density is positively linearly or non-linearly related to vegetation and/or soil carbon stock (Berzaghi et al. 2019), varying by forest habitat type and geographic region, and carbon stocks are lower in disturbed forests, (4) higher tiger densities are associated with forest carbon neutrality, and (5) tiger-carbon relationships are confounded by interaction with intermediate trophic level wildlife diversity/abundance.
Briefly, our methods included collating tiger distribution and density data across Asia, extracting remotely sensed and field-based carbon, biodiversity, and environmental data in the overlapping areas (forest only) and then running a series of statistical analyses aligned with our objectives above. Our work expands the scope of previous animal-carbon research by including underreported ecosystem functions, especially carbon removals, soil organic carbon, and net carbon flux, a gradient of predator density more than presence/absence, and aspects of context dependency including forest habitat type, region, human disturbance, and variable carbon densities (Brodie et al. 2024). In this study, a forest is defined as a continuous stand of trees. It includes both forested areas (generally with a closed canopy) and wooded areas (canopy more open), including primary and secondary forest habitats and forest edges/margins, as per the IUCN Habitats Classification Scheme (IUCN 2012) to which input data used to extract tiger habitat in this study were calibrated (Jung et al. 2020). Species range was determined using tiger conservation landscape (TCL) data (Sanderson et al. 2023), but see sensitivity analysis in Section 5.
2 Results
2.1 Carbon Stock and Flux in Tiger-Present and Tiger-Absent Forests Globally
Almost 90% of the tiger's current global range is forest, primarily temperate forest (44%), subtropical/tropical moist lowland forest (23%), subtropical/tropical dry forest (12%), and subtropical/tropical moist montane forest (9%) (Figure 1). Hence, our study investigates the dominant habitat types by total land area. Across the range, within forest habitat types and regions, aboveground biomass carbon (AGBC) per hectare was typically lower where tigers were absent (Cliff's Delta δ < −0.3), except in subtropical/tropical dry forests where AGBC density was higher in tiger-absent forest (Cliff's Delta δ = 0.39, 95% CI: 0.39–0.40, Figure 1, Figure S1). However, tiger-present and tiger-absent forests differed in underlying environmental conditions such that AGBC variation often matched variation in mean annual precipitation and mirrored evergreen forest dominance in all subtropical/tropical forest habitat types except mangroves. By contrast, soil organic carbon (SOC) per hectare was higher in tiger-present forests in some forest habitat types, higher in tiger-absent forests in others, and indifferent in others (|Cliff's Delta δ| < 0.3) and had no consistent relationship with mean annual temperature, precipitation, evergreen forest dominance, AGBC, or tree species richness. Net forest carbon flux also did not show consistent relationships with tiger presence/absence or environmental factors globally, but we highlight subtropical/tropical mangroves, which were substantial net carbon sinks and sequestered more (net) carbon per hectare where tigers were present, and there were several forest habitat types and regions that were net carbon sources.
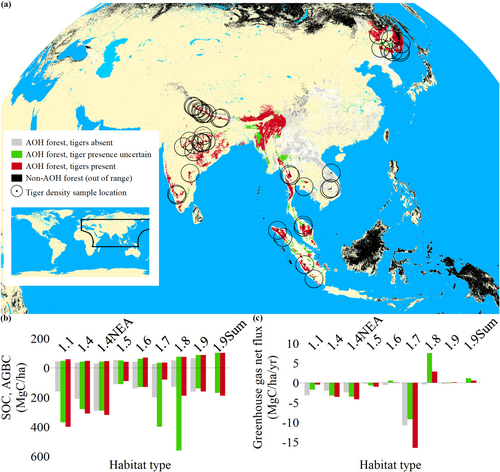
Based on average carbon emissions per hectare for each forest habitat type and region in tiger-present (1,411,669 km2) and tiger-absent (1,021,081 km2) forests, simple counterfactual forest emissions in tiger-present forests were 0.010 GtCO2/year lower (~9% lower) than expected if tigers were lost, mostly attributable to subtropical/tropical dry forests (Table 1). Per hectare, these so-called “avoided emissions” were also considerable in subtropical/tropical mangrove forests and, regionally, in subtropical/tropical moist montane forests in Sumatra. Carbon removals were 0.082 GtCO2/year higher (~28% higher) in tiger-present forests than expected if tigers were absent, most observable in temperate and mangrove forests but highly variable among forest habitat types and regions. Similarly, if tiger-absent forests still had free-living wild tigers and similar conditions as tiger-present forests, emissions would be 0.009 GtCO2/year lower (~10% lower) than observed and carbon removals 0.028 GtCO2/year higher (~12% higher).
| Habitat (subset) | Area of habitat (km2) | Forest greenhouse gas emissions (GtCO2/year) | Forest carbon removals (GtCO2/year) | |||||
|---|---|---|---|---|---|---|---|---|
| Extant range | Extinct range | Extant range | Extinct range | Assumed difference | Extant range | Extinct range | Assumed difference | |
| 1.1 Boreal forest, range-wide | 364 | 140 | 4.343 × 10−5 | 2.138 × 10−8 | 4.337 × 10−5 | 5.978 × 10−5 | 4.402 × 10−5 | −5.466 × 10−5 |
| 1.4 Temperate forest, range-wide | 693,559 | 364,975 | 0.015 | 0.005 | 0.005 | 0.264 | 0.080 | 0.113 |
| 1.4 Temperate forest, Northeast Asia | 601,864 | 169,772 | 0.014 | 0.004 | 0.001 | 0.265 | 0.045 | 0.104 |
| 1.4 Temperate forest, not Northeast Asia | 91,695 | 195,270 |
0.001 |
0.002 | 7.324 × 10−5 | 4.949 × 10−4 | 0.035 | −0.016 |
| 1.5 Subtropical/tropical dry forest, range-wide | 189,057 | 143,234 | 0.007 | 0.022 | −0.022 | 0.026 | 0.025 | −0.007 |
| 1.6 Subtropical/tropical moist lowland forest, range-wide | 369,858 | 385,358 | 0.059 | 0.057 | 0.004 | 0.056 | 0.080 | −0.021 |
| 1.7 Subtropical/tropical mangrove vegetation above high tide level, range-wide | 5588 | 918 | 4.809 × 10−5 | 1.495 × 10−4 | −8.619 × 10−4 | 0.009 | 0.001 | 0.002 |
| 1.8 Subtropical/tropical swamp, range-wide | 8848 | 2478 | 0.005 | 3.481 x 10−4 | 0.004 | 0.002 | 4.611 × 10−4 | 8.038 × 10−4 |
| 1.9 Subtropical/tropical moist montane forest, range-widea |
144,395 (136,102) |
123,978 (186,571) |
0.014 (0.015) |
0.012 (0.022) |
9.909 × 10−5 (−0.001) |
0.013 (0.013) |
0.016 (0.029) |
−0.006 (−0.009) |
| 1.9 Subtropical/tropical moist montane forest, Sumatraa |
49,377 (62,552) |
15,720 (8370) |
0.005 (0.009) |
0.003 (0.003) |
−0.004 (−0.016) |
0.002 (0.004) |
0.001 (0.003) |
−0.001 (−0.015) |
| 1.9 Subtropical/tropical moist montane forest, not Sumatraa |
95,018 (73,550) |
23,421 (178.201) |
0.009 (0.006) |
0.002 (0.018) |
−1.028 × 10−4 (−0.002) |
0.010 (0.009) |
0.005 (0.027) |
−0.008 (−0.002) |
| Totals, range-wideb | 1,411,669 | 1,021,081 | 0.100 | 0.097 | −0.010 | 0.370 | 0.202 | 0.082 |
- Note: Positive assumed differences indicate additional carbon emissions or removals, and vice versa. Extant and extinct range are determined from the Tiger Conservation Landscape (TCL) dataset, year 2020, respectively the “species” and “restoration” landscapes and fragments.
