Bumble bee responses to climate and landscapes: Investigating habitat associations and species assemblages across geographic regions in the United States of America
Abstract
Bumble bees are integral pollinators of native and cultivated plant communities, but species are undergoing significant changes in range and abundance on a global scale. Climate change and land cover alteration are key drivers in pollinator declines; however, limited research has evaluated the cumulative effects of these factors on bumble bee assemblages. This study tests bumble bee assemblage (calculated as richness and abundance) responses to climate and land use by modeling species-specific habitat requirements, and assemblage-level responses across geographic regions. We integrated species richness, abundance, and distribution data for 18 bumble bee species with site-specific bioclimatic, landscape composition, and landscape configuration data to evaluate the effects of multiple environmental stressors on bumble bee assemblages throughout 433 agricultural fields in Florida, Indiana, Kansas, Kentucky, Maryland, South Carolina, Utah, Virginia, and West Virginia from 2018 to 2020. Distinct east versus west groupings emerged when evaluating species-specific habitat associations, prompting a detailed evaluation of bumble bee assemblages by geographic region. Maximum temperature of warmest month and precipitation of driest month had a positive impact on bumble bee assemblages in the Corn Belt/Appalachian/northeast, southeast, and northern plains regions, but a negative impact on the mountain region. Further, forest land cover surrounding agricultural fields was highlighted as supporting more rich and abundant bumble bee assemblages. Overall, climate and land use combine to drive bumble bee assemblages, but how those processes operate is idiosyncratic and spatially contingent across regions. From these findings, we suggested regionally specific management practices to best support rich and abundant bumble bee assemblages in agroecosystems. Results from this study contribute to a better understanding of climate and landscape factors affecting bumble bees and their habitats throughout the United States.
1 INTRODUCTION
Bumble bees (Hymenoptera: Apidae: Bombus Latreille) are globally widespread pollinators of native and cultivated plant communities (Goulson, 2010; Klein et al., 2007; Kremen et al., 2002). There are more than 265 bumble bee species worldwide, 47 of which occur in the United States (Colla et al., 2011; Koch et al., 2012; Maebe et al., 2021; Williams et al., 2014). Despite their ecological and economic importance to wild and agricultural systems, bumble bee communities are undergoing drastic changes due to anthropogenic effects such as climate change and agricultural intensification and expansion (Fourcade et al., 2019; Goulson et al., 2015; Kerr et al., 2015; Kohler et al., 2020).
Global climate change has led to a rise in average temperatures, changes in precipitation patterns, and an increase in the frequency and intensity of extreme and localized weather events (Easterling et al., 2000; Meehl & Tebaldi, 2004). Changes in climate can have profound impacts on bumble bee species' abundances, distributions, and population dynamics as well as overall community structure (Easterling et al., 2000; Fourcade et al., 2019; Kerr et al., 2015; Parmesan, 2006). Temperature is a pervasive selective pressure that influences bumble bee evolution and adaptation (Hines, 2008; Jackson et al., 2020; Kerr et al., 2015; Martinet et al., 2018, 2021; Pimsler et al., 2020; Rasmont et al., 2015; Williams et al., 2018). Given their annual colony cycle, bumble bees are exposed to a wide range of temperatures as they develop from spring to autumn and gynes undergo winter diapause. Common throughout cool temperate, alpine, and arctic ecosystems, bumble bees can regulate their body temperature and generate heat in cold climates (Heinrich, 1976; Kerr et al., 2015; Pimsler et al., 2020), while also having adaptations to prevent overheating (i.e., wing fanning, thoracic, or evaporative cooling) during the summer (Heinrich, 1976; Westhus et al., 2013). Despite these adaptations, frequent, intense, and/or persistent heat waves can lead to increased mortality of bumble bees by inducing hyperthermic stress when foraging (for workers) or during nuptial behavior (for males), increasing risk of desiccation and reducing floral availability (Fourcade et al., 2019; Iserbyt & Rasmont, 2012; Martinet et al., 2015, 2021; Oyen et al., 2016; Rasmont & Iserbyt, 2012; Vanderplanck et al., 2019; Williams et al., 2009). Additionally, precipitation has direct (e.g., desiccation and foraging) and indirect (e.g., floral resource availability and soil moisture for nesting) effects on bumble bee fitness and can drive foraging periods and geographic ranges (Jackson et al., 2018; Koch et al., 2019; Pyke et al., 2011; Williams et al., 2015; Willmer & Stone, 1998). Further, bumble bee species within the same community can be differentially impacted by climate and floral resource availability, indicating species-specific responses (Ogilvie et al., 2017). Therefore, understanding how bioclimatic predictors (climate conditions related to species physiology) shape bumble bee assemblages (calculated as species richness and abundance) is of critical importance for evaluating climatic constraints. Climate change also interacts with other anthropogenic disturbances, such as land cover conversion and intensification, further altering species' responses to these environmental conditions (Easterling et al., 2000; Fourcade et al., 2019; Kerr et al., 2015; Marshall et al., 2018).
Within agricultural systems, intensification and expansion of high-yielding field crops have led to extensive changes in landscape composition and configuration, reducing the number and amount of distinct land cover categories and simplifying the way in which they are arranged (Meehan et al., 2011; Nelson & Burchfield, 2021; Pfeiffer et al., 2019). This landscape simplification often leads to increased homogenization, which reduces the availability, diversity, and distribution of nutritionally sufficient floral resources and nesting sites for bumble bees (Parys et al., 2021), leading them to be inadvertently extirpated in these agricultural environments (Hall et al., 2017; Westphal et al., 2003; Williams et al., 2012). While bumble bees can fly several kilometers to establish colonies and forage, they are also central place foragers with strong site fidelity (Goulson, 2010; Lepais et al., 2010; Ogilvie & Thomson, 2016; Rao & Strange, 2012). Therefore, increasing the diversity and connectedness of land use types that provide increased floral and nesting resources within areas surrounding agriculturally intensified lands can positively impact bumble bee assemblages and diversity (Kaiser-Bunbury et al., 2017; Miljanic et al., 2019).
While the individual effects of climate and various landscape factors have been studied, research is just starting to evaluate the effects of multiple stressors simultaneously, underscoring the need to understand how bumble bees are affected by a range of co-occurring environmental changes across geographic regions (Escobedo-Kenefic et al., 2020; Fourcade et al., 2019; Ganuza et al., 2022; Kohler et al., 2020; Marshall et al., 2018). Research incorporating land cover change with climate change continually reports that evaluating these factors together is important to understand environmental change impacts on pollinators, which can then be used to implement effective conservation practices (Kammerer et al., 2020; Naeem et al., 2019). As bumble bees have strong species-specific associations with land use and climate variables (Christman, Spears, Strange, et al., 2022; Liczner & Colla, 2020), it is important to study both individual species responses and community responses to gain a more holistic understanding of how bumble bees will be impacted by environmental changes (Whitehorn et al., 2022). For example, imperiled B. occidentalis (Greene, 1858) range-wide declines have been attributed to increasing temperatures during the warmest quarter of the year, severe drought years, and use of nitroguanidine neonicotinoid insecticides, while their occupancy is positively associated with increased forest and shrub area (Janousek et al., 2023). Meanwhile, previous research in Utah identified that bumble bee assemblages (calculated as species richness and abundance) were richer and more abundant at agricultural sites surrounded by more agricultural land cover, low temperatures, and high relative humidity, and lowest at agricultural sites with more urban land cover in the surrounding area, high temperatures, and low relative humidity (Christman, Spears, Strange, et al., 2022). Further, land use can amplify or offset changes in suitable habitat for bumble bees under various climate projections, emphasizing the importance of utilizing targeted land management practices to reduce the magnitude of bumble bee declines (Prestele et al., 2021). Overall, evaluating climate and land use factors together is critical for managing pollinator populations and their environments.
