Unravelling Changes in the Pinus radiata Root and Soil Microbiomes as a Function of Aridity
Funding: This study was supported by programme C04X2002 ‘The Tree Microbiome Project: at the root of climate proofing forests’, supported by the New Zealand Ministry of Business, Innovation and Employment (MBIE) and the Forest Growers Levy Trust.
ABSTRACT
Increased aridity is emerging as a key impact of climate change in terrestrial ecosystems globally. Forest biomes are particularly vulnerable to the impacts of changing environmental conditions due to their long-lived and sessile nature. Microbiomes have coevolved with plants under changing environmental conditions with shared fitness outcomes. However, both the movement of plants via domestication and rapid pace of environmental change may impact the ability of plants to recruit microbial symbionts that support environmental stress tolerance. This study investigates the effects of aridity on tree-root microbiome symbiosis, focusing on the widely planted Pinus radiata. By sampling a broad geographic range and diverse environmental gradients, we reveal how aridity, soil and climatic variables shape microbial communities in P. radiata roots and soils. Our findings highlight that while aridity significantly predicts microbial community assembly, other environmental variables such as soil pH and organic carbon, strongly influence bacterial diversity. Groups of both bacterial and fungal taxa were identified as conditionally present with aridity, underscoring their importance in P. radiata resilience under increasingly environmental stress. Based on the transition of current mesic ecosystems to arid conditions under climate change, we found these arid associated taxa vary in their frequency in bulk soils projected to become arid. These results highlight the risk that these taxa will need to be recruited by other means. Ecological filtering by the host and environmental conditions fosters a “friends with benefits” relationship, wherein certain microbial taxa provide key benefits, such as extension of phenotypic tolerance to water limitation, to the host. Both bacterial and fungal communities are shaped more by stochastic than deterministic assembly processes, suggesting a complex interplay of host and environmental factors in community structure formation. The insights gained have implications for understanding the resilience of tree species and the ecosystem services they provide under future climate scenarios.
1 Introduction
Increasing aridity causes significant water stress in trees and forests. As a bioclimatic metric, aridity integrates hydro-climatological and ecological variables, extending beyond drought, which focuses on short-term deviations from average precipitation (Zomer et al. 2022). Indeed, the Aridity Index (AI), calculated as the ratio of annual precipitation to potential evapotranspiration, is now widely used in studies examining the impacts of climate on species distribution, turnover, and productivity (Maestre et al. 2015; Delgado-Baquerizo et al. 2020; Hu et al. 2021; Berdugo et al. 2022; Shi et al. 2024). By the end of the century, rising aridity could transform one-fifth of all land, shifting ecosystems and causing widespread extinctions of plants, animals, and other life forms (Vicente-Serrano et al. 2024). As ecosystems increasingly face chronic aridity alongside periodic droughts, the AI is being adopted in global ecological and biogeographical models to assess climate impacts on species and ecosystem function (Dai 2013). However, the impacts of aridity on tree microbiomes remain largely unknown.
The tree microbiome, comprising the bacteria, fungi and a myriad of other microbial life existing on and in the tree tissues, can alter tree fitness and enable adaptation to changing environments (Addison et al. 2024). This is expected as a natural outcome of millennia of coevolutionary selection between trees and microorganisms (Morris 2018; Rosenberg and Zilber-Rosenberg 2018), where the fitness outcomes of the tree and its microbiome are shared (Rúa and Hoeksema 2024). Given that microbes evolve on time scales much quicker than their host plants, it is proposed that this coevolutionary selection allows the microorganisms to offer the plant physiological adaptations. These adaptations can act as an extension to the plant, allowing them rapid responses to environmental changes, especially those associated with ecosystem dryness (Trivedi et al. 2022), but empirical evidence remains inconclusive, and the rate of such adaptation is uncertain.
Tree and microbial associations have evolved together under conditions of aridity. These selective pressures potentially result in co-evolution of symbiosis that support aridity tolerance. However, the magnitude and mechanisms related to host-microbiome symbiosis under aridity-related stress remain unknown. A key gap in knowledge is whether plant microbiome assembly is influenced by increasing aridity and, if so, the direction and magnitude of this response. Such knowledge is an essential step towards advancing fundamental understanding of co-evolution and natural adaptation, and to developing future solutions for tree productivity as the intensity of ecosystem dryness (aridity) is expected to increase with climate change.
Ecological filtering encompasses both host and environmental filtering influencing microbiome selection (Aguilar-Trigueros et al. 2017; Cadotte and Tucker 2017). For plant root microbiomes, ecological filtering is expressed against the reservoir of microbial community present in the wider (bulk) soils. Importantly, these communities have been shaped through the expression of edaphic and climatic factors (e.g., Fierer et al. 2009; Serna-Chavez et al. 2013; Tedersoo et al. 2014). Furthermore, stronger ecological filtering of microbiomes from the soil to the root tends to reduce community α-diversity, with a dominance of taxa recruited through filtering (Ling et al. 2022). In some instances, geographical or dispersal limitation may influence the occurrence of some components of the microbiome (Bissett et al. 2010). However, there is a lack of empirical evidence for this.
There is substantial scientific evidence indicating that increasing aridity negatively affects soil microbial diversity and function (Delgado-Baquerizo et al. 2020; Lei et al. 2024; Ochoa-Hueso et al. 2024), yet there is limited information on how aridity impacts the root microbiomes of tree species. Future predictions show that land will be exposed to an increase in aridity of more than 20% by 2100 and is expected to have negative impacts on multiple ecosystem functions (Berdugo et al. 2020). This represents a critical knowledge gap, as tree microbiomes are understood to play a vital role in alleviating the effects of abiotic stresses and helping trees adapt to climate change. Understanding how aridity influences the microbiome assembly in plant root systems is crucial for opening opportunities to enhance the resilience of tree holobionts and support ecosystem stability under changing climatic conditions (Cheng et al. 2019; Trivedi et al. 2022; Singh et al. 2023).