- a In the underlying Tiger Conservation Landscape (TCL) data, there are no restoration landscapes or restoration fragments in Sumatra. Reported values for Sumatra and not Sumatra “Extinct” range instead use the “survey” landscapes and fragments in the TCL data, considered to be similar to “Presence Uncertain” of the IUCN range classification system. For better comparison between tiger-present and tiger-absent subtropical/tropical moist montane forests in Sumatra, outside of Sumatra, and range-wide, we additionally report values when IUCN extant and extinct range are used as the underlying data to prepare the Area of Habitat (AOH) from which carbon data were extracted; these values are reported in parentheses.
- b Totals are calculated as the range-wide sum and do not include Northeast Asia and Sumatra separately.
2.2 Testing for Top-Down Effects of Tiger Density on Forest Carbon Stocks
Of 43 digitized forest landscapes with corresponding tiger density estimates globally (Figure 1, Table S1), 29 were disturbed forests with tigers present (temperate forest: n = 7; subtropical/tropical dry forest: n = 7; subtropical/tropical moist lowland forest: n = 15). These were selected to test the top-down versus bottom-up research question.
First, silhouette analysis of multivariate carbon stock (AGBC, belowground biomass carbon [BGBC], SOC) found that landscapes within each forest habitat type were clustered into two groups, distinguished by AGBC/BGBC dissimilarity. These groups were then composited across forest habitat types to form one low/intermediate AGBC/BGBC group (n = 21) and one high AGBC/BGBC group (n = 8). In both groups, anosim and adonis tests confirmed that most variation in carbon stock was explained by forest habitat type (R/R2 > 0.70, p < 0.01, Table 2, Figure S2). In forests with low/intermediate AGBC/BGBC, but not high AGBC/BGBC, an additional 4% of carbon stock variation was probably explained by variation in tiger density, standardized by forest habitat type (R2 = 0.04, p = 0.07, n = 21). By contrast, standardized precipitation and tree diversity variation had no additional explanatory power than forest habitat type alone, whereas standardized temperature explained an additional 8% of carbon stock variability in low/intermediate AGBC/BGBC forests only (R2 = 0.08, p < 0.001, n = 21).
| Test | Carbon cluster | Variable | R/R2 | p |
|---|---|---|---|---|
| Anosim | Low/intermediate AGBC/BGBC | Forest habitat type | 0.70 | 0.001 |
| High AGBC/BGBC | Forest habitat type | 0.96 | 0.006 | |
| Adonis | Low/intermediate AGBC/BGBC | Forest habitat type | 0.87 | < 0.001 |
| Forest habitat type×tiger density | 0.04 | 0.07 | ||
| Forest habitat type×MAP | 0.03 | NS | ||
| Forest habitat type×MAT | 0.08 | < 0.001 | ||
| Forest habitat type×tree diversity | 0.01 | NS | ||
| High AGBC/BGBC | Forest habitat type | 0.96 | < 0.001 | |
| Forest habitat type×tiger density | 0.02 | NS | ||
| Forest habitat type×MAP | 0.02 | NS | ||
| Forest habitat type×MAT | 0.03 | NS | ||
| Forest habitat type×tree diversity | 0.01 | NS |
- Note: Adonis tests of interaction between forest habitat type and tiger density, MAP, MAT, and tree diversity, were evaluated sequentially, testing whether these variables explained significantly more variation not explained by forest habitat type alone. Tiger density, MAP, MAT, and tree diversity were continuous variables scaled by forest habitat type. R and R2 are statistical measures of group differences and explained variation, respectively, in anosim and adonis analyses.
- Abbreviations: AGBC, aboveground biomass carbon; BGBC, belowground biomass carbon; MAP, mean annual precipitation; MAT, mean annual temperature; SOC, soil organic carbon (0–100 cm depth).
We expect that failing to detect a significant effect of tiger density on carbon stock in high AGBC/BGBC forests was due to low statistical power (Type II error) and/or underlying ecological differences in trophic network complexity, which weaken or distort top-down effects of tigers on carbon stock; we expected low/intermediate AGBC/BGBC forests to be more like “empty forests” with more easily detectable direct tiger-carbon relationships. Among six diversity and abundance variables compared between the two carbon groups, all standardized by forest habitat type, low/intermediate- and high-AGBC/BGBC forests were most dissimilar in their ungulate biomass, lower in low/intermediate AGBC/BGBC forests (p = 0.05, Figure S3). Hence, to then test possible mechanisms of top-down control of tigers on carbon stock, subsequently splitting the data into low and high ungulate biomass groups, rather than low/intermediate- and high-AGBC/BGBC groups, gave a reasonable solution to overcome limitations of small sample sizes and an appropriate means to test hypothesized tiger density-ungulate biomass-carbon stock pathways. Low (n = 17) and high (n = 12) ungulate biomass groups represented below- and above-average ungulate biomass in each forest habitat type, assumed to roughly proxy the low/intermediate- and high-AGBC/BGBC forest groups, respectively, from the anosim and adonis analyses.
If our assumption that tigers exert top-down control of carbon stock via ungulate biomass in low/intermediate AGBC/BGBC forests was true, first, we expected to find a negative relationship between tiger density and ungulate biomass in the low ungulate biomass group but not in the high ungulate biomass group. There was some support for this, switching from top-down to bottom-up control as ungulate biomass increased (Figure 2). Second, we expected changes in ungulate biomass to correspond with changes in carbon stock, particularly in the low ungulate biomass group. For consistency, we tested ungulate biomass against the coordinate values of the two NMDS axes of the distance matrix used in the anosim and adonis analyses, NMDS1 aligning with SOC, and NMDS2 aligning with AGBC/BGBC. Indeed, NMDS2 had a significant non-linear relationship with ungulate biomass in the low ungulate biomass group (R2adjusted = 0.476, p = 0.01, RMSE (LOO-CV) = 12.74, n = 17) but not in the high ungulate biomass group (R2adjusted = 0.179, p = 0.35, RMSE (LOO-CV) = 29.87, n = 12). In these aggregated data, ungulate biomass–NMDS1 relationships were weak in both ungulate biomass groups.