In this study, we evaluated the effects of climate, landscape composition, and landscape configuration on bumble bee species assemblages (calculated as species richness and abundance) in agricultural fields throughout nine states in the United States. Specifically, we tested bumble bee responses to climate and land use by modeling species-specific habitat requirements and assemblage-level responses across geographic regions. Results from this study contribute to a better understanding of climate and landscape factors affecting bumble bees and their habitats throughout the United States. Detailed knowledge of species-specific relationships with climate and landscape variables across a geographic range is invaluable to improve targeted conservation and land management strategies to mitigate the effects of ongoing environmental changes.
2 MATERIALS AND METHODS
2.1 Overview
We combined richness, abundance, and distribution data for 18 bumble bee species with bioclimatic, landscape composition, and landscape configuration data to evaluate the effects of multiple environmental stressors on bumble bee species assemblages throughout the United States. Bumble bees were collected as bycatch within pest monitoring traps placed in 433 agricultural fields throughout nine states from 2018 to 2020. Bioclimatic data were derived at a 1-km spatial scale using historical Daymet monthly precipitation, minimum temperature, and maximum temperature data from 2000 to 2020 (Thornton et al., 2020). Landscape composition and configuration data were derived at a 1-km spatial scale from land cover values obtained from the USDA National Agricultural Statistics Service CropScape and Cropland Data Layer from 2018 to 2020 (USDA NASS CDL, 2018–2020). A canonical correspondence analysis and generalized additive mixed models were conducted to assess bumble bee assemblage-level responses in relation to climate and land use variables. All data were analyzed using R version 4.3.1 (R Core Team, 2022). The details for all methodological and analytical steps are described in detail in the following sections.
2.2 Collection of bumble bees
Bumble bees were collected within pest monitoring traps that were placed by state cooperators in agricultural fields across diverse regions in the United States as part of early-detection surveys for invasive lepidopterans following Spears et al. (2016) and U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Cooperative Agricultural Pest Survey approved methods for pest surveillance (Cooperative Agricultural Pest Survey [CAPS], 2022). Previous research identified that bumble bees are attracted to pest monitoring traps and suggested that these captures be used to advance knowledge of biodiversity, population fluctuations, and other ecological objectives (Buchholz et al., 2011; Christman, Spears, Strange, et al., 2022; Grocock et al., 2020; Grocock & Evenden, 2020; Parys et al., 2021; Sipolski et al., 2019; Spears et al., 2016, 2021; Spears & Ramirez, 2015; Whitfield et al., 2019).
This study included a total of 433 agricultural fields throughout Florida, Indiana, Kansas, Kentucky, Maryland, South Carolina, Utah, Virginia, and West Virginia from 2018 to 2020, where the number of sites varied by state, year, and target pest (Table 1). Target pests included Christmas berry webworm (CBW, Cryptoblabes gnidiella Milliere, 1867), cotton cutworm (CC, Spodoptera litura Fabricius, 1775), Egyptian cottonworm (EC, Spodoptera littoralis Boisduval, 1833), golden twin spot moth (GTS, Chrysodeixis chalcites Esper, 1789), Old World bollworm (OWB, Helicoverpa armigera Hübner, 1808), and silver Y moth (SYM, Autographa gamma Linnaeus, 1758). Following methodology outlined in Christman, Spears, Strange, et al. (2022) and CAPS (2022), multi-colored (green canopy, yellow funnel, and white bucket) bucket traps (International Pheromone Systems, Cheshire, UK) were placed 20 m apart and hung 1.5 m above the ground along the edge of vegetable or other commodity crop fields (e.g., alfalfa, corn, and small grain) as part of the CAPS monitoring program. Each trap contained a pheromone lure for a single target pest inside the lure basket of the trap canopy. An insecticide strip (Hercon Vaportape II: 10% dimethyl 2,2-dichlorovinyl phosphate, Hercon Environmental Corporation, Emigsville, PA) and a small, cellulose sponge were placed inside each bucket to kill the captured insects and absorb rainwater, respectively. Insecticide strips and pheromone lures for CBW, GTS, OWB, and SYM were replaced no more than every 28 days, whereas pheromone lures for CC and EC were changed no more than every 84 days. Although the collection period for traps varied by state, most traps were serviced biweekly (monthly in Kentucky) from May to August, but some states had an extended trapping season based on the period of expected pest activity (Table 1). Because lure comparisons were not the intent of this study (but see Spears et al., 2016 for lure-specific analyses), trap data were combined by study site and collection period.
| State | Number of sites | Target pest(s) | Collection period |
|---|---|---|---|
| 2018 | |||
| Kansas | 86 | CC, EC | July–October |
| Utah | 30 | CC, EC, OWB | April–September |
| West Virginia | 9 | CC, EC, GTS, OWB, SYM | June–September |
| 2019 | |||
| Florida | 16 | OWB | April–September |
| Indiana | 6 | CC, EC, GTS, OWB, SYM | May–August |
| Kentucky | 41 | CC, EC, GTS, OWB, SYM | May–October |
| Maryland | 26 | CC, OWB, SYM | June–July |
| South Carolina | 16 | CC, EC, OWB | June–October |
| Utah | 30 | CC, EC, OWB | June–August |
| Virginia | 13 | OWB | July–September |
| West Virginia | 32 | CBW, CC, EC, GTS, OWB, SYM | May–October |
| 2020 | |||
| Indiana | 6 | CC, EC, GTS, OWB, SYM | April–August |
| Kentucky | 63 | CC, EC, GTS, OWB, SYM | May–September |
| Utah | 30 | CC, EC, OWB | May–August |
| Virginia | 12 | OWB | August–September |
| West Virginia | 17 | CBW, CC, EC, OWB, SYM | May–September |
- Note: Target pests included Christmas berry webworm (CBW), cotton cutworm (CC), Egyptian cottonworm (EC), golden twin spot moth (GTS), Old World bollworm (OWB), and silver Y moth (SYM).
Trap contents were screened for target pests by state cooperators, and all non-target captures (bycatch) were sent to the Utah State University Department of Biology. Bumble bees were separated from all other non-target specimens and then stored in a freezer at −18°C until they could be pin-mounted, labeled, and identified to species using taxonomic keys (Colla et al., 2011; Koch et al., 2012; Williams et al., 2014). All specimens were deposited into a collection in the Department of Biology at Utah State University in Logan, Utah, USA, and records were entered into the USDA, Agricultural Research Service, Pollinating Insect—Biology, Management, Systematics Research Unit, National Pollinating Insects Database.
2.3 Bioclimatic variables
Historical weather data over the past 20 years (2000–2020) were extracted from each site at a 1-km spatial resolution for averaged monthly precipitation, minimum temperature, and maximum temperature using Daymet monthly climate summaries (Thornton et al., 2020). Nineteen bioclimatic variables from WorldClim were derived from the monthly precipitation and temperature values to generate more biologically meaningful variables with the dismo library (Fick & Hijmans, 2017; Hijmans et al., 2020). Using an expert-based selection process, this subset was reduced further by selecting six variables that represent annual climatic trends and extremes: annual mean temperature, maximum temperature of warmest month, minimum temperature of coldest month, annual precipitation, precipitation of wettest month, and precipitation of driest month (Fick & Hijmans, 2017; Hijmans et al., 2020; Jackson et al., 2020).
