Long-term soil warming decreases soil microbial necromass carbon by adversely affecting its production and decomposition
Abstract
Microbial necromass carbon (MNC) accounts for a large fraction of soil organic carbon (SOC) in terrestrial ecosystems. Yet our understanding of the fate of this large carbon pool under long-term warming is uncertain. Here, we show that 14 years of soil warming (+4°C) in a temperate forest resulted in a reduction in MNC by 11% (0–10 cm) and 33% (10–20 cm). Warming caused a decrease in the content of MNC due to a decline in microbial biomass carbon and reduced microbial carbon use efficiency. This reduction was primarily caused by warming-induced limitations in available soil phosphorus, which, in turn, constrained the production of microbial biomass. Conversely, warming increased the activity of soil extracellular enzymes, specifically N-acetylglucosaminidase and leucine aminopeptidase, which accelerated the decomposition of MNC. These findings collectively demonstrate that decoupling of MNC formation and decomposition underlie the observed MNC loss under climate warming, which could affect SOC content in temperate forest ecosystems more widespread.
1 INTRODUCTION
Soil microbial necromass carbon (MNC) plays a crucial role as a precursor in the formation and stabilization of soil organic carbon (SOC), constituting over half of the SOC (Buckeridge, Creamer, & Whitaker, 2022; Liang & Amelung, 2019; Whalen et al., 2022). Despite its potential ecological significance, the effects of global warming on MNC and its subsequent fate in the soil has remained inadequately explored (Buckeridge, Creamer, & Whitaker, 2022; Cao et al., 2023). Empirical and theoretical evidence has demonstrated that global warming can directly or indirectly trigger changes in soil nutrient availability (such as nitrogen [N] and phosphorus [P] content) (Bai et al., 2013; Tian, Shi, et al., 2023), soil extracellular enzyme activity (Chen et al., 2018), microbial physiological traits, and the composition and function of soil microbial communities (Donhauser et al., 2021; Melillo et al., 2017). These changes, in turn, can significantly impact the accumulation of MNC in soils (Buckeridge, Creamer, & Whitaker, 2022; Kästner et al., 2021; Whalen et al., 2022). Hence, comprehending the dynamics of MNC in a warming world is critical to improve our mechanistic understanding of SOC cycling in a warmer world.
Soil MNC refers to the organic carbon (C) fraction in soils that derives from the remains of dead microbial cells, including cell walls, excreted compounds, and remnants of microbial cells and fragments (Buckeridge, Creamer, & Whitaker, 2022; Liang et al., 2019; Whalen et al., 2022). The fate and dynamics of MNC is determined by the balance between MNC formation and decomposition and their interaction with MNC (de)stabilization (Buckeridge, Mason, et al., 2022; Liang et al., 2019). MNC formation is controlled by microbial physiological traits including microbial C use efficiency (CUE, the proportion of microbial C taken up that is allocated to the synthesis of new biomass), microbial growth rate, and microbial turnover rate (Buckeridge, Creamer, & Whitaker, 2022; Kästner et al., 2021). Multiple studies support that microorganisms with higher CUE and microbial turnover rate are more favorable for the accumulation of MNC (Buckeridge, Creamer, & Whitaker, 2022; Kästner et al., 2021), but they may also promote MNC decomposition through priming effects (Craig et al., 2022). These studies therefore indicated that the relationship between microbial physiological traits and MNC is more complex. Moreover, these microbial physiological traits are affected by warming, as shown in laboratory and field settings (Purcell et al., 2022; Walker et al., 2018; Zhang et al., 2022). For example, microbial CUE may decrease due to increased microbial maintenance costs under elevated temperatures (Zhang et al., 2022). However, the relationships between microbial physiological traits and MNC under climate warming have remained unresolved.
Soil nutrient (i.e., N and P) availability might also be an important modulator of MNC dynamics. This is because N and P are essential nutrients for microbial growth, playing key roles in energy transfer, protein and nucleic acid synthesis, cell membrane structure, metabolic reactions, and signal transduction (Kuypers et al., 2018; Walton et al., 2023). Both nutrients therefore were shown to constrain the growth of microbes in many ecosystems. Recent studies on P effects have shown that the response of MNC to P addition varies across ecosystems, with increased MNC accumulation in a subalpine forest due to reduced decomposition (Luo et al., 2022), decreased accumulation in alpine grasslands due to enhanced decomposition (Ma et al., 2023), and increased recycling and MNC abundance in a tropical coastal forest (Yuan et al., 2021). These studies have primarily relied on fertilization experiments that artificially increase P availability through the addition of mineral P, but this may not accurately represent the conditions encountered by soil microbial communities in natural ecosystems. In addition, warming promotes the transformation of available P to occluded P (Siebers et al., 2017) and was shown to reduce soil total and labile P pools due to increased P outputs from soils via increased plant P uptake and downward transport of colloidal and particulate P (Tian, Shi, et al., 2023). However, the consistency of the relationship between MNC and soil P availability remains poorly understood when it comes to climate warming.