In this study, we assessed the importance of climate and soil-related host-selective pressures for shaping P. radiata root-microbiome communities, focusing on the influence of aridity. We capitalize on the wide geographical extent of P. radiata (Monterey Pine) plantings across New Zealand and Australia, which span climates ranging from relatively mild to strongly arid. Furthermore, these populations originate from similar, genetically domesticated parent material (Dungey et al. 2009) helping limit intraspecies variation. We hypothesize that with increased aridity, ecological filtering by the host will be stronger, evident in more intense selection of bacterial and fungal root microbiomes from the bulk soils. Specifically, we predict that the proportion of deterministic to stochastic processes associated with root microbiome assembly will increase with aridity due to stronger host and environmental selection. We then aim to identify aridity-associated taxa conditionally enriched under arid conditions, hypothesizing that low-abundance members of today's mesic soil ecosystems may increase under climate change. Using functional microbial predictions, we hypothesize that metabolic pathways and traits of the microbial community will alter with increasing aridity, with increases in stress tolerance traits and functions becoming more pronounced. Finally, we used climate models to assess the presence of conditionally rare species associated with aridity required for future root microbiome associations to inform the risk P. radiata has for root-microbiome mismatches that may impact the microbiome potential functioning in the future.
2 Materials and Methods
2.1 Sites, Soils, and Climatic Data
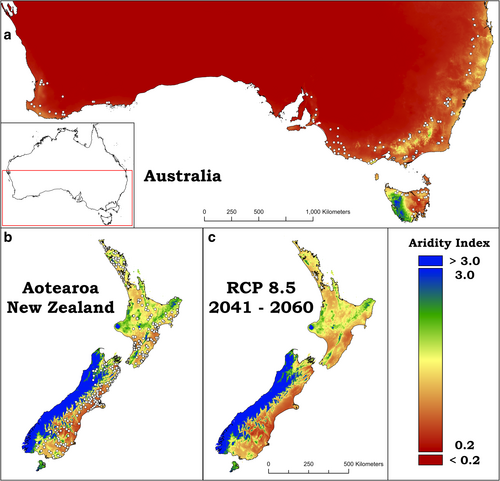
Samples of P. radiata bulk soil and root material were collected from 517 locations across New Zealand and Australia (Figure 1; with finer resolution in Figures S1 and S2). Sampling occurred across a latitudinal range spanning approximately 2600 km and a longitudinal distance of over 5600 km. This expansive geographic range provided a natural gradient in current aridity conditions (Figure 1a,b). Aridity spanned extremely dry (AI = 0.307; near Perth, Western Australia) through to wet (AI = 2.38; on the west coast of South Island, New Zealand). Naturally, the expansive sampling also covered a diverse range of soil types, fertility levels, and other environmental properties. These data are given in Data S1.
Soil from each location was characterised in physicochemical properties using Fourier-transformed, diffuse reflectance, mid-infrared spectroscopy (FT-IR). Predictions were made against the National Soil Survey Center–Kellogg Soil Survey Laboratory as described in (Sanderman et al. 2020). For total sulphur (Total_S) and available phosphate (Av_P), where the predictivity ability of FT-IR is low, empirical data was directly measured; Total_S was measured after microwave digestion of soil with nitric acid followed by ICP-AES, and Av_P was Mehlich 3 extracted and quantified using ICP-OES spectroscopy. Climate data were extracted at each sample location from the WorldClim 2.1 database (Fick and Hijmans 2017). Aridity values were then determined from the Global Aridity Index (AI) and Potential Evapotranspiration Database (V3; Zomer et al. 2022). Although aridity is a complex concept relating to multiple hydrological processes, it can be approximated as the ratio of mean annual precipitation (MAP) to mean annual evapotranspiration (ET0). Links to GitHub for the R code used to collect WorldClim data from the GPS locations are provided in the Data S1.
Bioclimatic and chemical variables were tested for multicollinearity and variable reduction was undertaken. The final variables used for analysis were as follows: available phosphorus (Av_P), total sulfur (Total_S), organic carbon (Organic_C), pH, Sand content %, Clay content %, exchangeable calcium (Exch_Ca), exchangeable magnesium (Exch_Mg), exchangeable sodium (Exch_Na), C/N ratio, aridity index (AridityIndex), mean annual temperature (MeanTemp), seasonal temperature (TempSeason), seasonal precipitation (PrecipSeason) (Final variables in Dataset https://doi.org/10.5061/dryad.6q573n688) (Addison et al. 2025).
2.2 Soil and Root Collection
Soil and root sampling methodology followed a previously established approach that captures tree-level root microbiome variation (Addison et al. 2023). The generalised protocol is summarised in Figure S3. Briefly, soil samples were collected at 1 m distance out from the base of the tree trunk, extending radially outwards towards the canopy edge. Soils were sampled using a 15 × 15 cm ‘spade square’ collection to a depth of 10 cm. Only mineral soil was collected; organic horizon material was removed. Samples were collected from each cardinal point around the tree (north, south, east and west) then bulked to produce a single sample per tree containing both bulk soil and roots. Samples were homogenised then the roots separated from the bulk soil (Addison et al. 2023).
We chose P. radiata as a model species for this study because it has a relatively limited endemic range comprising the central coastal region of California, USA and offshore islands of Baja California, Mexico (Burdon et al. 2017). Yet P. radiata has a broad Hutchinsonian niche space, thriving in conditions with annual rainfall ranging from 350 to 2500 mm and temperatures between 8°C and 17°C (Gallart et al. 2018a, 2018b). This wide niche occupancy characteristic has enabled domestication of P. radiata as a commercial soft wood tree species and now has a near global extended range (Lavery and Mead 1998). Importantly, the species is interacting with a diverse range of microbiomes across a very broad range of ecological settings. Consequently, this species provides an excellent, field-relevant and globally expansive model system in which processes underpinning the assembly of root microbiomes can be evaluated.
2.3 Microbial Community Analyses
All soil and root extractions were conducted in quadruplicates. The bulk soil was sieved to < 2 mm and ~0.25 g was placed into Qiagen Powerbead tubes for DNA extraction following the manufacturer's protocols (DNeasy PowerSoil Pro Kit, Qiagen, Hilden, Germany). Root fragments were washed in 30 mL sterile 0.1 M CaCl2 solution for 1 h, cut into ~2 mm portions, and ~0.25 g samples were placed into PowerBead tubes for DNA extraction. The four separate extractions were then combined to create a single soil and root DNA sample for each tree (Addison et al. 2023). DNA was then quantified using the Quant-iT PicoGreen dsDNA (ThermoFisher, USA).