2.4 Landscape composition and configuration
The elevation of each site was extracted from the North American Elevation 1-km resolution GRID (United States Department of the Interior, 2021). Land cover values from 2018 to 2020 were obtained from USDA National Agricultural Statistics Service (NASS) CropScape and Cropland Data Layer (CDL) (USDA NASS CDL, 2018–2020), which maps data at a 30-m spatial resolution.
The 255 Cropland Data Layer land cover classes were aggregated into five land cover categories: bumble bee-attractive crops, bumble bee-unattractive crops, rangeland, forest, and developed land (Table S1). The attractiveness of agricultural crops to bumble bees was determined using the Pollinator Attractiveness Crop List produced by the United States Department of Agriculture [USDA] (2017). Bumble bee-attractive crops included plants such as tomatoes, peppers, berries, and alfalfa, while bumble bee-unattractive crops included plants such as corn, sorghum, and wheat. Here, we note that in this study, alfalfa was included as a bumble bee-attractive crop since the field can flower up to 25% prior to harvest, providing a pulse of resources for bumble bees (USDA, 2017). Rangeland referred to lands suitable for grazing or browsing, including switchgrass, shrubland, wetlands (woody and herbaceous), grasslands, and pastures. Forest included deciduous, evergreen, and mixed forests. Developed land referred to urban environments that have been built up with impervious surface cover, including low-intensity (20%–49%), medium-intensity (50%–79%), and high-intensity (80%–100%) lands. The number of pixels of each land cover category was then extracted from a 1-km buffer surrounding each site and the percentage of bumble bee-attractive crops, bumble bee-unattractive crops, rangeland, forest, and developed land was quantified to determine the landscape composition surrounding our surveyed agricultural sites.
Additionally, landscape configuration indices (contiguity, and interspersion and juxtaposition) were calculated at a 1-km buffer surrounding each site using the 255 Cropland Data Layer land cover classes with the landscapemetric library (Hesselbarth et al., 2019). Contiguity refers to the spatial connectedness of land cover classes within a landscape. Interspersion and juxtaposition refer to the arrangement, relationship, and proximity of different land cover classes in a landscape (Hesselbarth et al., 2019).
2.5 Data analysis
Four aspects of bumble bee species composition were measured for each state: total count, richness (number of species), Pielou's evenness (abundance per species), and Shannon diversity (which accounts for evenness and richness) with the vegan and codyn libraries. The weekly bumble bee collection rate for each state was quantified each year to standardize differences among state collection periods. Bubble maps were used to visualize bumble bee distribution, abundance, and diversity throughout the surveyed states.
Variance inflation factor was used to test for multicollinearity among the selected bioclimatic and landscape variables. Variables with a variance inflation factor greater than 10 were removed in descending order until all values were lower than 10 to reduce collinearity among the explanatory variables. Maximum temperature of warmest month, minimum temperature of coldest month, precipitation of wettest month, precipitation of driest month, elevation, bumble bee-attractive crops, bumble bee-unattractive crops, forest, developed land, contiguity, and interspersion and juxtaposition were included as the bioclimatic variables and landscape indices within the following models.
A canonical correspondence analysis (CCA) was used to identify species-specific habitat requirements by assessing correlations among explanatory variables (bioclimatic variables and landscape indices) and response variables (Bombus species assemblages) from 2018 to 2020 with the vegan and picante libraries. Variance inflation factor was used again to test for multicollinearity in the CCA. Precipitation of driest month was removed to reduce collinearity among the explanatory variables. A permutation test was used to determine the significance of each variable and the overall model.
Generalized additive mixed models by geographic region were used to describe bumble bee species assemblages (richness and abundance) in relation to linear and nonlinear bioclimatic and landscape variables among all surveyed sites within the region, while accounting for spatial and temporal autocorrelated residuals with the mcgv and nlme libraries. Study sites were split into one of four USDA Farm Production Regions: Corn Belt/Appalachian/northeast, southeast, northern plains, and mountains. Corn belt/Appalachian/northeast included sites within Indiana, Kentucky, Maryland, Virginia, and West Virginia (n = 225). Southeast included sites within Florida and South Carolina (n = 32). Northern plains included sites within Kansas (n = 86), and mountains included sites within Utah (n = 90). Spatial and temporal autocorrelation captured potential imbalances and non-independencies that may be present within our samples across geographic coordinates and year. All predictor variables were initially smoothed using p-splines to account for nonlinearities. Variables with an effective degree of freedom of 1 suggested the term was reduced to a simple linear effect and thus did not need to be smoothed.
3 RESULTS
3.1 Collection of bumble bees
From 2018 to 2020, 5,004 bumble bees representing 18 species were collected across nine states (Table 2). Collection rates varied by state and year. For example, Florida had extremely low collection rates of one bumble bee per week in 2019, whereas over 40 bumble bees were collected per week in Utah (2018 and 2020) and West Virginia (2019) (Table 3). Bombus fervidus (Fabricus, 1798), B. bimaculatus (Cresson, 1863), B. impatiens (Cresson, 1863), B. pensylvanicus (De Geer, 1773), and B. huntii (Greene, 1860) were the five most abundant species within traps, comprising 84% of total captures (Figure 1). Bumble bee species diversity was consistently highest in Indiana, Kentucky, Utah, and West Virginia (Figure 2; Table 3).