Soil extracellular enzyme activities (EEA) might also be important predictors of MNC dynamics by promoting MNC decomposition, therefore differing from the effects of microbial physiological traits and soil nutrients on MNC formation (Cao et al., 2023; Luo et al., 2022). MNC can be stabilized by interacting with the surfaces of soil minerals or aggregate formation, or it can be decomposed and utilized by live microorganisms (Buckeridge, Creamer, & Whitaker, 2022; Kästner et al., 2021; Liang et al., 2019). Soil enzymes, such as N-acetylglucosaminidase (NAG), break down microbial residues like fungal-derived chitin and bacterial-derived peptidoglycan (Joergensen, 2018). Decomposition of MNC provides nutrients in favorable amounts due to its resemblance to microbial biomass in elemental and chemical composition (Buckeridge, Creamer, & Whitaker, 2022; Whalen et al., 2022). Previous studies have indicated that enzymes in colder regions exhibit greater sensitivity to temperature increases compared to those in warmer regions, with soil EEA at higher latitudes showing a stronger response to rising temperatures than enzymes at lower latitudes (German et al., 2012; Koch et al., 2007), but the actual relationship between MNC dynamics and soil enzymes in response to climate warming is still poorly understood.
To this end, very little research has directly assessed the impacts of in situ warming on MNC contents and dynamics (Cai et al., 2023; Ding et al., 2019; Liang & Balser, 2012). For instance, Ding et al. (2019) found that the contribution of MNC to SOC increased with warming, due to facilitated microbial growth and proliferation in an alpine meadow on the Qinghai-Tibet Plateau. In contrast, warming decreased the contribution of MNC to SOC through accelerated degradation of amino sugars after 9 years warming in a Californian annual grassland ecosystem (Liang & Balser, 2012). These results clearly show that our knowledge on the warming response of MNC formation and stabilization are incomplete. Moreover, the few studies published were performed in grassland ecosystems (Cai et al., 2023; Ding et al., 2019; Liang & Balser, 2012), excluding forest ecosystems, with a predominant focus on shorter-term responses (Liang & Balser, 2012). However, short-term and long-term warming may be different in terms of their effect on soil nutrient availability, soil microbial community structure, and soil enzymes (Melillo et al., 2017; Romero-Olivares et al., 2017). For example, a 26-year long-term soil warming study conducted at the Harvard Forest LTER site (USA) revealed substantial reorganization of the soil microbial community, characterized by a decrease in the abundance of fungal biomarkers and a shift toward Gram-positive bacteria (Melillo et al., 2017; Metcalfe, 2017). Nonetheless, the roles of microbial traits (i.e., CUE, growth rates, and turnover), soil nutrient availability, and EEA in regulating MNC dynamics under long-term in situ warming have not yet been explored in temperate forests. Identifying these drivers may provide a more mechanistic understanding of how soil C sequestration can be enhanced under climate warming conditions.
Temperate forests encompass approximately 25% of the world's forested area and store around 22% of terrestrial forest soil C, making them crucial for regional soil C accounting (Bonan, 2008; Lal, 2005). The projected temperature increases of 1–2°C from 1990 to 2050 in temperate forests, with the climate becoming warmer and drier, poses a significant threat to the functioning of the soils in temperate forest ecosystems (Adams et al., 2019; Seidl et al., 2017). To our best knowledge, currently only two warming experiments have been operative in temperate forests for >10 years (Melillo et al., 2017; Tian, Schindlbacher, et al., 2023), but in none of those MNC dynamics were studied and published. To fill this knowledge gap, we investigated the response of MNC dynamics and the underlying processes to long-term soil warming (>14 years) in a temperate forest in Achenkirch, Austria. Our specific objectives were (1) to investigate how soil warming impacts the content of soil MNC and (2) to identify the key factors and processes that underlie the observed changes in MNC content. Our previous work found that long-term soil warming increased belowground plant C inputs (Heinzle et al., 2023; Kengdo et al., 2023) but decreased SOC content through increased EEA (Tian, Schindlbacher, et al., 2023) and decreased the soil P pools (Tian, Shi, et al., 2023). Therefore, we hypothesized that long-term warming reduced MNC as a matter of decreased soil P and soil microbial biomass and that warming promotes the decomposition of MNC by increased soil EEA.