Sequencing of microbial communities followed methods established by the Earth Microbiome Programme (Thompson, et al. 2017). In brief, bacterial community amplicon libraries were prepared using primers 515F and 806R to provide coverage of the 16S rRNA V4–V5 hypervariable region (Parada et al. 2016). The fungal community was characterised via variation in the ITS1 gene region, amplified using primers ITS1f (Ihrmark et al. 2012) and ITS2 (White et al. 1990). PCR chemistry and conditions are described in full elsewhere (Addison et al. 2023). For all PCRs, primers included Illumina sequencing adaptors and pads, along with a unique Golay 12-mer barcode (on the forward primer for bacteria, and reverse primer for fungal PCR). No-template PCR controls were included in sequencing. Barcoding enabled individual samples to be identified following multiplexed sequencing of mixed amplicon pools.
PCR products were purified and standardised to equimolar concentrations using a SequalPrep Normalisation Plate, 96 Well (Invitrogen—Life Technology). Sequencing was performed using an Illumina MiSeq system at the Australian Genome Research Facility (AGRF). Bacterial libraries were sequenced using 2 × 250 bp paired-end (PE) read chemistry, and fungal libraries using 2 × 300 bp PE chemistry.
2.4 Bioinformatic Processing of MiSeq Reads
Sequences were filtered and trimmed using the DADA2 Big Data workflow (Callahan et al. 2016) implemented within R v 4.4.1 (R Core Team 2024). Samples were demultiplexed, and paired-end reads were filtered and trimmed (maxN = 0, truncQ = 2, rm.phix = TRUE and maxEE = 2) prior to dereplication and de novo consensus chimera checking. The fastq files of negative template controls had low or no reads, none of which passed quality control; these samples were removed from the dataset (Addison et al. 2025).
Amplicon sequence variants (ASVs) were calculated and taxonomy assigned using the RDP Classifier 13.5 trainset v18 for bacteria (Wang et al. 2007) and the UNITE database v10.0 for fungi (Abarenkov et al. 2024). Further filtering removed amplicons of plant-based origin (e.g., chloroplast and mitochondria), Archaea, and ASVs unclassified at the kingdom or phylum level. Raw sequences for both 16S rRNA and ITS rRNA libraries have been placed in the NCBI sequence read archive (SRA) under the BioProject accession PRJNA1217937. ASV processed sequences, including count and taxonomy tables, are freely available at https://doi.org/10.5061/dryad.6q573n688.
Rarefaction curves were generated (vegan package in R; (Oksanen et al. 2024)) to evaluate how well sequencing depth provided coverage of ASVs present for each sample (Figures S4–S7). Henceforth, each ASV is inferred as a unique taxon (phylotype) of bacteria or fungi, and the number of sequence reads within each ASV is the measure of abundance.
2.5 Analysis
Unless indicated, all analyses were conducted with R v 4.4.1 (R Core Team 2024).
2.5.1 Relationships of Soil and Root Microbiome With Edaphic and Climatic Variables
Phyla level distributions of bacterial and fungal communities were generated to visualise relative abundance changes between bulk soil and root compartments. The α-diversity (Shannon index; H′) of each microbiome sample was then calculated, with a comparison between the bulk and root microbiomes compared using the Mann–Whitney test.
Linear modelling (lm in the stats package) was used to find the best fit of soil and climatic variables to variation in bacterial and fungal community α-diversity (H′) across the root microbiome samples. Predictor variable selection was based on the AICc criterion. Standardised coefficients were extracted from the model, and confidence intervals were calculated. From the best model, as shown by AICc, the proportional effects of the predictor variables were plotted.
2.5.2 Associations Between Aridity, Soil Properties, and Environmental Variables in Pinus radiata Soil and Root Microbiome Assemblages
ASV data were rarefied using the median sequence depth (phyloseq package, R; McMurdie and Holmes 2013) before further analysis. Rarified data were square root transformed prior to calculating similarity between samples using the Bray–Curtis similarity measure.
We tested if variation in the structure of bacterial and fungal root and bulk soil communities was related to aridity and/or other soil or environmental variables. Distance-based linear modelling (DistLM) was used to fit soil and environmental predictor variables to microbiome community structure (Bray–Curtis similarity matrices). Models were run using a stepwise variable predictor selection procedure and AICc-based selection criteria (p < 0.05). The partial and marginal effects of the model terms in explaining variance in (i.e., within the best-fit reduced model) were determined using PERMANOVA. Predictor variables were tested in the PERMANOVA in order of DistLM selection, and testing was conducted using Type 1 (sequential) sum-of-squares. DistLM and PERMANOVA testing were conducted in PERMANOVA+ (PRIMER-E Ltd) using methods described in (Anderson et al. 2008).
To identify key aridity-associated root-microbiome taxa, we used differential abundance analysis with the ancombc function in the ANCOMBC R package (Lin and Peddada 2020). Only root ASVs that exhibited statistically significant responses to AI (α = 0.05) were used in further analyses. Linear models were built for significant ASVs by bootstrapping the 95% confidence intervals (2000×) to test whether the estimates overlapped 0 for aridity. The significant ASVs identified from root samples were used to produce clustered heatmaps for both root and those same ASVs within bulk soil samples (pheatmap function, R). Log abundances in the aridity-significant ASVs, by sample root and bulk soil sample type, were plotted based on high to low AI. Significant ASVs associated with high aridity were identified and their individual log abundance responses to AI plotted (high log abundances with high aridity reducing to low log abundances with low aridity).
2.5.3 Root Microbiome Assembly Processes Defined Across an Aridity Gradient
We tested the extent to which ecological filtering of microbial communities on the root differed across ecosystems varying in aridity. To determine the relationship between the root microbiome and environmental variables, particularly AI, we used the iCAMP framework (Stegen et al. 2013; as extended by Ning et al. 2020) in the R package iCAMP (v. 1.5.12). Phylogenetic turnover, calculated by comparing observed phylogenetic distances between communities to a null model, was determined using the Beta-Nearest Taxon Index (β-NTI) statistic. This provided quantitative measures of a range of deterministic and stochastic processes associated with root microbe assembly for each sample. We then modelled the changes in these selection processes with aridity and the other predictor variables using DistLM and PERMANOVA (as before).