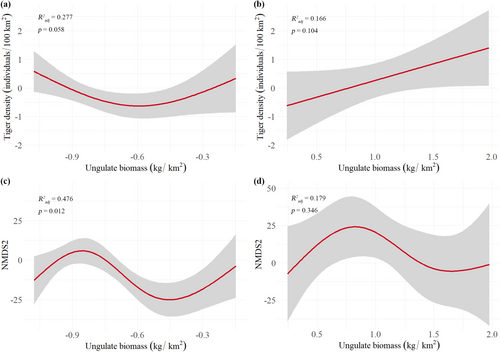
Finally, disaggregating data to test top-down effects in each forest habitat type individually, because tiger-carbon relationships differed between forest habitat types (Figure S4), subtropical/tropical moist lowland forest, as the only forest habitat type with sufficient data to split into low and high ungulate biomass groups for separate analyses, demonstrated that tigers exerted top-down control of carbon stock (NMDS1) via ungulate biomass, particularly in the low ungulate biomass group (Figure S5). Similarly, a positive ungulate biomass-NMDS1 relationship probably exists in subtropical/tropical dry forests (R2adjusted = 0.349, p = 0.08), but a logarithmic growth relationship between tiger density and ungulate biomass suggests that either both top-down and bottom-up processes are operating or that increases in ungulate biomass have diminishing effects on tiger density as tigers reach environmental carrying capacity. Random forest analysis, however, confirmed that ungulate biomass explained 75.10% ± 0.61% of the variation in tiger density so we assume that the tiger-carbon relationship in subtropical/tropical dry forests was primarily bottom-up. In temperate forests, our data show no support for dominant top-down effects of tiger density on ungulate biomass, and data were too few to reasonably test suspected breakpoints in shifts between top-down and bottom-up control.
2.3 Direct Tiger Density-Forest Carbon Models and Predictions, and Effects of Disturbance
Next, we tested for direct relationships between tiger density and carbon stock and flux in each forest habitat type and region individually so that carbon stock and/or flux could be predicted for individual landscapes and range-wide under conditions of tiger recovery. Specifically, we tested tiger-carbon relationships for AGBC density, SOC density, forest carbon removals, forest carbon emissions, and net forest carbon flux for each data subset (n = 13, described in Section 5), including presence only and disturbed forests only; BGBC density models were redundant as BGBC strongly positively correlated with AGBC (Figure S4). Additionally, generalized linear models (GLMs) were used to identify significant environmental factors influencing AGBC and SOC, interpreted alongside correlation analyses to help explain observed tiger-carbon relationships.
In temperate forests in Northeast Asia (n = 8), particularly in disturbed forests (n = 7), tiger density increased with AGBC density (R2adjusted = 0.792, p = 0.005, n = 7) and net carbon removals (R2adjusted = 0.772, p = 0.006, n = 7); note, AGBC density and net carbon removals were correlated (r = 0.90, p = 0.006, n = 7). Random forest analysis determined that AGBC density and net carbon removals explained much of the tiger density variance, respectively 63.44% ± 0.59% and 48.18% ± 0.37% (n = 7). In GLM analyses, AGBC density increased with annual precipitation (R2CV = 0.904, n = 7; Tables S2 and S3), and annual precipitation was positively correlated with tiger density (r = 0.77, p = 0.025, n = 7; Figure S4), questioning whether tiger density follows vegetation carbon density or precipitation, or both. SOC density decreased with temperature and elevation and was unrelated to tiger density.
In subtropical/tropical dry forests (n = 13), AGBC and tiger density were not directly related. Among significant variables influencing AGBC in the GLM, forest landscape integrity (FLII) did, however, present a possible link with tiger density as it correlated with savanna/woody savanna dominance, itself a significant driver of tiger density (Figure S6); data suggest that forest landscapes with the highest dominance of “carbon poor” savanna/woody savanna habitat have the lowest tiger densities. Recalling the anomaly of higher AGBC density in the tiger-absent range in this forest habitat type at the global level and consulting GLM results, we conclude that subtropical/tropical dry forests currently without tigers probably have higher AGBC density because of at least moderately higher precipitation, tree species richness, evergreen forest dominance, and lower savanna dominance; these tiger-absent forests are mainly in southeast Asia, a region with high wildlife snaring “empty forests.” In disturbed tiger-present forests, tiger density increased with SOC (R2adjusted = 0.509, p = 0.04, n = 7). This can be explained by a positive correlation between tiger density and evergreen forest cover (r = 0.77, p = 0.041, n = 7) and a positive effect of evergreen forest cover on SOC (R2CV = 0.805, n = 7).
In subtropical/tropical moist lowland forests (n = 15), AGBC was not related to tiger density but increased with NDVI. SOC density was non-linearly related to tiger density (four-degree polynomial; R2adjusted = 0.3104, p = 0.103, n = 15); retaining only tiger densities less than ~7 tigers/100 km2 (the 75th percentile) improved the model, peaking at around 3 tigers/100 km2 (two-degree polynomial; R2adjusted = 0.5794, p = 0.013, n = 11; increasing function: p = 0.006; decreasing function: p = 0.004; Figure 3). Random forest analysis confirmed that tiger density explained 4.32% ± 0.73% (n = 15) or 23.52% ± 1.19% (n = 11) of the variance in SOC density. Excluding the effects of tiger density, GLM analysis indicated that SOC was determined by tree diversity, savanna/woody savanna cover, NDVI, and mixed forest cover.
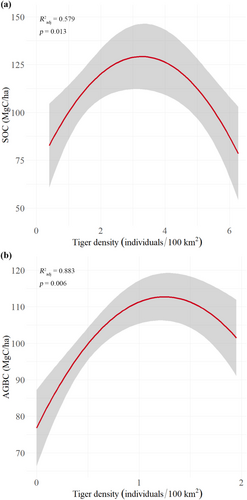
In subtropical/tropical moist montane forests (n = 7), tiger density range-wide was related to AGBC density in a non-linear relationship (two-degree polynomial; R2adjusted = 0.8826, p = 0.006, n = 7; increasing function: p = 0.003; decreasing function: p = 0.004), peaking at around 1 tiger/100 km2. These data were excluded from the earlier top-down versus bottom-up analyses as they were from relatively intact forests and included tiger absence data; so here, we add that ungulate biomass increased (weakly) with AGBC density (r = 0.69, p = 0.086, n = 7) although the tiger density-ungulate biomass relationship was unclear. In the GLM, we did not find an acceptable model of AGBC density probably because of substantial deforestation in the studied landscapes. SOC was not related to tiger density and decreased with temperature. Additionally, in relatively intact subtropical/tropical moist montane forests globally, net flux decreased with tiger density (r = −0.87, p = 0.024, n = 6), while in Sumatra, emissions increased with tiger density (r = 0.98, p = 0.025, n = 4).