2 MATERIALS AND METHODS
2.1 Experimental design
This study was conducted in the Achenkirch soil warming experiment, located in a temperate forest in the Austrian Alps (11°38′21″ E; 47°34′50″ N) at an altitude of 910 m. The area has a temperate continental climate, with an annual mean air temperature of 7°C and mean annual precipitation of 1493 mm (from 1988 to 2017). The soils in the area are classified as shallow Rendzic Leptosols, developed on dolomite bedrock, and the dominant tree species in the ~130-year-old forest is Norway spruce (Picea abies L. H. Karst.), with European beech (Fagus sylvatica L.), and silver fir (Albies alab Mill.) admixed.
The soil warming experiment was designed in a paired plot design. Twelve 2 m × 2 m plots were grouped into six blocks (pairs), established in 2004 (n = 3 blocks) and 2007 (n = 3 blocks). Two treatments, ambient temperature (control) and warming by 4°C, were assigned to the two plots of each block. Soil heating cables (Etherma, Salzburg, Austria) were installed at a soil depth of 3 cm, with 7.5 cm distance between cables, in the warming and the control plots (the control plots were not heated but cables installed to control for the disturbance effect). Soil temperature in the warming plots was maintained at a constant 4°C higher than in the paired control plots throughout the snow-free period (April–December). Soil temperatures were recorded at half-hourly intervals at depths of 5 and 15 cm below the soil surface. Further details about the site and the experimental setup can be found elsewhere (Kengdo et al., 2022; Schindlbacher et al., 2015; Tian, Shi, et al., 2023).
2.2 Soil sampling, soil parameters, and microbial biomass analysis
Soil samples were collected from the control and the warmed plots at the Achenkirch soil warming experiment in May, August, and October 2019. Seven randomized soil cores per plot were taken from depths of 0–10 and 10–20 cm using a 2.5-cm-wide hand auger. The soil depth increments within the same plot were combined and passed through a 2-mm sieve. Measurements of important soil physicochemical and biological properties (including microbial biomass, soil nutrients, soil enzymes, microbial growth, and turnover) were performed on the same samples, and the data published recently (Tian, Schindlbacher, et al., 2023; Tian, Shi, et al., 2023) and used here. The measurement techniques are therefore only shortly depicted below.
Soil water content was determined gravimetrically by drying aliquots of 5 g of fresh soil at 105°C for 48 h. Soil pH was measured using an ISFET pH sensor (Sentron, The Netherlands) in a 1:5 (w:v) slurry of air-dried soil and ultra-pure water. To determine soil organic C, acid pretreatment (2 M HCl) was carried out to remove carbonates (dolomite), followed by CN analysis using an elemental analyzer (Carlo Erba EA1110, Thermo Fisher, USA). and were extracted with 1 M KCl (1:7.5 [w:v]) and determined colorimetrically. Dissolved organic N was calculated as the difference between total dissolved N (TDN, determined by a Shimadzu TOC/TN analyzer) and the sum of and .
Soil microbial biomass C was determined by the chloroform-fumigation extraction method (Vance et al., 1987). Total dissolved organic C in 0.5 M K2SO4 extracts of fumigated and non-fumigated soils were measured using a dissolved organic C analyzer (Shimadzu TOC/TN analyzer), and microbial biomass C was calculated as the difference in dissolved organic C between the fumigated and unfumigated samples using a conversion factor (kc) of 0.45 (Joergensen, 1996).
To determine bioavailable phosphorus (P) in the form of Olsen inorganic P (Olsen Pi) and Olsen organic P (Olsen Po), soil bicarbonate extraction (0.5 M NaHCO3, pH 8.5, 1:7.5 w:v) and the malachite green method were used (D'Angelo et al., 2001; Rowland & Haygarth, 1997), employing (or not) the acid persulfate digestion method for Olsen total P (Olsen Pt). The ignition method (450°C for 5 h) followed by 0.5 M H2SO4 extraction was used to determine soil total P (TP), total inorganic P (TIP), and total organic P (TOP) by difference (Kuo, 1996).