FUNGuild was used to investigate the functional prediction of fungal communities within bulk soil and root compartments (Nguyen et al. 2016). Nine guilds were classified according to three trophic modes: pathotrophs, saprotrophs and symbiotrophs. Guilds considered ‘probable’ and ‘highly probable’ were selected for further analysis. All other ASVs were classified as ‘unassigned’. We modelled the relationship between functional guild relative abundance changes with aridity and the other predictor variables using DistLM and PERMANOVA (as before). To predict bacterial function, the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was used with KEGG functional predictions (Langille et al. 2013). Spearman rank correlation with Benjamini-Hochberg applied was performed to assess the relationships between abundance of pathway classes and the AI.
2.5.4 Potential of Today's Bulk Soil Microbiomes to Support Future Arid-Associated Root Microbiomes
To model future aridity conditions over the sites sampled, we considered future aridity under the four ICPP representative Concentration Pathway (RCP) scenarios: 2.6, 4.5, 6.0, and 8.5. These represent a low-emission scenario (RCP 2.6), two stabilising emissions (2040 and 2080) scenarios (RCP 4.5 and 6.0), and a high emissions scenario (RCP 8.5). Future aridity indices were obtained from the CMIP6 climate model scenarios from the Australian Community Climate and Earth System Simulator (ACCESS) using the highest available resolution (30 arcseconds). Where sampling locations had no available data, such as along coastlines where the resolution of gridded datasets leads to data gaps, we assigned the values of the nearest available cell.
Taxa within groups (clusters; Figures 2 and 3) of root microbiome associations that were identified as having significantly enriched (log-fold abundance changes as described previously) in present-day arid conditions (< 0.65 AI) were identified. These comprised the bacterial ASVs across clusters 3, 4, 7, and 8, and the fungal ASVs in cluster 1. We then tested to see if these aridity-associated root microbiome ASVs were present in the local bulk soil species pool (presence/absence) of samples projected to transition to dryland areas (samples with < 0.65 AI) by 2050 under the 8.5 RCP 2050 scenario.
3 Results
3.1 Environmental and Soil Chemical Properties Influence on Microbiome Diversity and Community Structure
After bioinformatic processing, a total of 81,590 bacterial and 41,906 fungal ASVs were recovered. These were dispersed across 34 bacterial and 17 fungal phyla. Bulk soil was richer in bacteria and fungi than the comparative root microbiomes: 63,731 vs 40,734 ASVs for bacteria and 30,082 vs 17,873 ASVs for fungi. Only 21% of bacterial ASVs were shared between compartments, whereas for fungi 28.1% of ASVs were shared. A full description of the microbial communities is included in Figures S8 and S9.
Root microbiomes had lower mean α-diversity (Shannon Index H′) than bulk soil for both bacterial (p < 0.001) and fungal communities (p < 0.001; Figure S10). Variation in α-diversity of the bacterial root microbiome was best predicted by organic C (β = 0.425, p < 0.001), pH (β = 0.151, p < 0.001), MAT (β = 0.035, p = 0.004), and precipitation seasonality (PrecipSeason) (β = 0.191, p = 0.133) (Table S1). While inclusion of seasonal variation in precipitation increased the overall fit of the best reduced model (adjusted R2 = 0.131), large variation in this term reduced the statistical significance of this term (p = 0.133).
Fungal root microbiome α-diversity was most strongly predicted by declining aridity (i.e., increased AI values) (β = 0.422, p < 0.001; overall model adjusted R2 = 0.130) (Table S2). Further variation in α-diversity was evident in association with MAT (β = 0.095, p < 0.001), seasonal temperature (TempSeason) (β = 0.016, p = 0.007), and C:N ratio (β = 0.007, p < 0.001). The relationship with precipitation was inversely associated with aridity (β = −1.138, p < 0.001), as inter-seasonal variation in rainfall increased, fungal dominance also increased (α-diversity decreasing) indicating an overall lower strength of filtering processes.
Variation in both bacterial root and soil microbiome structures (β-diversity) was best described by changes in the pH of the local soil; the effect size was similar for the two compartments (bulk soil PPERM < 0.001, 8.32%; root PPERM < 0.001, 8.66%) (Table S3). Once variation in soil pH was accounted for, mean annual temperature (MAT) (bulk soil; PPERM < 0.001, 6.15% and roots; PPERM < 0.001, 7.31%) and AI (bulk soil; PPERM < 0.001, 6.52% and roots; PPERM < 0.001, 5.89%) best described significant portions of the remaining variation in bacterial communities (DistLM models).
For fungal microbiomes, variation in community structure in the bulk soils and roots was both best predicted first by contributions from mean annual temperature (MAT) (bulk soil; PPERM < 0.001, 7.61% and roots; PPERM < 0.001, 8.75%) and AI (bulk soil; PPERM < 0.001, 6.43% and roots; PPERM < 0.001, 6.87%) (Table S3). The assemblage of fungi on roots was also strongly associated with variation in seasonal precipitation (close in effect size to the contribution from aridity) (Table S3).
Overall, DistLM models were marginally better fitted for bacterial than for fungal communities and for bulk soils compared with roots (total cumulative effects; bacterial roots 46.67%, bacterial bulk soils 54.85%, fungal roots 34.37% and fungal bulk soils 43.24% variance explained). More variables contributed to overall community structures within the bulk soils than to roots.