| Species | Abundance by state | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| FL | IN | KS | KY | MD | SC | UT | VA | WV | |
| Bombus appositus | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 |
| B. auricomus | 0 | 16 | 2 | 88 | 0 | 8 | 0 | 2 | 70 |
| B. bimaculatus | 0 | 171 | 13 | 67 | 148 | 15 | 0 | 5 | 776 |
| B. californicus | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| B. centralis | 0 | 0 | 0 | 0 | 0 | 0 | 38 | 0 | 0 |
| B. fervidus | 0 | 121 | 0 | 6 | 31 | 0 | 964 | 3 | 99 |
| B. fraternus | 3 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| B. griseocollis | 0 | 8 | 29 | 33 | 4 | 1 | 73 | 5 | 76 |
| B. huntii | 0 | 0 | 0 | 0 | 0 | 0 | 323 | 0 | 0 |
| B. impatiens | 5 | 243 | 7 | 235 | 12 | 114 | 0 | 41 | 345 |
| B. insularis | 0 | 0 | 0 | 0 | 0 | 0 | 36 | 0 | 0 |
| B. morrisoni | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 |
| B. nevadensis | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 0 | 0 |
| B. pensylvanicus | 9 | 104 | 155 | 272 | 8 | 52 | 1 | 40 | 11 |
| B. perplexus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 |
| B. rufocinctus | 0 | 1 | 0 | 0 | 0 | 0 | 107 | 0 | 0 |
| B. vagans | 0 | 34 | 0 | 2 | 0 | 0 | 0 | 0 | 87 |
| B. vancouverensis | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 0 | 0 |
| Total | 17 | 698 | 207 | 703 | 203 | 191 | 1591 | 96 | 1488 |
| State | Total count | Richness | Pielou's evenness | Shannon diversity | Collection rate by week |
|---|---|---|---|---|---|
| 2018 | |||||
| Kansas | 207 | 6 | 0.47 | 0.85 | 14.21 |
| Utah | 746 | 11 | 0.49 | 1.18 | 47.47 |
| West Virginia | 237 | 8 | 0.69 | 1.43 | 19.07 |
| 2019 | |||||
| Florida | 17 | 3 | 0.91 | 1.00 | 1.37 |
| Indiana | 310 | 7 | 0.79 | 1.54 | 25.53 |
| Kentucky | 535 | 7 | 0.71 | 1.37 | 32.01 |
| Maryland | 40 | 5 | 0.92 | 1.47 | 11.20 |
| South Carolina | 191 | 6 | 0.59 | 1.05 | 12.38 |
| Utah | 217 | 9 | 0.45 | 0.98 | 22.34 |
| Virginia | 103 | 6 | 0.65 | 1.17 | 14.71 |
| West Virginia | 932 | 8 | 0.71 | 1.47 | 48.33 |
| 2020 | |||||
| Indiana | 388 | 8 | 0.78 | 1.62 | 23.82 |
| Kentucky | 168 | 6 | 0.78 | 1.40 | 7.30 |
| Utah | 594 | 11 | 0.56 | 1.34 | 51.98 |
| Virginia | 10 | 3 | 0.58 | 0.64 | 2.00 |
| West Virginia | 309 | 8 | 0.57 | 1.18 | 18.18 |
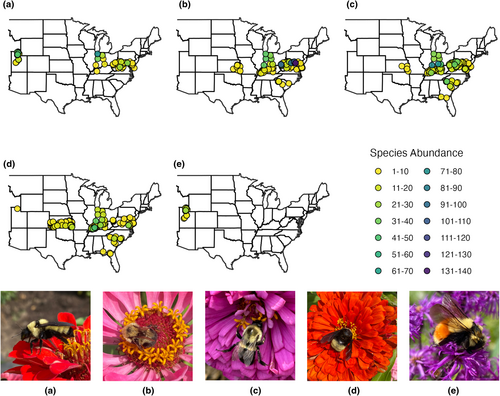
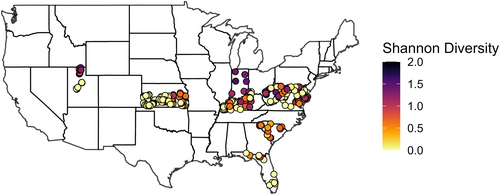
3.2 Bumble bee habitat associations
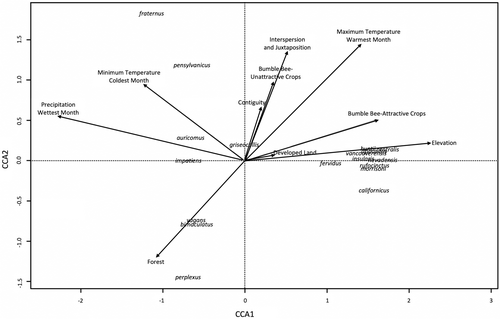
The permutation test determined that the overall canonical correspondence analysis model was statistically significant (F10, 292 = 16.70, p-value = .001). Additionally, the permutation test by term (i.e., explanatory variables) determined that bumble bee abundances were significantly correlated with maximum temperature of warmest month, minimum temperature of coldest month, precipitation of wettest month, elevation, bumble bee-attractive crops, bumble bee-unattractive crops, forest, developed land, and interspersion and juxtaposition (Table 4). Over the 3-year study period, these variables explained 36.38% of variation in bumble bee assemblages (richness and abundance). Bombus appositus (Cresson, 1878), B. californicus (Smith, 1854), B. centralis (Cresson, 1864), B. fervidus, B. huntii, B. insularis (Smith, 1861), B. morrisoni (Cresson, 1878), B. nevadensis (Cresson, 1874), B. rufocinctus (Cresson, 1863), and B. vancouverensis (Cresson, 1878) were clustered together (Figure 3). These western species shared similar habitat requirements, particularly high-elevation environments with high proportions of bumble bee-attractive and unattractive crops within the surrounding area, high maximum temperatures of warmest month, and increased interspersion and juxtaposition. All eastern bumble bee species were associated with low-elevation environments (Figure 3). Additionally, Bombus auricomus (Robertson, 1903), B. fraternus (Smith, 1854), B. impatiens, and B. pensylvanicus were associated with high minimum temperatures of coldest month and increased precipitation of wettest month (Figure 3). Further, B. bimaculatus, B. perplexus (Cresson, 1863), and B. vagans (Smith, 1854) were associated with habitats with high values of forest in the surrounding area. Bombus griseocollis (De Geer, 1773) was not associated with high or low values of any of the environmental variables, meaning they were found ubiquitously throughout the habitats regardless of the bioclimatic variable, landscape composition, and configuration indice values (Figure 3). Given the distinct groupings observed in the canonical correspondence analysis, we then evaluated bumble bee habitat associations by geographic region.
| Variable | df | F | p-value |
|---|---|---|---|
| Maximum temperature of warmest month | 1 | 48.165 | .001 |
| Minimum temperature of coldest month | 1 | 46.157 | .001 |
| Precipitation of wettest month | 1 | 33.512 | .001 |
| Elevation | 1 | 12.997 | .001 |
| Bumble bee-attractive crops | 1 | 4.379 | .001 |
| Bumble bee-unattractive crops | 1 | 2.224 | .023 |
| Forest | 1 | 7.666 | .001 |
| Developed land | 1 | 4.314 | .003 |
| Contiguity | 1 | 1.817 | .066 |
| Interspersion and juxtaposition | 1 | 5.789 | .001 |
3.3 Climate and landscape impact on bumble bee assemblages by geographic region
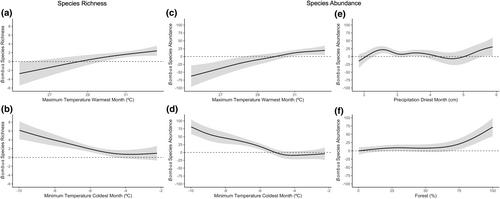
The generalized additive mixed model for the Corn Belt/Appalachian/northeast geographic region determined that bumble bee species richness was significantly associated with maximum temperature of warmest month and minimum temperature of coldest month (Tables 5 and 6). Bumble bee species abundances were also significantly associated with maximum temperature of warmest month and minimum temperature of coldest month in addition to precipitation of driest month and forest land cover (Tables 7 and 8). Based on partial effects plots, bumble bee species assemblages (richness and abundance) increased as maximum temperature of warmest month approached 32°C (Figure 4a,c). Meanwhile, as minimum temperature of the coldest month increased, bumble bees became less species rich and less abundant, plateauing at approximately −5°C (Figure 4b,d). Additionally, bumble bee species became more abundant as precipitation of driest month and forest land cover increased (Figure 4e,f).
| Smoothed predictor variable | EDF | F-value | p-value |
|---|---|---|---|
| Corn Belt/Appalachian/Northeast | |||
| Minimum temperature coldest month | 1.509 | 5.725 | .043 |
| Precipitation wettest month | 2.290 | 7.861 | .001 |
| Elevation | 2.210 | 2.665 | .078 |
| Contiguity | 1.811 | 0.994 | .315 |
| Southeast | |||
| Bumble bee-unattractive crops | 3.796 | 9.088 | .001 |
| Forest | 2.124 | 2.027 | .150 |
| Northern plains | |||
| Elevation | 2.611 | 2.110 | .068 |
| Forest | 4.417 | 3.567 | .006 |
| Mountains | |||
| Interspersion and juxtaposition | 1.753 | 1.905 | .273 |
- Note: Model results include the effective degrees of freedom (EDF), F-values, and p-values for each of the p-spline smoothed (non-linear) effects.