2.3 Soil microbial activity
Four hydrolytic enzymes (β-glucosidase [BG], N-acetylglucosaminidase [NAG], leucine aminopeptidase [LAP], and acid phosphatase [AP]) were measured by the fluorescent substrate addition method described by Kaiser et al. (2010). Soil samples (1 g) were sonicated in sodium acetate buffer (100 mM, pH 5.5) and assayed with 4-methylumbelliferyl-β-d-glucopyranoside for BG, 4-methylumbelliferyl-N-acetyl-β-d-glucosaminide for NAG, l-leucine-7-amido-4-methylcoumarin hydrochloride for LAP, and 4-methylumbelliferyl-phosphate for AP.
2.4 Soil amino sugar analysis
Soil amino sugar hydrolysis and quantification followed Zhang and Amelung (1996) and Salas et al. (2023). Aliquots (0.5 g) of air-dried soil were hydrolyzed with 10 mL 6 M HCl at 105°C for 8 h. The hydrolysates were filtered through cellulose acetate filter membranes (Sartorius, Goettingen, Germany) into 20 mL scintillation vials, evaporated to dryness with an N2 dryer, and then re-dissolved in 12 mL Milli Q water. The hydrolysates were transferred into 50 mL falcon tube, and the pH was adjusted to 6.6–6.8 with 1 M KOH. The samples were then centrifuged (1600g) for 15 min to remove iron precipitates and the supernatant freeze-dried. This residue was dissolved in 8 mL methanol and cleared by centrifugation (1600g) for 10 min. The methanol supernatant was transferred to a 10 mL (BPA-free) cryovial (Simport, polypropylene T310-10A) and dried in a N2 dryer. The samples were re-dissolved in 1 mL Milli Q water. Amino sugars in samples were prepared for LC–MS analysis via 1-methyl-3-phenyl-2-pyralozone (PMP) derivatization, as described by Salas et al. (2023). In brief, samples were derivatized using a 0.5 M PMP solution and the reaction was conducted in a water bath at 70°C. Following the reaction, formic acid was added for neutralization. To eliminate excess PMP, a liquid–liquid extraction with chloroform and water was performed. The resulting aqueous layer was carefully filtered through a 0.2 μm acetate membrane syringe filter (VWR International™). For analytical assessment, the filtered samples were introduced into a UPLC Ultimate 3000 system coupled to an Orbitrap Q Exactive HRMS system. Chromatographic separation was carried out on a Waters AccQ.Tag Ultra C18 column, and the mass spectrometer was operated in ESI positive mode, scanning a mass range from 150 to 1000 m/z.
2.5 Data analysis
Statistical analysis was performed in R statistical software (version 4.2.3). Before conducting further analyses, we assessed the homoscedasticity and normality of the data for MNC, FNC, BNC, FNC-to-BNC ratio, and their contributions to SOC, and applied log or sqrt transformations if necessary. To investigate the main effects of season, depth, and warming and their interactions, we used mixed-effects models with warming, depth, and season as fixed factors, and block and warming duration as random factors. Given the significant interactions we identified between warming and depth for both FNC and MNC variables, we proceeded with pairwise comparisons. Additionally, we assessed the impact of warming on MNC, FNC, BNC, and their contribution to SOC by using paired t-tests. For these tests, we set the significance level at α = .05.
Residuals from a mixed effects model, considering season and depth as fixed factors and block and warming duration as random factors, were used to eliminate the influence of season and depth on soil N, soil P, microbial traits, EEA, and MNC. Only the residual data were then employed for multiple regression models and linear regression analysis. We used multiple regression models to examine the combined effects of soil N, soil P, microbial traits, and EEA on MNC, as well as its two constituent components (BNC and FNC). To identify the most concise model that explains the variation in MNC best, we constructed four competing models, which consider an increasing level of biological complexity, to identify the set of predictors that provide the most parsimonious model for explaining the variation in MNC. (1) a “soil nutrient” model (includes soil , , dissolved organic N, total inorganic P, total organic P, Olsen organic P, and Olsen inorganic P); (2) a “microbial trait” model (includes microbial biomass C, CUE, and microbial turnover time); (3) a “EEA” model (includes three hydrolytic enzymes, BG, LAP, and AP); and (4) full model (includes all the predictors).
Initially, we used a backward stepwise regression procedure using R software to determine the best-fitting models with the lowest AICc values. Starting with the full model, we successively removed variables that had the least significant impact on the AICc. Next, based on the best model selected from the initial models (1–4), we conducted a model-averaging procedure using AICc (with ΔAICc <2) to determine the parameter coefficients for the most suitable final set of predictors for MNC. This procedure was performed using the “dredge” function in the R package Multi-Model Inference (MuMIn). All predictors and response variables were standardized prior to analysis, employing the Z-score method to interpret parameter estimates on a comparable scale. To assess the relative importance of the predictors as drivers of MNC, we calculated the relative effect of the parameter estimates for each predictor in comparison with the effect of all parameter estimates in the models. Finally, all figures were prepared by R statistical software (version 4.2.3) and/or Adobe Illustrator 2021.