3.2 Aridity's Influence on Key Members of Root and Soil Microbiomes
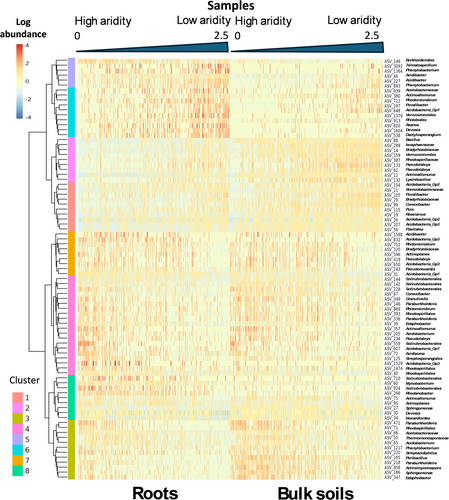
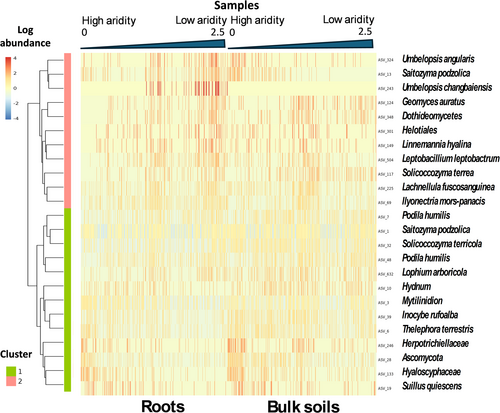
The P. radiata root microbiome hosted 85 bacterial (0.1% of the total 81,590 ASVs; Figure 2) and 24 fungal ASVs (0.06% of the total 41,906 ASVs; Figure 3) that exhibited significant changes (q value < 0.05) in differential abundance across the present-day AI continuum (Tables S4 and S5). All of the significant bacterial ASVs and most of the fungal ASVs (except for one) in the roots are found within the bulk soil samples (Figures 2 and 3). However, these ASVs were not present in the same proportions for the root and bulk soils.
Within the root microbiome, the 85 bacterial ASVs associated with high present-day AI made up 10.7% of the relative abundance, while the 24 fungal ASVs represented 12.9% of the relative abundance on average. For the bulk soils, this number drops to 8.9% for AI-associated bacterial ASVs and 15.5% for the AI-associated fungal ASVs. Consequently, even though these ASVs make up a small percentage of total ASVs, their cumulative relative abundance makes them significant overall contributors to both the bacterial and fungal plant-root microbiomes.
Within root bacterial communities, the significant ASVs grouped into clusters, and several of these were more prevalent in drier conditions: clusters 3, 4, 7, and 8 (Figure 2). Within root fungal communities, two main clusters of fungal ASVs shift with aridity but in opposing directions; cluster 1 decreased with higher AI values, while cluster 2 is more prevalent in less arid conditions (higher AI) (Figure 3). Bulk soils bacterial communities demonstrate a similar trend to root bacterial communities. However, the changes for clusters 1, 2, and 3 are more pronounced in bulk soils than those seen for roots. Clusters 5, 6, and 8 show less pronounced changes within the bulk soils than the roots (Figure 2).
Fungal communities demonstrate similar patterns to those observed in roots; however, they show more spread across the entire aridity gradient than aligning to either high or low aridity (Figure 3). ASV_243 (Umbelopsis) was the only significant ASV to be found in roots that was completely absent from all samples within the bulk soils.
3.3 Recruitment Processes Influencing Root Microbiome Assembly From the Bulk Soil
Deterministic processes, including both heterogeneous and homogenous selection, accounted for on average 23.1% (ranging 13.4%–31.9%) of community assembly processes for bacterial root microbiomes (Figure S11a). Heterogeneous selection averaged 1.1% and homogeneous selection 22.0% of community assembly processes. For fungal root microbiomes, the average contribution of deterministic processes to community assembly averaged 23.6% (ranging 10.6%–51.7%; Figure S11b), with heterogeneous selection averaging 7.8% and homogeneous selection averaging 15.8% contribution to community assembly processes.
Deterministic processes were split for bacterial communities; the strong dominance of homogenous selection was used in the model (DistLM summary; Table S6). Soil pH was shown to have a dominant effect on homogenous selection for bacterial root communities (16.47% contribution; p = 0.001). Aridity was included in the bacterial model explaining 5.89% of the total variance in the amount of deterministic processes across the sites. For fungal communities, the homogeneous and heterogenous selections modeled different drivers affecting the community's deterministic processes. Fungal homogeneous selection was most explained by C/N ratio (7.37% contribution; p = 0.023) whereas variation in assembly processes linked to heterogeneous selection was best explained by exchangeable calcium (14.78% contribution; p = 0.001).
3.4 The Influence of Aridity on Functional Processes
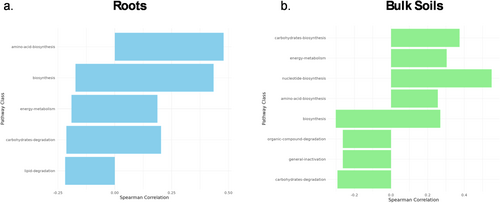
PICRUSt was used to predict gene functions of root and bulk soil bacterial microbiomes across the aridity gradient (Figure 4; Tables S7 and S8). Within bacterial root microbes, amino acid biosynthesis increased with less arid sites (higher AI values) (Figure 4 and Table S8), with the methionine salvage pathway being the most significantly correlated with increasing aridity. Lipid degradation decreased in more arid environments (lower AI values). In bulk soil samples, there were more pathway correlations with carbohydrate biosynthesis, energy metabolism, nucleotide biosynthesis, and amino acid biosynthesis increasing with lower aridity (high AI values; Figure 4 and Table S7). Organic compound degradation, including carbohydrate degradation, decreased in more arid environments.
Most fungal ASVs were classified into ectomycorrhizal, undefined saprotroph, pathogen/parasite, and ‘unassigned’ functions. In root microbiomes, ectomycorrhizal and plant saprotrophs on roots increase with aridity alongside a large decrease in the occurrence of taxa with unassigned functions (Figure 5a). For bulk soils, an increase in aridity was associated with a large decline in ectomycorrhiza but an increase in pathogens/parasite and undefined saprotrophs (Figure 5b). Variation in response of guilds to changes in aridity was greater in the root communities than bulk soils (Figure 5), suggesting root fungal guilds are more responsive to aridity.