Then, carbon predictions for different conservation scenarios globally (Table S4) and at the landscape scale (Table S5) showed that carbon stocks in tiger recovery landscapes/sites were generally similar or higher at target tiger densities compared with observed baseline values; Sathyamangalam is the exception tiger recovery site because of its extraordinarily high baseline SOC. Global predictions in forest habitat types with non-linear tiger-carbon relationships predicted that carbon stocks would be lower if tiger densities were recovered, but these conclusions were refuted by landscape-level predictions, emphasizing the need for context-specific data, models, and targets to predict localized restoration outcomes. Regarding model performance and human disturbance, AGBC and SOC were underestimated in relatively intact forests unless they were included in the statistical models (Table S6).
2.4 Tiger Density and Forest Carbon Neutrality
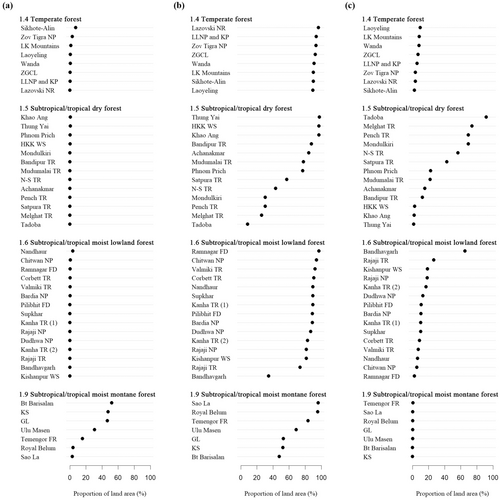
Fine-scale carbon fluxes were evaluated as the proportion of land area within each landscape that was a net carbon source, sink, or with no change (“carbon neutral”, Figure 4), revealing that subtropical/tropical moist montane forests in Sumatra were characterized as a net carbon source, temperate forest and subtropical/tropical moist lowland forest landscapes as a net carbon sink, that tiger-absent forest landscapes can occur anywhere along the sink/neutrality gradient, and that source/sink/neutrality had no apparent relationship with tiger density unless we assumed habitat-specific conservation target density—the top three landscapes with the most land area as “carbon neutral” in subtropical/tropical dry forest had densities of 2.29–4.45 tigers/100 km2 and target density is 3 tigers/100 km2, suggesting a possible link between carbon neutrality and target tiger density. Correlation analyses confirmed that total net flux was determined by emissions in subtropical/tropical moist montane forests and by removals in the three other habitat types. Lastly, forests with higher FLII had lower emissions in two forest habitat types—in other words, forest degradation preceded forest loss.
2.5 Indirect Tiger-Carbon Relationships via Interactions With Other Wildlife Diversity
First, in temperate forests, we found that SOC had a weak positive linear relationship with ungulate biomass per tiger (R2adjusted = 0.352, p = 0.094, n = 7). Second, in subtropical/tropical moist lowland forests, AGBC was partially explained by the non-linear interaction between tiger density and ungulate biomass (R2adjusted = 0.260, p = 0.101, RMSE (LOO-CV) = 17.63, n = 15) or, stronger statistically, by the non-linear interaction between tiger density and carnivore diversity (R2adjusted = 0.419, p = 0.030, RMSE (LOO-CV) = 15.66, n = 15, Figure S7). Inspecting the model plots revealed that increasing tiger density had a positive effect on AGBC where carnivore diversity is high and a negative effect where carnivore diversity is low; similarly, increasing tiger density where ungulate biomass was low probably reduced AGBC, while increasing tiger density at moderate ungulate biomass densities corresponded with increasing AGBC. Third, in subtropical/tropical moist montane forests, SOC was related to the non-linear interaction of tiger density and biodiversity richness intactness (R2adjusted = 0.915, p = 0.003, RMSE (LOO-CV) = 12.36, n = 7) or the linear interaction between tiger density and carnivore diversity if analyzing presence-only data and excluding one extreme tiger density outlier (R2adjusted = 0.929, p = 0.005, RMSE (2-fold CV) = 31.38, n = 5); note that these results are likely distorted by lower intactness/diversity and higher SOC in Sumatra compared with outside of Sumatra, and data were too few to analyze either geographic region independently. Finally, in subtropical/tropical dry forests, just as we could not find a direct link between AGBC and tiger density, we could not find a link between AGBC and a tiger density-biodiversity interaction term either.
3 Discussion
3.1 Tiger Presence, Absence, and Carbon Storage and Flux
First, AGBC was higher in tiger-present forests than in tiger-absent forests of the same type, even where mean annual precipitation, tree diversity, or mean annual temperature were similar. Theory supports this (Rizzuto et al. 2024), as do case studies of higher vegetation carbon stock where mammalian predators (Enydra lutris) in marine environments (Wilmers et al. 2012) and invertebrate predators (Pisaurina mira) in meadows (Strickland et al. 2013) are present. In our study, we did, however, assume that AGBC in subtropical/tropical forests was at least partially explained by evergreen forest dominance, not just tiger presence, and recommend that future comparative investigations assess finer levels of habitat classification to verify these claims. We also expected higher SOC where tigers were present (Rizzuto et al. 2024), but this was only true in some forest habitat types; whole community complexity, including animal diversity and trophic interactions, likely has a larger role in determining SOC variation than the individual effects of the presence/absence of a single given species (Sobral et al. 2017).
Second, our counterfactuals, although an oversimplification, suggest that tiger presence in some cases has some sort of “guardian” effect, protecting forests from deforestation and related emissions. While other factors may be at play, such as forest type, topography, and community ties and use of forests, we recall that enhanced management, protection, monitoring, and funding in specially designated tiger reserves in India have been linked with lower deforestation-related emissions (Lamba et al. 2023), and habitat loss in Cambodia, Laos, and Vietnam increased by 77%, 401%, and 410%, respectively, after losing tigers in the early 2000s (Sanderson et al. 2023). So, we assume that tiger presence, as a well-recognized flagship species, can have significant safeguarding effects on forest ecosystems, stemming (but not halting) emissions.