3 RESULTS
3.1 Soil microbial necromass carbon response to long-term soil warming
The linear mixed effects models revealed that long-term soil warming had significant negative effects on the bacterial necromass C (BNC), fungal necromass C (FNC), and MNC, but exerted no significant effect on the FNC-to-BNC ratio (Figure 1; Table 1). Both season and soil depth exhibited significant effects on BNC, FNC, MNC, and the FNC/BNC ratio. However, soil depth did not have a significant effect on BNC. Interestingly, none of the measured necromass parameters showed significant interactions between warming and season, highlighting that the observed warming effects were consistent across sampling periods. However, both FNC and MNC exhibited significant interactions between warming and soil depth suggesting that the effect of warming on FNC and MNC is contingent upon depth (Table 1). Pairwise comparisons revealed that warming had a more pronounced effect on FNC and MNC at 10–20 cm soil depth compared to 0–10 cm depth (Figure 1). In the warming plots, the MNC content decreased by 11% (marginally significant, t = 1.75, p = .09) and BNC content dropped by 32% (t = 3.53, p = .002) at a soil depth of 0–10 cm, compared to the control plots (Figure 1a,c). Conversely, at 10–20 cm soil depth, the content of BNC, FNC, and MNC declined by 30%, 36%, and 33%, respectively (Figure 1e–g). The FNC to BNC ratio at 0–10 cm depth was 1.79 in the control and 2.22 in the warming treatment (Figure 1d). In contrast, at 10–20 cm depth, the ratios were closely aligned, with 1.67 for the control and 1.53 for the warming plot (Figure 1h).
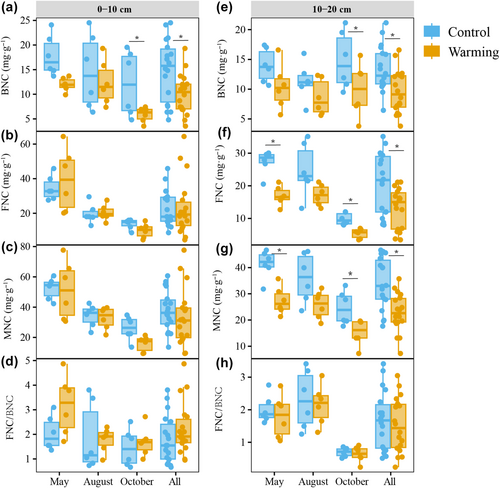
| BNC | FNC | MNC | FNC/BNC | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| Warming | 28.708 | <.001 | 6.105 | .016 | 24.644 | <.001 | 0.795 | .376 |
| Season | 4.474 | .015 | 65.611 | <.001 | 70.563 | <.001 | 18.224 | <.001 |
| Depth | 1.479 | .228 | 18.376 | <.001 | 20.301 | <.001 | 6.107 | .016 |
| Warming × Season | 1.347 | .267 | 0.071 | .931 | 0.492 | .614 | 1.133 | .328 |
| Warming × Depth | 0.430 | .514 | 8.002 | .006 | 4.981 | .029 | 3.176 | .079 |
| Season × Depth | 7.484 | .001 | 10.684 | <.001 | 11.382 | <.001 | 6.423 | .003 |
| Warming × Season × Depth | 0.258 | .773 | 2.238 | .115 | 1.706 | .189 | 1.469 | .237 |
- Abbreviations: BNC, bacterial necromass carbon; FNC, fungi necromass carbon; MNC, microbial necromass carbon. Bold values denote statistical significance at p < .05 level.
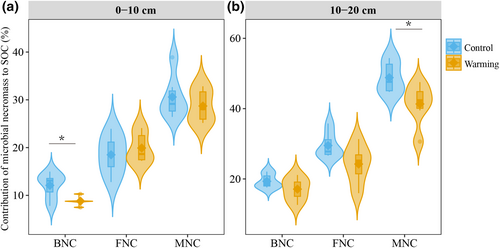
Overall, the contribution of BNC to SOC (BNC/SOC) was significantly lower in warming plots than in control plots (t = 3.11, p = .006, Figure 2a) at 0–10 cm depth. In contrast, soil warming did not affect FNC/SOC and MNC/SOC at 0–10 cm depth. At the 10–20 cm depth increment, the contribution of MNC to SOC decreased by 14% due to soil warming (t = 1.98, p = .04, Figure 2b), but there were no observable effects of warming on FNC/SOC and BNC/SOC (Figure 2b).