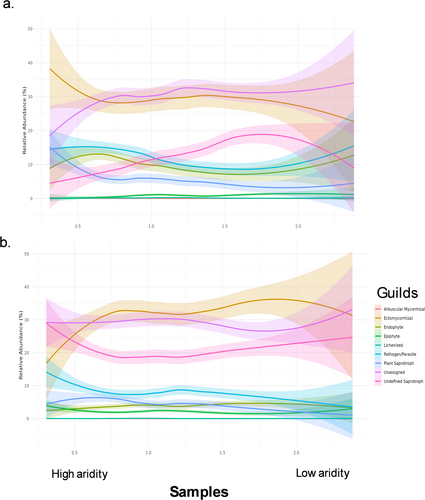
Guild changes were more prevalent in very high aridity sites (< 0.65 AI) and low aridity conditions (> 2 AI). For bulk soils across all the functional guilds, aridity only explained a small amount of variation, with overall R2 values low (0.032–0.099). MAT explained more variance for bulk soil functional guilds, with 0.8%–5.56% variance explained. Relationships within the roots were more varied (R2 = 0.045–0.166) with aridity explaining a meaningful portion of variation for the guilds pathogen/parasite (4.1%; increasing with increasing aridity) and within undefined saprotrophs (8.0%; decreases with increasing aridity). Ectomycorrhizal fungi within the bulk soils were explained best with MAT (5.56%) and within the roots exchangable sodium (Exch_Na; 1.56%).
3.5 Future Aridity Predictions
The relationship between current ecosystem aridity and future predictions is shown in Figure S12. Points sampled in present day areas of low aridity mapped well to areas sampled in the higher end of the aridity index. However, the magnitude of increases in aridity will be greater for sites that currently have lower aridity relative to higher aridity sites today (Figure S12). In the present-day climate, 12.6% of the total sampling sites were classified as dryland areas (< 0.65 AI). This number increases to 18% (a 30% increase in dryland states) with the future prediction aridity index 8.5 RCP 2050. Overall, environmental conditions currently present in the driest sampling sites in this study adequately represent modelled aridity climates (RCP 8.5 future predictions for 2041–2060; Figure 1c) for many of the wettest sample sites today. As such, the sampling gradient represents a robust space-for-climate model systems to test for P. radiata growth and root-microbiome interactions.
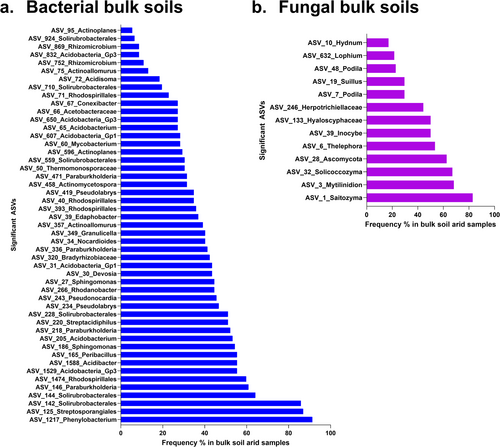
In the P. radiata root microbiomes, 49 bacterial and 13 fungal ASVs were identified as being conditionally enriched (log abundance) in arid sites. For bacterial communities, these arid enriched ASVs were dominated by the phylum Proteobacteria (~39%) and Actinobacteria (~37%). These represent similar proportions as seen in the overall phyla relative abundance for root communities. The enriched ASVs for fungal communities were dominated by Basidiomycota (~62%) and Ascomycota (~38%). These proportions were similar to those seen for the total root community relative abundances.
We assessed if the ASVs enriched in the microbiome of roots of trees growing in present day arid conditions were present in the local species pool (bulk soil) of sites forecast to become arid by 2050 (Figure 6a,b). No bacterial or fungal taxa that were identified as ‘aridity dependant’ in their root microbiome associations (defined in previous sections) were absent and therefore available for recruitment from the bulk soil species pool for all sites. On average, aridity-dependant root bacteria were present in 39.3% of bulk soils expected to become arid by 2050. These ranged from 5%–91% frequency. Similarly, aridity-dependant root fungi varied in occurrence in bulk samples from 17%–83% frequency (average 46.07%).
4 Discussion
4.1 Aridity's Influence on Ecological Filtering
Plants utilise ecological filtering to direct the assembly of root-associated microbiomes (Berdugo et al. 2022; Hodgson et al. 2024). This filtering, driven by both abiotic factors and biotic interactions, may have a role in supporting symbiosis with microbial taxa and functional traits that increase plants fitness. We assessed for evidence of ecological filtering of the root-microbiome from the bulk soil microbiome in relation to increased aridity, beginning with α-diversity. For both bacteria and fungi, there was evidence for increased dominance of root-microbiome associations with aridity; i.e., the enrichment of microbial taxa from the local species pool (soil) to the root microbiome was stronger for trees growing in more arid conditions.
Variation in bacterial α-diversity was primarily explained by edaphic properties, particularly organic carbon. While including seasonal variation in precipitation improved model fit, the influence of precipitation variation was secondary to soil properties. This indicates environmental filtering, but the first-order effect is that of soil characteristics. This is consistent with other studies in the recruitment of bacterial communities across the Arctic (Malard et al. 2021), itself an arid environment. In this study, aridity best explained variation in the fungal root microbiome α-diversity, consistent with previous findings on its dominant influence in richness and diversity in arid ecosystems (Guevara-Araya et al. 2022). Thus, aridity was more directly linked to fungal root-microbiome α-diversity than to bacterial root associations. This aridity-dependent microbial selection can lead to distinct community structures across ecosystems, for example where more extreme aridity limits overall diversity but enhances abundances of groups of drought-adapted taxa (Xu et al. 2018; Marasco et al. 2021). Our study underscores the central role of ecological filtering in shaping distinct root-microbiome community structures across aridity gradients, highlighting how differential bacterial and fungal responses to environmental stress may drive adaptive ecosystem resilience for P. radiata.
Microbiome composition often associates more closely with ecosystem properties than species α-diversity metrics (Shi et al. 2016; Hillebrand et al. 2017; Spaak et al. 2017). In bacterial communities, soil pH accounted for the most variation in both soil and root microbiome composition. Broad ecological surveys typically find pH to be a first-order factor associated with bacterial community assemblage (Lauber et al. 2008; Wakelin et al. 2008; Delgado-Baquerizo et al. 2017). In studies that naturally encompass differences in soil pH across the sample set, the influence of factors such as management, plant composition, and climate are often evident once variation due to pH has been accounted for (e.g., Kuramae et al. 2012). Our bacterial results here reflected this tenet; in both soil and root symbiosis, after variation due to soil pH was accounted for, aridity was significantly related to changes in bacterial community composition. Aridity was strongly related to fungal community composition, with environmental factors more pronounced than soil factors. These results support previous findings showing how climate variation influences, and particularly temperature, is associated with fungal distribution and endemicity globally (Větrovský et al. 2019; Tedersoo et al. 2022).