| Linear predictor variable | Coefficient estimate | Std. error | p-value |
|---|---|---|---|
| Corn Belt/Appalachian/Northeast | |||
| Precipitation of wettest | −0.033 | 0.103 | .752 |
| Precipitation of driest month | 0.239 | 0.283 | .398 |
| Bumble bee-attractive crops | 0.014 | 0.014 | .339 |
| Bumble bee-unattractive crops | −0.002 | 0.012 | .894 |
| Forest | 0.003 | 0.010 | .765 |
| Developed land | −0.001 | 0.013 | .939 |
| Interspersion and juxtaposition | −0.009 | 0.019 | .647 |
| Southeast | |||
| Maximum temperature warmest month | 1.049 | 0.368 | .011 |
| Minimum temperature coldest month | −0.101 | 0.203 | .595 |
| Precipitation of wettest month | 0.034 | 0.119 | .775 |
| Precipitation of driest month | 1.215 | 0.457 | .017 |
| Elevation | −0.001 | 0.002 | .952 |
| Bumble bee-attractive crops | −0.045 | 0.009 | .001 |
| Developed land | −0.038 | 0.018 | .057 |
| Contiguity | 0.005 | 0.026 | .846 |
| Interspersion and juxtaposition | 0.008 | 0.014 | .593 |
| Northern plains | |||
| Maximum temperature warmest month | −0.435 | 0.374 | .249 |
| Minimum temperature coldest month | 0.184 | 0.230 | .427 |
| Precipitation of wettest month | −0.046 | 0.055 | .402 |
| Precipitation of driest month | 0.383 | 0.759 | .615 |
| Bumble bee-attractive crops | −0.015 | 0.008 | .062 |
| Bumble bee-unattractive crops | −0.002 | 0.006 | .789 |
| Developed land | 0.014 | 0.016 | .399 |
| Contiguity | 0.022 | 0.027 | .403 |
| Interspersion and juxtaposition | 0.034 | 0.017 | .054 |
| Mountains | |||
| Maximum temperature warmest month | −2.989 | 0.722 | .001 |
| Minimum temperature coldest month | 0.461 | 0.302 | .131 |
| Precipitation of wettest month | −0.255 | 0.359 | .479 |
| Precipitation of driest month | −11.552 | 3.907 | .004 |
| Elevation | 0.001 | 0.002 | .491 |
| Bumble bee-attractive crops | 0.026 | 0.024 | .268 |
| Bumble bee-unattractive crops | −0.049 | 0.027 | .079 |
| Forest | 2.500 | 2.171 | .253 |
| Developed land | −0.045 | 0.031 | .145 |
| Contiguity | 0.310 | 0.109 | .006 |
- Note: Model results include the coefficient estimate, standard error, and p-value.
| Smoothed predictor variable | EDF | F-value | p-value |
|---|---|---|---|
| Corn Belt/Appalachian/Northeast | |||
| Maximum temperature warmest month | 1.391 | 14.738 | .001 |
| Minimum temperature coldest month | 4.660 | 10.132 | .001 |
| Precipitation of driest month | 6.051 | 4.200 | .001 |
| Forest | 3.792 | 6.448 | .001 |
| Southeast | |||
| Maximum temperature warmest month | 4.448 | 16.05 | .004 |
| Minimum temperature coldest month | 4.469 | 19.93 | .003 |
| Precipitation of wettest month | 2.692 | 15.22 | .007 |
| Forest | 7.832 | 31.80 | .001 |
| Northern plains | |||
| Maximum temperature warmest month | 0.999 | 0.231 | .633 |
| Minimum temperature coldest month | 0.999 | 1.497 | .225 |
| Bumble bee-attractive crops | 1.710 | 9.189 | .008 |
| Bumble bee-unattractive crops | 2.672 | 5.095 | .015 |
| Forest | 3.514 | 1.360 | .149 |
| Interspersion and juxtaposition | 2.338 | 4.839 | .007 |
| Mountains | |||
| Bumble bee-unattractive crops | 3.383 | 8.543 | .001 |
- Note: Model results include the effective degrees of freedom (EDF), F-values, and p-values for each of the p-spline smoothed (non-linear) effects.
| Linear predictor variable | Coefficient estimate | Std. error | p-value |
|---|---|---|---|
| Corn Belt/Appalachian/Northeast | |||
| Precipitation of wettest month | 0.197 | 1.277 | .878 |
| Elevation | 0.002 | 0.005 | .704 |
| Bumble bee-attractive crops | 0.211 | 0.159 | .185 |
| Bumble bee-unattractive crops | 0.082 | 0.131 | .513 |
| Developed land | 0.111 | 0.149 | .458 |
| Contiguity | −0.992 | 0.611 | .106 |
| Interspersion and juxtaposition | 0.005 | 0.233 | .983 |
| Southeast | |||
| Precipitation of driest month | 6.514 | 2.124 | .031 |
| Elevation | −0.017 | 0.007 | .059 |
| Bumble bee-attractive crops | −0.175 | 0.048 | .016 |
| Bumble bee-unattractive crops | −0.087 | 0.047 | .133 |
| Developed land | −0.609 | 0.059 | .001 |
| Contiguity | 0.274 | 0.077 | .739 |
| Interspersion and juxtaposition | 0.056 | 0.044 | .269 |
| Northern plains | |||
| Precipitation of wettest month | −0.141 | 0.244 | .565 |
| Precipitation of driest month | 4.202 | 3.307 | .208 |
| Elevation | −0.001 | 0.001 | .306 |
| Developed land | 0.006 | 0.073 | .930 |
| Contiguity | 0.221 | 0.126 | .084 |
| Mountains | |||
| Maximum temperature warmest month | −23.658 | 5.579 | .001 |
| Minimum temperature coldest month | 1.437 | 2.396 | .550 |
| Precipitation of wettest month | −1.081 | 2.837 | .704 |
| Precipitation of driest month | −65.711 | 30.662 | .035 |
| Elevation | 0.007 | 0.014 | .613 |
| Bumble bee-attractive crops | −0.289 | 0.194 | .139 |
| Forest | −11.742 | 16.941 | .490 |
| Developed land | −0.309 | 0.240 | .203 |
| Contiguity | 3.947 | 0.898 | .001 |
| Interspersion and juxtaposition | 0.328 | 0.397 | .411 |
- Note: Model results include the coefficient estimate, standard error, and p-value.
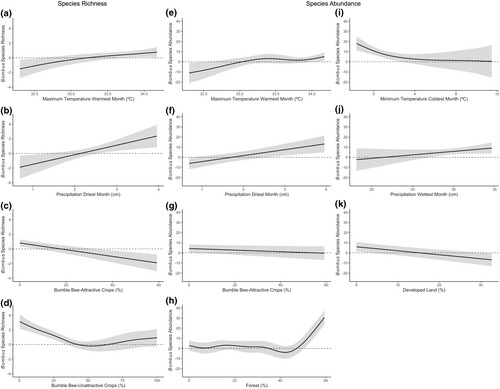
The generalized additive mixed model for the southeast geographic region determined that bumble bee species richness was significantly associated with maximum temperature of warmest month, precipitation of driest month, bumble bee-attractive crops, and bumble bee-unattractive crops (Tables 5 and 6). Meanwhile, bumble bee species abundances were significantly associated with maximum temperature of warmest month, minimum temperature of coldest month, precipitation of wettest and driest month, bumble bee-attractive crops, developed land, and forests (Tables 7 and 8). Based on partial effects plots, bumble bee species assemblages became more species rich and abundant as maximum temperature of warmest month increased to 34°C (Figure 5a,e). Bumble bee species assemblages also became richer and more abundant as total precipitation during the driest month increased (Figure 5b,f). Bumble bee species assemblages decreased slightly in richness and abundance with increasing bumble bee-attractive crops (Figure 5c,g). Further, bumble bee richness decreased as bumble bee-unattractive crops reached 50% within the surrounding area before plateauing as unattractive crop cover increased (Figure 5d). Meanwhile, bumble bee abundances decreased with increasing minimum temperature of coldest month and developed land, but increased with increasing forest land cover and precipitation of wettest month (Figure 5h–k).