3.2 Tiger Density and Carbon Stock, Top-Down and Bottom-Up
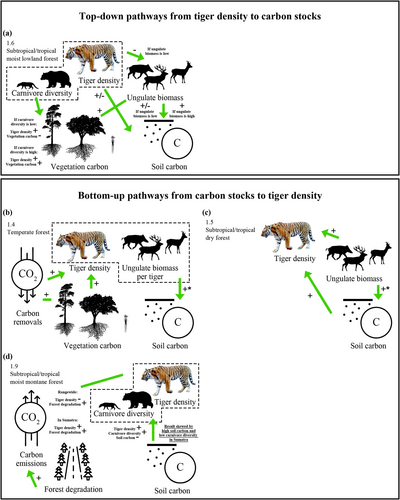
In all four forest habitat types studied, we found positive linear or non-linear relationships between tiger density and carbon stock in soil or vegetation, suggesting that large carnivores follow similar relationships with carbon stocks as large herbivores (Berzaghi et al. 2019; Holdo et al. 2009). Based on observed tiger densities (Table S1) and densities at which tiger-carbon thresholds occur in these habitat types (Figure 3), we assume that our models can predict carbon densities for most baseline and conservation targets in four major forest habitat types, including for “maximum recovery” scenarios (Harihar et al., unpublished data).
Regarding top-down control, at least in disturbed forests, our analyses suggest that tigers influence carbon stocks where AGBC/BGBC/ungulate biomass is relatively low but not high. Hence, we conclude that tigers have stronger top-down effects where forests are less well established, as found for lower trophic levels in disturbed versus undisturbed tropical forests (Granados et al. 2017), in restoration versus relatively undegraded terrestrial and aquatic systems (Xu et al. 2023), and as predicted where trophic pyramids are inverted (McCauley et al. 2018). In forests with higher net primary productivity, species interactions are also known to become more evenly distributed (She et al. 2025). Thus, in our results, it also follows that (1) ungulate biomass effects on AGBC/BGBC were more pronounced in forests with low AGBC/BGBC/ungulate biomass, (2) disaggregated data show stronger top-down dominance in subtropical/tropical moist lowland forests than in other forest habitat types, which may be more prone to degradation (Gray et al. 2023), (3) in subtropical/tropical moist lowland forests, top-down effects of tigers on carbon stock diminished at the highest tiger densities, (4) the tiger density-ungulate biomass relationship in relatively intact subtropical/tropical montane forests was unclear (but this could be an artefact of pooling Sumatra and non-Sumatra data), and (5) where tigers occurred at conservation target densities, most forest area was “carbon neutral,” i.e., lower (temporal) variability.
In three of four forest habitat types studied, we do, however, suppose that tiger-carbon relationships are bottom-up dominant, at least at the scale studied (summarized in Figure 5). The positive tiger-prey relationship in subtropical/tropical dry forests follows earlier models (Karanth et al. 2004), and it is also unsurprising to find higher ungulate biomass in the high AGBC/BGBC forests or the positive relationship between AGBC/carbon removals and tiger density in temperate forests, given previous studies (Jiang et al. 2017; She et al. 2023). That said, even within these three forest habitat types, we might still expect to find evidence of the top-down effects of tigers on carbon stocks at finer spatial scales, resulting from spatially heterogeneous predation risk (Atwood et al. 2018; Monk et al. 2024).
3.3 Context Dependency and Research Gaps
First, forest habitat type is an important determinant of carbon stock variation, tiger-carbon relationships, and the relative importance of abiotic variables. Interpretation in this light is advised. Understanding non-forests, mosaic landscapes, and habitat fragments not studied in our analysis is also recommendable. Second, carbon stocks in relatively intact forests were above our estimates derived from disturbed forest data; so, quantifying tiger-carbon relationships in relatively intact forests, even if bottom-up dominant, is an important supplement to this work (Brodie et al. 2024). Third, although animal-carbon peaks likely exist for individual animal species, as the intermediate disturbance hypothesis predicts (Connell 1978), we expect that extremely high densities of large carnivores are unlikely to deplete vegetation carbon stocks in the way that large herbivores can (Berzaghi et al. 2019) because of different feeding modes and top-down regulation of lower trophic levels (She et al. 2023). Fourth, our sensitivity analysis confirms that conclusions are robust to variations in methodological choices, including tiger range base data.
Robust long-term monitoring initiatives provide opportunities to answer important research questions about animal-carbon relationships and underlying ecological mechanisms, including breakpoints in top-down/bottom-up controls, herbivore release effects, and temporal dynamics of predator, prey, and forest carbon stock/flux changes. Field-based forest measurements are also needed to validate conclusions drawn from Earth observation data and integrate density-dependent animal effects on plant recruitment, mortality, and growth into analyses to improve carbon models (Capellesso et al. 2020; Rizzuto et al. 2024). National tiger surveys, already initiated in most tiger range countries, periodic site-level population estimates, and national forest inventories could provide the data needed for such analyses. In view of restoring intact large mammal assemblages, we note that some 400,000 km2 of forests globally can be fully restored by the reintroduction of tigers only (Vynne et al. 2022), which, given all due considerations and preparations (IUCN SSC 2013), could validate top-down effects in more intact ecosystems and improve counterfactual analyses. Additionally, studying the disease-mediated impact of African Swine Fever within the tiger range in around 2020 on forest carbon dynamics (Luskin et al. 2023) would complement analyses of rinderpest disease effects on carbon in African savanna (Holdo et al. 2009) and existing defaunation-carbon research (Brodie et al. 2024). Integrating forest degradation- and soil-related emissions into future analyses is also encouraged (Lapola et al. 2023).
3.4 Conservation and Management Implications and Recommendations
First and foremost, reducing deforestation and degradation in subtropical/tropical forests is the immediate, high-impact action to derive short-term gains (UNEP and IUCN 2021) in line with the UN COP26 Glasgow Leaders Declaration on Forests and Land Use to halt deforestation by 2030, including turning net carbon sources into net carbon sinks. Prioritizing the largest, most vulnerable, most biodiverse carbon stocks that indigenous peoples and local communities steward is logical (Hansen et al. 2016; Jung et al. 2021; Noon et al. 2022; Zhu et al. 2021), and giving higher protection to forests occupied by tigers probably means protecting higher vegetation carbon stocks. Legal status, geographic remoteness, presence of indigenous peoples, and preservation-oriented income (i.e., ecosystem service payments) are important factors determining forest protection success (Busch and Ferretti-Gallon 2017); Indonesia's recent (2016/2017–2022) progress offers inspiration (Forest Declaration Assessment Partners 2022). However, selective protection of such areas must not come at the expense of degradation and loss elsewhere. Instead, forests in the poorest conditions should be valued for their high carbon sequestration and/or wildlife recovery potential, which is particularly important for carbon drawdown. Consequently, replicating the protection and management conditions of tiger-present forests in tiger-absent forests should be expedited to secure these areas early, recalling that international targets to halve global emissions by 2030 roughly equate to a 7% reduction per year, a similar order of magnitude as our “what if” “avoided emissions” counterfactual results (~10%).