3.2 Identification of biogeochemical predictors for microbial necromass carbon and its components
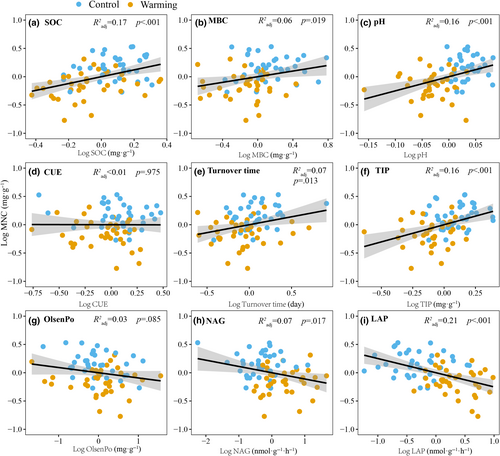
We used two statistical approaches (multiple regression models and partial linear regression analysis) to identify the most important predictors of MNC, as well as of its two components, FNC and BNC. Among the parameters we tested microbial traits (microbial biomass C, CUE, and microbial turnover time), soil phosphorus (P) pools (total inorganic P, total organic P, Olsen organic P, and Olsen inorganic P), soil N pools (, , and dissolved organic N), and soil extracellular enzyme activities (EEA). Linear regression analysis showed that MNC, BNC, and FNC were positively correlated with SOC, soil pH, microbial biomass C, microbial turnover time, and total inorganic P, while negatively with soil N-acetylglucosaminidase (NAG) and leucine aminopeptidase (LAP) activity (Figure 3; Figure S1). However, soil MNC, BNC, and FNC were not directly related to microbial CUE and Olsen organic P (Figure 3; Figure S1).
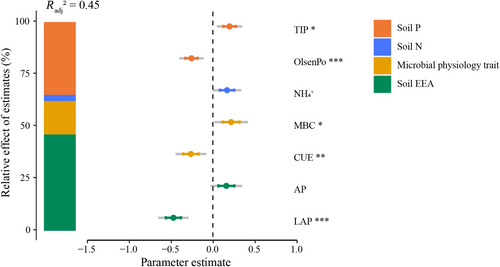
The optimal multiple regression model explaining MNC included , total inorganic P, Olsen organic P, microbial biomass C, CUE, acid phosphatase (AP), and LAP (Figure 4). Within the selected Akaike information criterion threshold (ΔAICc ≤2), we found the full model to explain a high proportion of the variance in MNC (adjusted R2 = 0.45, Figure 4; Table S1). Soil N, soil P, microbial physiological traits, and EEA were responsible for 3.3%, 34.6%, 13.2%, and 48.9% of the explained variance in MNC, respectively (Figure 4). Both FNC and BNC shared similar predictors, though their relative importance differed (Figure S2). For FNC and BNC, both the optimal models conferred to model structure 4 that includes soil P and EEA (Figure 2a,b). The BNC model had TIP, OlsenPo, AP, and LAP as the strongest predictors, which explained 22% of the variation (Figure 2a). Soil P and EEA were responsible for 35% and 65% of the explained variance in BNC, respectively. In the FNC models, the strongest predictors were total inorganic P, Olsen organic P, β-glucosidase (BG), and LAP, which explained 24% of the variation (Figure 2a). Soil P and EEA were responsible for 46% and 54% of the explained variance in the FNC model, respectively. Interestingly, we found that adding microbial traits to the multiple regression models did not improve the model performance in the BNC and FNC models (Figure S2; Table S1). We also statistically tested direct and indirect effects of warming on MNC through microbial functional traits using structural equation models (SEM), but all tested SEM models did not work out properly as the model structures used did not comply to the test parameter values commonly used to indicate a successful SEM (e.g., χ2 test, standardized root mean square residual). However, we found a significant positive correlation between microbial CUE, microbial turnover time, and microbial biomass C (Figure S3c,d). Microbial CUE was positively correlated with total organic P (Figure S3a), while microbial turnover time was positively correlated with total inorganic P and total organic P (Figure S3b). Therefore, part of the negative warming effects on MNC were likely indirect, through negative effects of soil P limitation on microbial biomass C and CUE and thereby on MNC.