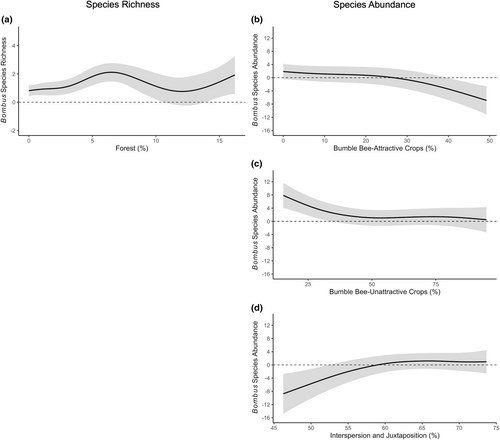
The generalized additive mixed model for the northern plains geographic region determined that bumble bee species richness was significantly associated with forests (Tables 5 and 6), whereas species abundances were significantly associated with bumble bee-attractive crops, bumble bee-unattractive crops, and interspersion and juxtaposition (Tables 5–8). Based on partial effects plots, bumble bee species richness increased with increasing forest land cover (Figure 6a). Bumble bee abundances decreased with increasing bumble bee-attractive and unattractive crops (Figure 6b,c). Further, as interspersion and juxtaposition increased, bumble bee species became more abundant (Figure 6d).
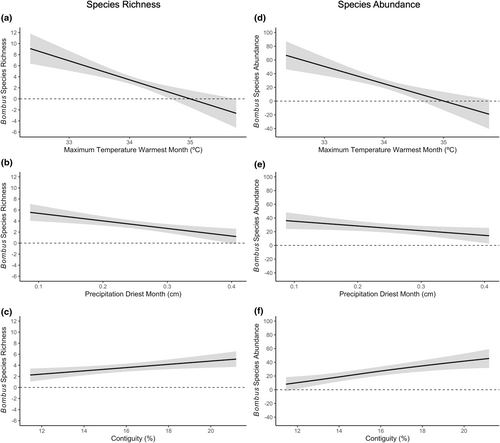
The generalized additive mixed model for the mountain geographic region determined that both bumble bee species richness and abundances were significantly associated with maximum temperature of warmest month, precipitation of driest month, and contiguity (Tables 5–8). Based on partial effects plots, bumble bee species assemblages (richness and abundance) decreased as maximum temperature of warmest month approached 36°C (Figure 7a,d). Further, as precipitation of driest month increased, bumble bees became less species rich and less abundant (Figure 7b,e). Meanwhile, bumble bee species assemblages became more species rich and abundant as contiguity approached 20% (Figure 7c,f).
4 DISCUSSION
In this study, we tested bumble bee responses to climate and land use by modeling species-specific habitat requirements, and assemblage-level responses across geographic regions. Similar to previous studies, we found that temperature, precipitation, and surrounding landscape structure significantly drive bumble bee assemblages in agricultural landscapes (Naeem et al., 2019). Distinct east versus west groupings emerged when evaluating species-specific habitat associations. These were further defined when assessing assemblage-level responses to climate and landscape by geographic region. In the eastern geographic regions (Corn Belt/Appalachian/northeast, southeast, and northern plains), bumble bee species richness and abundance increased with increasing maximum temperature of warmest month and precipitation of driest month, while bumble bee species richness and abundance in the mountain geographic region declined with the increase of these variables. Further, increasing amounts of forest land surrounding the agricultural fields resulted in more species-rich and abundant bumble bee assemblages, while bumble bee-attractive crops, bumble bee-unattractive crops, and developed land decreased bumble bee abundance and richness in the east. This substantiates our findings from the canonical correspondence analysis, which identified that eastern species were associated with higher levels of forest and lower levels of bumble bee-attractive crops, bumble bee-unattractive crops, and developed land. Overall, climate and land use combine to drive bumble bee assemblages, but how those processes operate is idiosyncratic and spatially contingent across geographic regions.
4.1 Bumble bee habitat associations
Species–environment associations identified that geographic region played a large role in species-specific habitat requirements, with distinct groupings in the east and west. In the east, species were associated with lower elevations along with increased minimum temperatures of coldest month, precipitation of wettest month, and forest land surrounding the agricultural fields. These associations make sense because the east typically has lower elevations, warmer winters, and more precipitation compared with the west. It is important here to note that the west is solely represented by Utah, an intermountain state characterized by high elevation, irrigated agricultural valleys. Therefore, results would likely be different if other western states (i.e., California, Oregon, Washington, and Colorado) were included in this dataset as climate and landscape differ widely when compared to Utah. In the west, Utah bees were associated with higher elevations along with increased levels of bumble bee attractive and unattractive crops, maximum temperatures of warmest month, and interspersion and juxtaposition. Again, from a geographic standpoint these associations make sense because the west has higher elevations, and warmer and drier summers than many of the eastern states, suggesting these bumble bees have adapted to these conditions. Further, the association of Utah bumble bee species with higher values of interspersion and juxtaposition suggests that these bumble bees are more abundant in landscapes that have a mix of land cover types that are well-dispersed (less contiguous) within the surrounding area. One species that emerged as being of particular interest is B. griseocollis, which was ubiquitous throughout the study areas, following its known distribution (Colla et al., 2011; Koch et al., 2012). This species may be more resilient to land cover and climate change as it is able to survive well throughout a range of habitat types (i.e., open farmland and fields, urban parks and gardens, and wetlands) and climates across the United States (Kingsolver et al., 2013; Koch et al., 2012; Williams et al., 2014). Overall, this model identified that geographic region impacted species-specific habitat requirements, emphasizing the importance of evaluating species on a regional scale.