Functionally, if deforestation and degradation are not controlled in subtropical/tropical Asia, we expect a major reshaping of species occupancy as forest specialists and savanna-dwelling mammals shift distributions, as analyses in South America (Armstrong McKay et al. 2022; Sales et al. 2020) and our results suggest (Figure S6). Also, forest loss and degradation, including fragmentation, diminish forests' ability to recycle moisture, reducing rainfall, increasing air temperature (Lawrence et al. 2022), and changing forests into savanna; there are also considerable CO2 emissions as trees die from this “savannization” process. Hence, there are additional benefits of acting on forest loss and degradation for managing wildlife populations and their distributions and local climate regulation services.
Animal-mediated effects on carbon stocks are a growing interest in land management research and practice, and we identify circumstances under which top-down control by tigers is probably operating. Managing these effects to derive certain benefits, such as carbon accumulation, must understand that manipulating animal populations, whether herbivores or predators, has variable outcomes, as modelled by non-linear relationships, and, independently, presence versus absence comparisons. While animal presence is likely to align with higher vegetation carbon stocks than animal absence, carbon stocks could either increase or decrease with population density changes, or not change at all, depending on the current animal and carbon densities, wildlife community composition, habitat type, and other factors. Following the intermediate disturbance hypothesis, it would seem theoretically possible to manage wildlife populations to increase carbon stocks in degraded forests, such as in wildlife corridors and areas outside of core conservation areas, helping achieve the Bonn Challenge (IUCN Bonn Challenge 2020) and other locally relevant objectives. Inside core conservation areas, top-down effects of single species probably diminish as forests establish, so bottom-up restoration is likely the more logical approach (Jiang et al. 2017); our tiger-carbon models provide managers with answers needed to optimize habitat suitability for tiger recovery. Beyond improving habitat suitability, recovering tigers also typically requires ending illegal poaching and snaring (Belecky and Gray 2020; Harrison et al. 2016; Tilker et al. 2019), increasing landscape contiguity, size, connectivity, and permeability (Ministry of Environment, F. A. C. C., Government of India 2017; Wang et al. 2023), managing human-wildlife conflict (Figel et al. 2023; IUCN 2023), and upholding high levels of political and institutional support for conservation (Gray et al. 2023; Maheshwari and Maheshwari 2021).
Within the tiger restoration landscapes/sites predicted (Table S5), carbon stocks in vegetation or soil were generally similar or higher at target tiger densities compared with baseline, suggesting some alignment of biodiversity and carbon interests. However, predicted carbon stocks were well below climate potentials. Some of this carbon gap could be explained by underestimating underlying remotely-sensed carbon data; however, minimizing human disturbance and reversing biodiversity loss are still likely to increase carbon stocks and reduce this gap (Weiskopf et al. 2024). Finally, our data and others in temperate forests in Northeast Asia identified positive links between wildlife recovery and forest carbon cycling and growth (Jiang et al. 2017; She et al. 2024), but our data in subtropical/tropical dry forests suggest that target tiger densities may be associated with carbon neutrality. It is, therefore, imperative to pursue research in this direction that more closely examines relationships between wildlife recovery and net carbon flux, acknowledging the significance of diminishing carbon sequestration rates, such as forest development, for carbon drawdown.
4 Conclusions
This work confirms five main new findings. First, forests with tigers living within them store more vegetation carbon per hectare than forests without tigers, near-consistently across forest types, even where precipitation, tree diversity, and/or temperature are similar. Also, tiger presence seems to have a “guardian” effect, lessening global CO2 emissions from deforestation and increasing CO2 removals, but this is highly variable among forest types. Second, tiger density increases can have significant top-down effects on carbon stocks in disturbed forests, particularly where AGBC/BGBC/ungulate biomass is relatively low. The direction of effects is determined by baseline tiger densities and wildlife community composition, including ungulate biomass density. Third, carbon stocks in vegetation and soils are closely linked with tiger densities and are higher where deforestation is less. In particular, tiger density-carbon stock relationships were positive in two forest habitat types, thresholds found in two other forest habitat types, and can be distinguished as either top-down or bottom-up dominant based on tiger density-ungulate biomass relationships. Fourth, tiger-carbon relationships can be confounded by interactions with other wildlife species' abundance/diversity. Lastly, there is some indication that net forest carbon flux is linked with tiger density, but results vary between habitat types. This area of research, in particular, needs further study because of the implications for carbon drawdown.
Protecting and restoring forest ecosystems and the wild animal populations living within them, more than “empty forests”, is an actionable and investable solution to climate change. It is currently available, scalable, quantifiable, and rooted in basic animal and plant ecology with a straightforward narrative and large co-benefits (Berzaghi et al. 2023; IPCC 2021; Schmitz et al. 2014). However, we are reminded that carbon is only part of the climate change mitigation story, and assessing additional relationships between biodiversity, forest condition, and the global climate—such as albedo effect, global cooling, nutrient availability, permanence, and landscape heterogeneity (Brienen et al. 2020; Ellison et al. 2024; Fernández-Martínez et al. 2014; Lawrence et al. 2022; Malhi et al. 2022; Trepel et al. 2024)—will contribute to a more holistic understanding of Earth systems and refining climate actions that maximize benefits to local and global society. This includes when atmospheric CO2 concentrations drop after surpassing the carbon neutrality checkpoint mid-century.
5 Materials and Methods
5.1 Data Inputs and Pre-Processing
5.1.1 Tiger Distribution, Density, and Area of Habitat (AOH)
We first determined the tiger's area of habitat (AOH) (Brooks et al. 2019) for the tiger present, absent, and presence uncertain range. Recognizing that results could potentially be influenced by the base species range data used, two data sources were used in parallel, the IUCN Red List Assessment and the Tiger Conservation Landscape (TCL) data sets; finally, TCL data were used (see Section 5.4 below). Tiger density estimates were drawn from published literature that used comparable research methods that were temporally consistent with the carbon data sets and had minimal temporal variability among tiger density estimates (Figure 1, Table S1). The corresponding studied landscapes were manually digitized in ArcGIS, from which the AOH was clipped and dominant forest habitat type determined, retaining only landscapes with > 75% of their AOH belonging to a single forest type and AOH > 133.3 km2 (see Supporting Information Methods S1). “Tiger-absent” (tiger density = 0) landscapes were supplemented to the data set as non-detections of tigers within the species range. Additionally, tiger “recovery sites” (Harihar et al. 2018) and habitat- and landscape-specific conservation targets (Harihar et al. 2018; Qi et al. 2021; Wikramanayake et al. 2011) provided the basis for conservation scenario predictions. Refer to Supporting Information Methods S1 for further details.