4.2 Selective Microbial Enrichment With Aridity
We hypothesised that there would be (1) taxa conditionally enriched in response to aridity and (2) that low-abundance members of today's moist soil ecosystems may increase under climate change. Although enriched root-associated aridity taxa represent a small fraction of the total ASVs present, their high abundances contributed significantly to overall composition, representing a similar trend to previous studies (Allsup et al. 2023). This indicates they are not numerically rare members of the plant-root microbiome but rather comprise important keystone aridity-related taxa, known to be highly influential in the stability of microbial networks (Wang et al. 2023, 2024).
The results show support for strong environmental influences on microbiome assembly, particularly of the tree-root microbiome. Clusters of common patterns of associations were evident with aridity, ranging from linear through to idiosyncratic responses. This is true for both bacterial and fungal communities and was more pronounced in the extremes of aridity (< 0.65 and > 1.5 AI). The significant root ASVs associated with higher prevalence in arid conditions were found to have a wide range of prevalence within future arid predicted sites. For low-frequency ASVs in samples, these taxa will need to be recruited outside their current bulk soil bounds for the trees to be able to partner with them in future conditions.
Approximately 37% of bacterial ASVs strongly associated with aridity were Actinobacteria. Actinobacteria represented about 25% of all significant ASVs, with roughly 70% of these taxa specifically associated with high aridity (low AI). This aligns with previous studies that show Actinobacteria at the root-soil interface in response to drought (Naylor et al. 2017; Xu and Coleman-Derr 2019; Karray et al. 2020; George et al. 2024). Notably, Actinobacteria were identified as one of the dominant phyla in Populus tree studies, where drought stress led to significant shifts in microbial community composition (Xie et al. 2021).
One limitation of using sequence read numbers as a measure of abundance for individual ASVs is that it does not account for various biases. While this approach is common in microbiome-related studies, it is imperfect given variation in rRNA operon copy numbers among taxa, PCR primer amplification bias, DNA extraction efficiency, and other factors (e.g., Regueira-Iglesias et al. 2023). These can be mitigated for, to varying extents, using experimental and/or bioinformatic tools. For example, our choice of methodology based on Earth Microbiome Protocols (Thompson, et al. 2017) helps ensure that bias present in PCR primers is expressed uniformly across studies using these methods, allowing for comparative analysis. Similarly, the use of mechanical disruption (bead-beating) during DNA extraction was focused on ensuring Gram-positive or spore-forming bacteria were not underestimated in the sequencing data. To this extent, our results based on variation in abundances of Actinobacteria (Gram +ve) indicate that significant limitations that might have influenced had been mitigated. However, care still needs to be taken in direct inference of absolute abundance of taxa based on NGS read numbers in this (and other) studies.
4.3 Aridity Shapes Microbial Functional Potential
Consistent with previous studies, bacterial functions shifted along environmental aridity gradients, reflecting adaptations to changing conditions (Song et al. 2019). In less arid environments, increased biosynthetic activity may enhance nutrient availability, while in drier conditions, stress-related pathways become more prominent. Root-associated microbiomes showed greater amino acid biosynthesis in less arid soils, while the methionine salvage pathway correlated with increasing aridity. The correlation between the methionine salvage pathway and increasing aridity reflects microbial strategies for maintaining sulfur metabolism under drought stress, consistent with prior evidence of stress-related functional adaptations (Song et al. 2019). Lipid degradation declined under drier conditions, indicating shifts in microbial activity. In bulk soil, biosynthetic pathways were more active in less arid environments, while organic compound degradation decreased with aridity, potentially slowing nutrient cycling. These findings highlight microbial adaptations to aridity with implications for plant–microbe interactions and ecosystem function.
Of all fungal root symbionts, mycorrhizal associations are the most widely studied due to their important contributions to plant fitness and ecosystem processes (Smith and Read 2008). These provide not only nutritive advantages to the plant, but enhance resistance to biotic and abiotic stress, including drought (Allen 2007). Indeed, increased aridity is expected to result in greater nutrient exchange rates between mycorrhizal fungi and plant roots (Pérez-Ramos et al. 2021). Both Basidomycota and Ascomycota fungal phyla include a diversity of ectomycorrhizal species (Tedersoo et al. 2010; Martin et al. 2018) and are well represented in the fungal guild analysis. Ectomycorrhizal associations with P. radiata roots increased in relative abundance with aridity. If pine forests are lost, their associated microbial biodiversity is at risk. Indeed, due to climate change ectomycorrhizal fungal species in North American pine forests are predicted to decline by up to 26% within the next 50 years (Steidinger et al. 2020). This is contrary to our results that show ectomycorrhiza increase in relative abundance with increased aridity, meaning that while overall species numbers will decrease, keystone ectomycorrhiza may increase in relative abundance to maintain symbiotic relationships with tree roots.
The impacts of aridity on wider plant-microbiome interactions may result in either a strengthening or weakening of symbioses (Philippot et al. 2021; Angulo et al. 2022). More arid conditions appeared to lead to more reliance on key plant-microbe symbiotic relationships which exhibit a larger role (higher abundance of these taxa) (Naylor et al. 2017; Naylor and Coleman-Derr 2018; Marasco et al. 2021; Wang et al. 2024). Such changes in root microbiome symbioses due to aridity could have cascading effects on forest ecosystem services spanning nutrient cycling and biodiversity, through to carbon sequestration. Given the importance of these symbioses to the functioning and fitness of forest ecosystems globally, this represents a key outcome.
4.4 Stochastic Processes Dominate Recruitment
We hypothesised that the strong environmental selective pressures induced by aridity would transition root microbiome assembly towards increased deterministic processes. However, our results did not support this hypothesis, as root microbiome assembly appeared to be driven predominantly by stochastic processes, even under increasing aridity. Stochastic mechanisms allow for greater variability in community composition where unguided events influence the microbiome assembly. In our study, the dominance of stochastic over deterministic processes suggests that even as aridity increases, randomness in assembly for large portions of the root microbiome assembly endures. This persistence of stochasticity may support a degree of resilience to adverse environmental conditions, suggesting that environmental filtering, while important, does not fully dictate microbial community assembly under arid conditions.