4.2 Climate and landscape impact on bumble bee assemblages by geographic region
In the Corn Belt/Appalachian/northeast geographic region, bumble bee species richness and abundances were positively associated with maximum temperature of warmest month, precipitation of driest month, and forests, but negatively associated with minimum temperature of coldest month. Even as a cold-adapted genus, bumble bees can tolerate a wide range of temperatures and successfully fly and forage between 16 and 36°C (Couvillon et al., 2010). This likely explains the increase in bumble bee richness and abundance as temperatures approached 32°C during the warmest month. While this range is well below critical thermal maxima (Christman, Spears, Koch, et al., 2022; Oyen et al., 2016), it is important to note that temperature thresholds were not identified in this model. This is of significance as extreme heat can pose challenges for bumble bee survival, particularly if high temperatures persist for extended periods (Martinet et al., 2015, 2021; Rasmont et al., 2015). This concern is further confounded by the finding that increasing levels of precipitation during the driest month resulted in increased bumble bee abundance, meaning bumble bees were negatively associated with drier environments. Therefore, while bumble bees can tolerate desiccation, their ability to maintain their water content during droughts and/or heat waves may not be enough to tolerate these unprecedented pressures (Burdine & McCluney, 2019; Ferry & Corbet, 1996; Maebe et al., 2021; Nicolson, 2009). Of further concern, bumble bee richness and abundance decreased with increasing levels of minimum temperature of coldest month. This is likely a result of bumble bees being a cold-adapted genus that relies on the colder temperatures to slow their metabolism and delay their development during the overwintering stage (Denlinger, 2002; Hahn & Denlinger, 2007, 2011; Maebe et al., 2021; Vesterlund et al., 2014). As winter temperatures continue to increase because of climate change, this may put bumble bees that have insufficient fat reserves at risk or lead to phenological changes in emergence periods (Maebe et al., 2021). Further, warmer winters can lead to reduced snowpack, which can buffer overwintering bumble bee gynes from external temperatures via a layer of insulating snow (Domine et al., 2012). This loss in snowpack can cause bumble bees to experience increased soil temperature variation and cold beyond their thermal minima, especially if events such as polar vortexes occur, leading to increased overwintering mortality (Domine et al., 2012; Lindsay et al., 2024). Overall, these findings suggest that both warm, wet summers and colder winters are necessary for bumble bee assemblages to thrive. Further, bumble bee assemblages increased in terms of richness and abundance as forest land increased in the surrounding landscape. This is likely a result of forests providing abundant floral resources, nesting sites, resting locations, shelter from wind and predators, and favorable microclimate conditions (Betts et al., 2019; Everaars et al., 2011; Mola et al., 2021; Pfeiffer et al., 2019; Potts et al., 2005; Sõber et al., 2020; Sydenham et al., 2016). Overall, these results emphasize the importance of maintaining diverse forest habitats around agricultural fields on top of establishing targeted land management practices (i.e., restoring habitats, diversifying crops, and implementing cover cropping) to mitigate the impacts of climate change on bumble bees in agroecosystems (Prestele et al., 2021).
In the southeast, bumble bee species richness and abundance were positively associated with maximum temperature of warmest month, precipitation of driest and wettest month, and forests. Meanwhile, bumble bee assemblages were negatively associated with minimum temperature of coldest month, bumble bee-attractive and unattractive crops, and developed land. Many of the climatic trends observed in the Corn Belt/Appalachian/northeast geographic region also held true in the southeast. Specifically, bumble bee species became richer and more abundant as temperatures approached 34°C, which is within the 16–36°C temperature range in which bumble bees successfully fly and forage (Couvillon et al., 2010). However, temperatures at which bumble bees were found were higher in the southeast than in the Corn Belt/Appalachian/northeast region. While temperatures are historically hotter in the southeast than the Corn Belt/Appalachian/northeast region, potentially making bumble bees in this region more heat adaptive, this also makes rising temperatures more of a concern for the few species that are found within this region. Additionally, increased levels of precipitation during the driest and wettest months led to a rise in abundance and richness for bumble bee assemblages. Precipitation is necessary for plant growth, which can therefore increase the amount of available food resources for bumble bees, thereby increasing their prevalence. Precipitation can also help moderate extreme temperatures (Trenberth & Shea, 2005), providing more stable and favorable microhabitats for bumble bees. However, projected changes in precipitation within the southeast can lead to both drier environments or flooding, which are of concern for bumble bees via increased risk of desiccation, changes in floral phenology and abundance, disruption of foraging ability, and loss of nesting habitat (Burdine & McCluney, 2019; Ferry & Corbet, 1996; Goulson et al., 2018; Harder, 1986; Koch et al., 2019; Liczner & Colla, 2019; Maebe et al., 2021; Nicolson, 2009; Peat & Goulson, 2005; Sanderson et al., 2015). There was also an observed decline in both the richness and abundance of bumble bees with higher minimum temperature of coldest month. This preference for colder winter climates aligns with their cold-adaptive abilities and their reliance on colder temperatures during the overwintering stage of their life cycle (Denlinger, 2002; Hahn & Denlinger, 2007, 2011; Maebe et al., 2021; Vesterlund et al., 2014). Further, as stated above, forests adjacent to agricultural areas can enhance both the richness and abundance of bumble bees by offering diverse flowering plants, nesting sites, refuge, and favorable microclimates (Betts et al., 2019; Everaars et al., 2011; Mola et al., 2021; Pfeiffer et al., 2019; Potts et al., 2005; Sõber et al., 2020; Sydenham et al., 2016). Meanwhile, bumble bee-attractive crops, unattractive crops, and developed land reduced bumble bee richness and abundance. While bumble bee-attractive crops can provide additional, favorable floral resources for bumble bees, their impact may be similar to bumble bee-unattractive crops in that they create more continuous agricultural land, thereby reducing alternative resources for bumble bees. Additionally, while developed land can increase floral resources via roadside strips, residential gardens, community gardens, and city parks (Bennett & Lovell, 2019; Kelemen & Rehan, 2020; Martins et al., 2017; Wenzel et al., 2020), the impact can also be negative by reducing stable floral resources, diminishing under-ground and above-ground nesting sites, and increasing heavy metal contamination (Ahrné et al., 2009; Geslin et al., 2016; Glaum et al., 2017; Sivakoff et al., 2020). Overall, these findings further underscore the significance of preserving and restoring forests surrounding agricultural fields to provide alternative resources alongside implementing land management practices to lessen bumble bee declines in the face of climate change.
In the northern plains region, bumble bees species richness was positively associated with forests, while species abundances were negatively associated with bumble bee-attractive crops and bumble bee-unattractive crops. Observed landscape trends in the southeast regions were again documented in the northern plains region with forests providing additional, alternative resources for bumble bees (Betts et al., 2019; Everaars et al., 2011; Mola et al., 2021; Pfeiffer et al., 2019; Potts et al., 2005; Sõber et al., 2020; Sydenham et al., 2016) that are not available in bumble bee-attractive and unattractive crops. This can explain why increasing these crop types surrounding agricultural fields does not support abundant bumble bee populations. Further, the increase in these crops within the surrounding area may instead increase homogeneity, reducing crucial resources needed to support bumble bee assemblages within agricultural landscapes (Hall et al., 2017; Parys et al., 2021; Westphal et al., 2003; Williams et al., 2012). This is further supported by the finding that bumble bee abundances were positively associated with increased interspersion and juxtaposition. As landscapes became increasingly diverse and heterogenous, more bumble bees were abundant likely due to the increase in resources from surrounding forested habitats. Overall, incorporating diverse forest patches surrounding agricultural fields can contribute to the conservation of bumble bees by providing alternative floral and nesting resources (Mola et al., 2021; Pfeiffer et al., 2019; Sõber et al., 2020).