5.1.2 Carbon Data
At the time of writing, the most up-to-date, accurate, highly regarded, and fit-for-purpose global carbon data sets were from 2010 for carbon stocks (Hengl and Wheeler 2018; Spawn and Gibbs 2020; Spawn et al. 2020) and 2001–2021 for carbon flux (Harris et al. 2021). The Spawn et al. data set (Spawn and Gibbs 2020; Spawn et al. 2020) was selected for AGBC and BGBC because it is more comprehensive than other available data (e.g., Santoro 2018), including woody and herbaceous vegetation. These data, which integrate remote sensing and machine learning, have been shown to correlate highly with independent field-based forest inventory data at the state level across the conterminous USA (Law et al. 2023) and here in our validation, with field measurements in Asia (Donato et al. 2011; Slik et al. 2013; r = 0.31, p = 0.01, n = 67; Figure S8), although higher values in the dataset are assumed to be underestimated, as for an alternative global carbon dataset (Rozendaal et al. 2017; Santoro 2018). SOC data were downloaded as individual depth layers (Hengl and Wheeler 2018) and mosaicked in ArcGIS to represent soil depth 0–100 cm. Global potential carbon data sets (Walker, Gorelik, Baccini, et al. 2022; Walker, Gorelik, Cook-Patton, et al. 2022) were used to verify that predicted carbon storage under different conservation scenarios was within physical bounds in a baseline climate and future climate using GCM noresm1-m (NO) for RCP8.5 by 2050; potential SOC density under future climate was not available, and the baseline climate is for 0–200 cm soil depth. Forest carbon emissions, removals, and flux were downloaded as measured values per pixel, not per hectare.
5.1.3 Wild Ungulate Biomass Density and Wildlife Diversity Data
Using published AOHs (Lumbierres et al. 2022a, 2022b) and corresponding global biomass estimates (Greenspoon et al. 2023; Greenspoon and Noor 2023), wild ungulate biomass density (kg/km2) was approximated as a newly mosaicked global spatial data set representing 243 ungulate species. Similarly, spatial data sets of species richness were derived for ungulates (n = 243), combined ungulates and lagomorphs (n = 335), and carnivores (n = 251). See Supporting Information Methods S1 for further details.
5.1.4 Environmental Data
From the WorldClim bioclimatic variables (Fick and Hijmans 2017), only mean annual temperature (MAT) and mean annual precipitation (MAP) were used in our analyses due to high correlations among mean, maximum, minimum, and so forth. Elevation, normalized difference vegetation index (NDVI), evapotranspiration, tree species richness (Liang et al. 2022), and net primary productivity were among other environmental factors considered in this paper (Table S7), as well as latitude (or distance from the equator), taken as the y coordinate of the centroid of each landscape. A second habitat classification system (International Geosphere-Biosphere Programme, IGBP) was used to investigate finer-scale habitat relationships with carbon dynamics and tiger densities, calculated as the proportion of the landscape belonging to the respective habitat type. Because human activities were assumed to impact carbon stocks and flux (Pyles et al. 2022), the proportion of the landscape that was part of the intact forest landscapes (IFL) in 2013 (Potapov et al. 2017) was also included in analyses; this year was closest to that of the carbon and tiger data in this paper. These proportionate values were determined by the ‘Tabulate Area’ function and/or pixel counts in ArcGIS. FLII was also included to evaluate disturbance effects, representing observed and inferred human modification of ecosystem structure, composition, and function (Grantham et al. 2020).
5.1.5 Data Extraction and Tabulation
All spatial data were brought into a standard projection (WGS 1984 World Mercator) and resampled to pixels measuring 1 km2 for consistency. To extract summary statistics, such as sum, mean, standard deviation, and pixel count of AGBC, BGBC, and SOC, forest carbon emissions, and so forth, the ‘Zonal Statistics as Table’ tool was used in ArcGIS, rescaling where necessary: AGBC, BGBC, and SOC were rescaled to MgC/ha and the FLII mean was divided by 1000 because of the scaling factor of the original dataset. Mean forest carbon removals, emissions, and net flux in a landscape were calculated as the sum of all pixels divided by the size of the AOH and then divided by 21 to derive average annual values, following the data author's guidance. The size of AOH was determined from the pixel count. In the Zonal Statistics as Table operation, the input zone data was either a raster layer of habitat types within a range (tiger presence, absence, and presence uncertain) or a table of digitized landscapes; specifically, the AOH masks (specifically, forest only) were the inputs.
5.2 Data Subsets
Because of probable context dependency, we performed habitat-specific analyses of tiger density-carbon relationships as recommended (Thapa et al. 2023). Additionally, Northeast Asia and Sumatra were analyzed separately from the remainder of the same habitat type by masking the AOH layer to respective regional polygons because of distinct climatological differences between temperate forests in Northeast Asia and elsewhere, and ecological differences between subtropical/tropical moist montane forests in Sumatra and elsewhere, not least of all for the fact that Sumatra is outside of the range of the sympatric leopard (Panthera pardus); in our case specifically, the tiger density data in Sumatra were also calculated differently to those outside of Sumatra, further justifying the distinction. Beyond forest habitat type and global/regional subsets, we further divided the data into a total of 13 subsets, specifically (1) whether analyses included tiger-absent landscapes or used tiger-present landscapes only; (2) whether analyses included landscapes with all levels of disturbance, relatively intact landscapes only, or disturbed landscapes only, and; (3) whether analyses filtered data based on an AOH ≥ 250 km2 or ≥ 133.3 km2; 250 km2 and 133.3 km2 represent the 75th and 90th percentile of the minimum area required for five non-overlapping female home ranges in subtropical/tropical forests (Sanderson et al. 2023). We analyzed all possible subsets, reporting results together with corresponding subset details. Finally, references to “disturbed forests” mean forests in which zero pixels within the landscape/range overlapped with the IFL layer; conversely, “relatively intact forests” overlapped with IFL. When extracting carbon data from disturbed forests in global prediction analyses (Table S4), habitat that overlapped the IFL layer was first removed from the raster data set using the ‘Erase’ tool in ArcGIS.
5.3 Statistical Analysis
5.3.1 Comparing Tiger-Present and Tiger-Absent Forests
To compare carbon stocks, bioclimatic variables, and tree species richness in the tiger-present and tiger-absent forests, pixel values in these two groups were first extracted using the “Sample” tool in ArcGIS and then analyzed using Cliff's Delta (δ) with 95% confidence intervals calculated by bootstrapping with 1000 resamples. Counterfactuals were calculated as the emissions/removals across the AOH of the first state (i.e., tigers present, MgCO2e) minus the rate of emissions/removals in the second state (i.e., tigers absent, MgCO2e/km2) multiplied by the AOH of the first state (km2) and then divided by 21 to give values per year and rescaled to GtCO2e/year; the reverse was calculated for a “what if” scenario if tiger-absent forests still had free-living wild tigers, calculated by subtracting the emissions/removals in the second state (i.e., tigers absent, MgCO2e) from the rate of emissions/removals in the first state (i.e., tigers present, MgCO2e/km2) multiplied by the AOH of the second state (km2).