4 DISCUSSION
An improved mechanistic understanding of how longer-term soil warming affects MNC dynamics can be a cornerstone toward improved soil C sequestration predictions. Our analysis considers the impact of long-term soil warming on soil microbial traits (microbial biomass C, CUE, and turnover time), soil nutrients (N and P), and soil EEA to provide a depth-informed understanding of MNC dynamics. Consistent with our hypotheses, we found that MNC and its components decreased (by 10%–30%) at 0–10 and 10–20 cm soil depth under long-term soil warming (Figure 1; Table 1). This is in line with the findings of Liang et al. (2015), who observed a 53% decrease in total amino sugar concentrations following 9 years of experimental warming in a dry California annual grassland ecosystem. Long-term soil warming affected MNC in the studied temperate forest through two mechanisms (Figure 5): (i) decreased soil-available P limits microbial biomass production (growth), reducing CUE and microbial biomass C, leading to decreased MNC production; and (ii) increased soil EEA promote the decomposition of soil organic matter and MNC, leading to increased MNC depolymerization and mineralization.
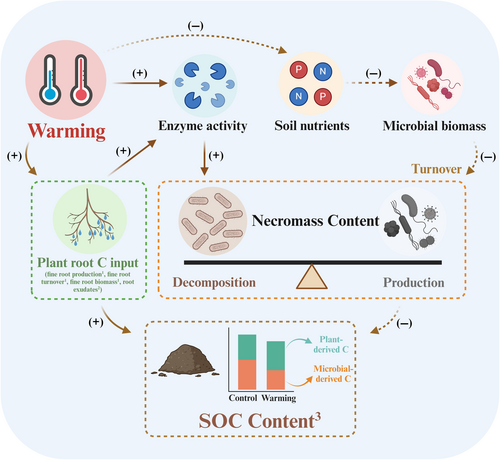
4.1 Warming decreased microbial necromass carbon production by reducing available soil P
Quantifying MNC production in soils presents significant challenges due to its diffuse nature and limitations of available methods (Buckeridge, Creamer, & Whitaker, 2022; Camenzind et al., 2023; Kallenbach et al., 2016). However, microbial physiological traits, such as microbial turnover time, microbial biomass C, and CUE, can serve as indirect indices of MNC production and provide valuable insights into MNC dynamics (Cotrufo et al., 2013; Kallenbach et al., 2015, 2016; Prommer et al., 2020). In this study, linear regression and multiple regression models showed that MBC was positively related to MNC (Figures 3b and 4), which is consistent with other studies (Jia et al., 2022; Prommer et al., 2020; Wang, Qu, et al., 2021). At our site, the microbial biomass C content declined by 22% after 14 years of soil warming (Tian, Schindlbacher, et al., 2023), which indicates that the yield of MNC declined because MNC originates from the decomposition and breakdown of C-rich organic molecules in dead microbial cells (Camenzind et al., 2023). The decrease in microbial biomass C content observed in the warming plot was in part associated with a decline in CUE, as indicated by previous research at the site (Tian, Schindlbacher, et al., 2023). This relationship was corroborated by our regression analysis depicting a positive relationship between microbial biomass C and CUE (Figure S3c). This suggests that soil microbes tend to channel more C toward respiration for energy provisioning rather than directing toward growth investments when environmental conditions disfavor growth and biomass accumulation, reducing microbial CUE and biomass concurrently.
Interestingly, the relationship between MNC and CUE displayed inconsistency in our regression analysis (Figures 3d and 4). Linear regression analysis (Figure 3d) did not reveal a significant correlation, while multiple regression models (Figure 4) indicated a negative relationship. It was not surprising that CUE and MNC showed different or no relationships in both regression models, given that multiple regression models reflect the concurrent effects of multiple parameters and their interaction (direct and/or indirect effects) on MNC. This further suggests that the effects of CUE on MNC may be indirect and influenced by other factors, such as soil P availability and soil EEA (see below for discussion). In addition, we observed that microbial physiological traits (microbial biomass C, CUE, and turnover time) were not included in the optimal model to explain specific BNC and FNC dynamics (Figure S2b). Accordingly, microbial traits alone were not a good predictor for BNC and FNC dynamics in this study, which was also partially supported by linear regression analysis that showed no significant direct relationship of CUE with BNC or FNC. The inability of microbial physiological traits to account for variations in BNC and FNC implies that community-level parameters alone are insufficient to explain the dynamics of microbial necromass in temperate forests. This could partially be attributed to the differences in CUE between bacteria and fungi, with fungi generally exhibiting higher CUE than bacteria (Silva-Sánchez et al., 2019; Soares & Rousk, 2019), and fungi often being dominant in forest ecosystems. This result underscores the critical importance of evaluating microbial physiological traits at more refined than bulk microbial level to predict microbial necromass dynamics in future research.