The high stochasticity observed in this study is not surprising, given the dynamic and opportunistic nature of the rhizosphere microbiome (Pantigoso et al. 2022). On a given sampling day, much of the root microbiome will comprise general rhizosphere colonists—fast-growing copiotrophic taxa enriched from the soil in response to the increased availability of nutrients leaked or exuded from the roots (López et al. 2022). These microbes rapidly respond to short-term environmental changes, such as variations in moisture or temperature immediately prior to sampling, creating a significant degree of ‘microbial white noise’ in this habitat (Hartman and Tringe 2019). This stochasticity underscores the challenges of identifying a subset of the microbiome conditionally related to long-term drivers such as aridity or other environmental factors. Without sampling high numbers of individual plants across environmental (or other) gradients, our ability to characterise elements within the root microbiome that are deterministically assembled as a result of long-term pressures (e.g., aridity) as opposed to those either ‘randomly enriched’ due to the generalist rhizosphere responses associated enriched labile nutrients or to short term environmental change (e.g., recent rainfall) would be unlikely to be successful in the field.
Against this backdrop of stochastic processes and transient generalists, we still observed considerable deterministic drivers influence root microbiome assembly. This suggests that, despite the microbiome assembly due to recent environmental responses, persistent factors occurring over the longer term due to host, soil, and environmental variables still exert strong selective pressures, fostering a temporally stable fraction of taxa within the overall root microbiome. These deterministic processes likely influence the core microbiome, consisting of microbial taxa consistently associated with the host plant under varying conditions. This core microbiome may represent the “friends that are always there for you”, providing critical ecological services such as nutrient acquisition, stress tolerance, and pathogen suppression, even amid fluctuating conditions (Fierer 2017; Llado et al. 2017). This interplay of stochastic and deterministic processes underscores the complexity of microbial community assembly processes in root microbiomes.
4.5 The Microbial Associations of the Future
Populations of trees are thought to have co evolved with the local microbial communities over long evolutionary timescales, with the symbioses established playing an important role in enhancing resilience and fitness. The ‘old friends’ hypothesis suggests that co evolved symbiosis are important for fitness (sensu immune system regulation in the human-microbiome model for which this hypothesis was proposed; Rook 2013). Supported by our results, we propose that important elements of microbiome symbiosis can be established conditionally in response to local selective pressures (environmental or otherwise), acquiring adaptation from the wide pool of microorganisms in the bulk soil. This ‘friends with benefits’ hypothesis can be applied to tree root-microbiomes potential for adaption to future climate. In this regard, we queried the bulk soil microbiome libraries to determine if taxa associated with beneficial symbiosis in highly arid soils are present, at sites projected to increase in aridity in the future based on the climate change scenarios. Looking across all the soils, our results show these key taxa from the roots were present (excluding a single fungal taxa) at the global scale (not necessarily present in every individual sample). Their presence in the soils ensures these ‘friends’ are available in the local species pool for potential selection into the root microbiome community. If conditions change and these taxa are not present then they (1), may need to make new friends or (2), need some help being re-introduced to the necessary microbes.
4.6 Conclusions
Aridity strongly influences the β-diversity of bacterial and fungal communities across root microbiomes and soil compartments for P. radiata, though it does not dominate their overall assembly dynamics (deterministic vs. stochastic processes). A subset of bacterial and fungal ASVs showed strong responses to aridity, contributing disproportionately to community composition. Functional predictions highlighted that root communities are more sensitive to aridity than bulk soils, with extreme conditions triggering notable shifts in functional guilds. These findings emphasize aridity's profound influence on the root microbiome's structure, diversity, and functional potential, particularly under environmental extremes. This analysis demonstrates that the necessary microbial partners for future arid climates may already be present in the surrounding soils, positioning tree roots to selectively filter and recruit microbes they need to withstand future environmental pressures. These findings reveal a nuanced interaction between ecological processes and evolutionary dynamics, with aridity, environmental conditions, and nutrient availability playing pivotal roles in shaping microbial communities. This complexity has important implications for predicting how future environmental changes, such as increased aridity or shifts in soil chemistry, will impact root-microbiome composition and functioning. It also suggests that the plant root is exerting less selective filtering for specific microbial partners that might enhance drought resilience than we hypothesized. The unpredictable nature of resource availability and environmental stressors may reduce the effectiveness of plant control mechanisms, allowing microbial communities to assemble more randomly. This could result in less specific microbiomes being consistently favored, as the stochastic events may override the plant's selection based on functional traits.
Author Contributions
Sarah L. Addison: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing – original draft, writing – review and editing. Zhen-Zhen Yan: investigation, methodology, writing – original draft, writing – review and editing. Tom Carlin: data curation, methodology, software, writing – original draft, writing – review and editing. Megan A. Rúa: investigation, methodology, supervision, writing – original draft, writing – review and editing. Simeon J. Smaill: conceptualization, supervision. Kaitlyn Daley: investigation, methodology. Brajesh K. Singh: supervision, writing – original draft, writing – review and editing. Steve A. Wakelin: conceptualization, formal analysis, funding acquisition, investigation, supervision, writing – original draft, writing – review and editing.
Acknowledgements
We are grateful to Nina Eichler for her help with sampling collections and a very large collection of forest and landowners for allowing access across both New Zealand and Australia. We also want to thank the reviewers for their helpful comments for improving this manuscript. Open access publishing facilitated by Western Sydney University, as part of the Wiley - Western Sydney University agreement via the Council of Australian University Librarians.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in Dryad at https://doi.org/10.5061/dryad.6q573n688 (bacterial (16S), fungal (ITS) datasets, chemical and environmental data). Climate data and future climate scenarios were obtained from WorldClim at https://www.worldclim.org/data/worldclim21.html (version 2.1) and https://www.worldclim.org/data/cmip6/cmip6_clim30s.html, respectively. Present and future aridity estimates were obtained from Figshare at https://doi.org/10.6084/m9.figshare.7504448.v6. Raw sequencing data is available from the NCBI sequence read archive (SRA) under the BioProject accession PRJNA1217937.