5.3.2 Top-Down Effects of Tiger Density on Carbon Stock
Our analysis framework is adapted from Tobias-Hünefeldt et al. (2021). First, silhouette analysis determined clustering within the data, using the ‘factoextra’ package (Kassambara and Mundt 2020), accepting silhouette width > 0.4. Second, anosim and adonis tests, using ‘vegan’ package (Oksanen et al. 2020), analysed drivers of carbon variation in the Euclidean distance matrix; continuous variables were scaled by forest habitat type, and adonis tests were run with 9999 permutations. Third, wildlife diversity/abundance comparisons between the two carbon clusters were performed using exact permutation tests from the ‘coin’ package (Hothorn et al. 2006, 2008). Fourth, tiger density-ungulate biomass and ungulate biomass-carbon stock relationships were tested by generalized additive models (GAMs), validated with leave-one-out cross-validation. Tiger density-ungulate biomass relationships were also verified by random forest analysis with 500 trees run in triplicate using the ‘randomForest’ package (Liaw and Wiener 2002), reported as mean ± SD percentage variance explained by the model.
5.3.3 Direct Tiger-Carbon Relationships and Predictions
Tiger density-carbon stock/flux relationships were analyzed by linear regression and non-linear polynomial models. Where the number of data points was sufficient, polynomial models were evaluated with k-fold cross-validation (k = 10), and the best model was fitted after inspecting the (lowest) mean square error (MSE) of each degree of polynomial fit. We tested tiger-carbon relationships for AGBC density, SOC density, carbon removals, carbon emissions, and net flux for each data subset described above, including presence-only and disturbed forests. As described above, tiger-carbon relationships were verified by random forest analysis with 500 trees. Predictions for different conservation scenarios were computed according to the best models with the ‘predict’ function in R, specifying the target tiger density and a logical argument to return confidence intervals. Similar methods were followed to test the sensitivity of models to human disturbance. AGBC and SOC density was modeled by multivariate generalized linear modeling (GLM), first using the ‘fuzzySim’ package to check for collinearity between covariates (Table S8) and to select the variables to include in the global model based on a correlation threshold of 0.7 and VIF < 10. The ‘dredge’ function was used to find the best model with the lowest AICc. Model quality was determined by inspecting QQ and residual plots, Cook's Distance, variance explained by the model, and R2 and k-fold cross-validated R2 (k = 10, or lower where data points were fewer). Variables were standardized before GLM analysis. Pearson's correlation computed correlations between environmental variables and tiger density, assuming statistical significance at p < 0.05.
5.3.4 Tiger Density and Carbon Neutrality
For the net carbon source/sink/neutral analyses, the ‘IsNull’ and ‘Less Than’ functions in ArcGIS were used to determine the proportion of “neutral” pixels (the number of NoData cells divided by the total number of cells in the flux layer), net sink (the number of cells with flux less than zero divided by the number of cells in the flux layer), and net source (the remaining portion); values were reported as percentages.
5.3.5 Indirect Tiger-Carbon Relationships
Where we could not find a direct link between tiger density and carbon stock, we tested both linear and nonlinear interactions between tiger density and six diversity/abundance metrics as predictors of AGBC or SOC, namely, ungulate biomass, ungulate species richness, combined ungulate and lagomorph species richness, carnivore species richness, biodiversity richness intactness, and biodiversity abundance intactness, as well as ungulate biomass per tiger. Linear interaction was modeled with linear regression models containing only the interaction term, and nonlinear interaction was modeled with GAMs with tensor product smooth terms. Model validations were performed with k-fold cross validation for linear interaction or leave-one-out cross validation for nonlinear interaction.
5.4 Sensitivity Analysis
The core analyses in this paper were repeated with variations in the underlying data, testing the sensitivity of the results to methodological choices, principally (1) whether IUCN range data or Tiger Conservation Landscape (TCL) data were used to determine the AOH used in all subsequent analyses; (2) whether tiger landscapes were digitized as the SCR state space or effective trapping area or as the minimum convex polygons (MCPs) of camera trap arrays; (3) whether a data filtering threshold of 80% or 75% was used to determine that a sufficient proportion of the landscape was of the habitat type being evaluated; (4) whether only tiger density estimates derived from spatially-explicit capture-recapture (SCR) likelihood methods were selected or data filtering included both SCR likelihood and SCR Bayesian methods; (5) whether TCL restoration landscapes were used as the “tiger density = 0” data or original publications of non-detections; and (6) whether best models were selected according to step-wise regression and AIC values or AIC adjusted for small sample sizes (AICc). Broadly, the conclusions were very similar, and we report results based on the second selection in each case above because the TCL data are more up-to-date than the IUCN data and allow year-specific AOHs to be determined. In Sumatra, there were no TCL restoration landscapes, so tiger-present versus tiger-absent comparison was not valid. So, we attach the results based on IUCN data of extant and extinct ranges to figures and tables where appropriate for better comparison and interpretation. The more lenient filtering process meant that a higher number of landscapes/tiger densities could be included in analyses, strengthening statistical power.
All statistical analyses were performed in R Version 4.1.2 or 4.4.1. Data visualization was aided by ‘psych’ (Revelle 2023), ‘ggplot2’ (Wickham 2016), and ‘ggpubr’ (Kassambara 2020) packages. See Figure 6 for summarized methods.

Author Contributions
Nathan James Roberts: conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing – original draft. Abishek Harihar: data curation, investigation, validation, writing – review and editing. Xuhui Zhou: methodology, validation, visualization, writing – review and editing. Wen She: validation, visualization, writing – review and editing. Guangshun Jiang: conceptualization, funding acquisition, investigation, methodology, project administration, supervision, validation, visualization, writing – review and editing.
Acknowledgements
This research was funded by the National Key R&D Program of China (2023YFF1305000) and Fundamental Research Funds for the Central Universities (2572022DS04). N.J.R. was supported by a Chinese government scholarship. We sincerely thank Tom Gray (WWF Tigers Alive Initiative) for feedback, direction, and discussion, which strengthened the alignment and interpretation of the work in this manuscript, and we thank colleagues at Northeast Forestry University, especially Jinzhe Qi, Jiayin Gu, Heng Bao, Joka Dengata Lemu, and Tika Ram Poudel for routine comments, assistance, and advice concerning methodology, data, and analysis. Large data processes were supported by the College of Computer and Control Engineering, Northeast Forestry University. Graphical abstract artwork was prepared by Dakota Harr. We also thank Anand M. Osuri from the Nature Conservation Foundation for reviewing the manuscript. Finally, thank you to all those who prepared and shared the data analyzed in this study, and the anonymous reviewers who challenged us to draw more from the data and guided manuscript improvements.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data and code that support the findings in this study are openly available in Dryad at https://doi.org/10.5061/dryad.cjsxksnhj. The secondary data sources used in this study can be found in Tables S1 and S7 of the Supporting Information files.