In the mountain region, bumble bee species richness and abundances were negatively associated with maximum temperature of warmest month and precipitation of driest month, but positively associated with contiguity. The canonical correspondence analysis identified that bumble bees were positively associated with maximum temperature of warmest month in Utah. However, the generalized additive mixed model provided more in-depth information, indicating that bumble bee richness and abundance declined as temperature rose from 32 to 36°C. While this temperature range is well below critical thermal maxima for many species (Christman, Spears, Koch, et al., 2022; Oyen et al., 2016), this is still a high-temperature range for cold-adapted bumble bees to sustain if held constant over long periods of time. This observed decline is also of concern given that temperatures are projected to continue to increase in Utah (Khatri & Strong, 2020), which could lead to a reduction in the richness and abundance of bumble bees. Additionally, increasing levels of precipitation of driest month resulted in less rich and abundant bumble bee assemblages. However, it is important to note that precipitation ranged from 0.1 to 0.4 cm during the driest month, indicating overall aridness. While this may suggest that bumble bees are adapted to dry environments, this could also be a product of land management practices within Utah. Utah is the second driest state in the United States (Utah Division of Emergency Management, 2022); therefore, much of the agricultural production heavily depends on irrigation (Barker et al., 2023). As such, the surveyed fields likely are not as dry as recorded in the models due to microclimates formed from these irrigation practices. This can explain why bumble bees assemblages are rich and abundant even in these increasingly dry environments. This emphasizes the importance of irrigated agricultural crops in Utah and other increasingly dry geographic locations as this practice is not only essential for increasing crop yields but is also seemingly imperative for supporting bumble bee assemblages. However, targeted research is needed to further parse out this relationship. It is important to note again that the mountain geographic region is represented by Utah, an intermountain state characterized by high elevation, irrigated agricultural valleys. Therefore, these results would likely differ if western states that experience higher levels of precipitation (i.e., Oregon and Washington) were included in this dataset. Further, increasing contiguity was associated with rich and abundant bumble bee assemblages. However, contiguity was low (11.5%–21.2%) throughout our study sites, meaning the landscapes were comprised of several different land cover types (Hesselbarth et al., 2019), which could increase the amount of floral and nesting resources available to bumble bees within the surrounding area. Future research identifying the specific land use types would provide a more fine-scale, localized assessment of the resources available to bumble bees.
4.3 Limitations
Although this study has some limitation inherent to its design (i.e., location of sites being monitored, state-level differences in sample size, collection dates and period, target pests, and agriculturally focused trapping sites), analyzing trap bycatch reduces associated costs by allowing more efficient use of time and resources while utilizing data that would otherwise be discarded (Spears et al., 2021). Further, this study's substantial spatial coverage and high number of replicates within and across years resulted in a large dataset that enriches our knowledge of bumble bee assemblages across geographic space and time (Kohler et al., 2020). However, it is important to remember that all these species were collected in agricultural fields, which influences the composition of species represented within these surveys. Regardless, the low proportion of singletons in this dataset indicates a strong sampling regime (Kohler et al., 2020; Williams et al., 2001). Finally, the inclusion of climatic and landscape composition and configuration variables into one model introduces sources of uncertainty but yields more realistic results about their cumulative effects on Bombus species assemblages (Conlisk et al., 2013; Louca et al., 2015).
4.4 Broad-scale management implications
This study further emphasizes the importance of conducting broad-scale surveys. From this study, we identified that B. pensylvanicus was one of the most abundant species collected. This is of particular interest from a conservation perspective because its numbers have drastically declined since 2000 (~89%), to the point that B. pensylvanicus is being evaluated to determine whether it warrants federal protection under the U.S. Fish and Wildlife Service Endangered Species Act (USFWS, 2021). While the finding that B. pensylvanicus was one of our most abundant species may be due to its populations increasing, it could also be a result of this species being more attracted to pest monitoring traps. Further research is needed to determine which, if either, is true since both have significant management implications. If the former is true, B. pensylvanicus populations may be rebounding, so listing under the Endangered Species Act may not be warranted. If the latter is true, having an influx of B. pensylvanicus captured passively as bycatch could contribute to its declining populations. Regardless, this finding highlights the need for interagency cooperation and research collaborations to understand impacts of pest monitoring traps on bumble bee species and to develop and implement recovery plans and protective regulations.
While research is underway to understand the broader impacts of pest monitoring traps on bumble bees at a national scale (Christman, Spears, Strange, et al., 2022; Spears et al., 2016, 2021; Spears & Ramirez, 2015), science policy and interagency collaboration is needed to make actionable change. With informed science policy, innovative management practices and mandates can be employed that meet multiple federal agency's goals, while also preventing the least amount of harm to agriculture, natural resources, and imperiled species. Further, given the diversity of insects captured within pest monitoring traps, improving policies and increasing interagency collaboration could protect a range of insect and plant species, ecosystem services, and habitats.
5 CONCLUSIONS
Overall, results from this study contribute to a better understanding of climate and landscape factors affecting bumble bees and their habitats throughout the United States. Climate and land use combine to drive bumble bee assemblages, but how those processes operate is idiosyncratic and spatially contingent across regions. More specifically, within the Corn Belt/Appalachian/northeast and southeast regions, bumble bee assemblages are richer and more abundant with increasing temperatures and precipitation. However, this leads to concerns for bumble bee assemblage persistence as thresholds are met with rising temperatures and lessening precipitation. Therefore, this not only emphasizes the need for continual research monitoring these species under new climate regimes but also highlights the importance of developing targeted land management practices to mitigate the effects of climate change on bumble bees. Additionally, within the Corn Belt/Appalachian/northeast, southeast, and northern plains regions, forest land was identified as being the most important land cover type to support rich and abundant bumble bee assemblages. This stresses the importance forests play in the future conservation of bumble bees by providing them with alternative floral and nesting resources. Similar to the east, continual monitoring and implementing targeted land management practices are necessary in the west. One practice, irrigation, may be of critical importance within Utah and other arid states. Irrigation practices may create favorable microclimates within agricultural fields for bumble bees by reducing the temperature and increasing humidity, emphasizing that these practices should continue to both increase crop yield (as intended) and support bumble bee assemblages (unintended). Further, maintaining diverse landscapes surrounding agricultural fields is important to increase the amount of floral and nesting resources available to bumble bees. Moreover, detailed knowledge of species-specific relationships and assemblage associations with climate and landscape variables across a broad geographic range is invaluable to improve targeted conservation and land management strategies to mitigate the effects of ongoing environmental changes.
AUTHOR CONTRIBUTIONS
Morgan E. Christman: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; software; validation; visualization; writing – original draft. Lori R. Spears: Conceptualization; funding acquisition; methodology; project administration; resources; supervision; writing – review and editing. Emily K. Burchfield: Conceptualization; formal analysis; methodology; software; supervision; writing – review and editing. William D. Pearse: Conceptualization; formal analysis; methodology; supervision; writing – review and editing. James P. Strange: Supervision; writing – review and editing. Ricardo A. Ramirez: Conceptualization; funding acquisition; methodology; project administration; resources; supervision; writing – review and editing.
ACKNOWLEDGMENTS
We thank Harold Ikerd for his assistance with data management; Soli Velez, Kami Lay, and Anna Fabiszak for their assistance with processing insects; Todd Gilligan, Brian Christman, Zachary Schumm, and the anonymous reviewers for invaluable comments that improved this manuscript; and the following state cooperators for providing bycatch samples: Larry Bledsoe, Bradley Danner, Eric Day, David Gianino, Mathew Howle, Janet Lensing, Laura Miller, Laurinda Romonda, and Gaye Williams. This work was made possible, in part by Cooperative Agreements AP18PPQFO000C100, AP19PPQFO000C269, AP19PPQS&T00C056, AP20PPQFO000C074, AP20PPQS&T00C065, and AP21PPQS&T00C056 from the United States Department of Agriculture's Animal and Plant Health Inspection Service (APHIS) and National Science Foundation Grant No. 1633756. Work may not necessarily express APHIS' views.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data and code supporting the findings of this study are available on Zenodo at https://doi.org/10.5281/zenodo.6363812.