Another possible reason for reduced MNC formation with warming could be the indirect effect of warming on soil-available P. Several in situ P addition experiments have examined the influence of soil-available P on MNC (Luo et al., 2022; Ma et al., 2023; Yuan et al., 2021). The results of these studies have produced inconsistent findings, largely because the impact of P addition on MNC was contingent upon the ecosystem type and the pre-existing soil P condition. Both linear regression and multiple regression models showed that MNC (Figures 3b and 4), BNC (Figures S1e and S2a), and FNC (Figures S1e and S2b) were positively correlated with soil total inorganic P, while soil total inorganic P contents declined after 14 years soil warming (Tian, Shi, et al., 2023). This decrease in soil total P pools was likely due to long-term warming effects that increased net plant P uptake and enhanced the downward transportation of colloidal and particulate P, resulting in higher losses of P from soils (Tian, Shi, et al., 2023). These results indicate that soil-available P plays a pivotal role in governing the reduced MNC formation under soil warming conditions. As mentioned above, there is a strong positive relationship between MNC and microbial biomass C, as shown here and elsewhere (Wang, An, et al., 2021). Additionally, soil P availability is an important moderator of microbial growth. For example, the addition of P significantly increased the microbial biomass in old-growth tropical forests where P availability was low, while P addition had no effect on the microbial biomass in pine forests where P availability was high (Liu et al., 2012). Therefore, adequate P supply is essential for microorganisms due to its key roles in genetic encoding (DNA and RNA), energy transfer (e.g., ATP), cell structure (phospholipids), metabolism, and signaling (Kuypers et al., 2018; Walton et al., 2023). The critical role of P availability gains further substantiation from correlated research carried out at the same forest experimental site, indicating that sustained warming prompted a shift in soil microorganisms from being primarily C-limited to experiencing co-limitation by both C and P (Shi et al., 2023). This shift was attributed to an aggravation of abiotic P immobilization within warming soil environments and increased long-term soil P losses (Tian, Shi, et al., 2023). In addition, Luo et al. (2022) showed that P enhances the effectiveness of microbial N use and reduces the synthesis of N-acquiring enzymes, thereby decreasing unnecessary consumption and reducing the decomposition of microbial necromass. Conversely, reducing P availability appears to increase the synthesis of N-acquiring enzymes, potentially enhancing the decomposition of microbial necromass. In our study, soil warming reduced soil P availability, increased the activity of N- and P-acquiring enzymes (i.e., LAP and NAG, and AP) (Tian, Schindlbacher, et al., 2023; Tian, Shi, et al., 2023) and thereby accelerated the decomposition of MNC, as discussed below. Moreover, thus far mining of MNC for nutrients has been mostly discussed in terms of N gain, due to the high N content of microbial cell wall fragments containing peptidoglycan and chitin, as well as cell wall-bound proteins. However, microbial necromass does not only represent cell wall-bound residues (as indicated by e.g. amino sugars), but also substantial yet unknown contributions of intracellular and extracellular residues (Shi et al., 2024). Intracellular residues certainly come with a high content of DNA and RNA, though they are thought to turn over rapidly. Extracellular polymeric compounds (EPS) consist of proteins, polysaccharides, lipids, and DNA (eDNA), with exopolysaccharides often colocalizing with eDNA in biofilms. Bacterial cell walls can also contain a substantial fraction of organic P in the form of teichoic acids, representing polymers consisting of repeating polyol phosphate units occurring in most Gram-positive bacteria (Shiraishi et al., 2016). Though not investigated in a targeted way thus far increased decomposition of microbial (cellular, cell wall, and extracellular) necromass may greatly benefit soil microbes in terms of P nutrition under P-limiting conditions.
4.2 Warming accelerates microbial necromass carbon decomposition by stimulating soil EEA
In line with MNC production, the effects of warming on MNC decomposition received less attention due to limitations in methodological approaches (Buckeridge, Mason, et al., 2022; Wang et al., 2020). Hence, soil EEA frequently serve as an indicator for assessing the decomposition of MNC in numerous studies (Cai et al., 2023; Luo et al., 2022; Ma et al., 2023). For instance, increased soil EEA, such as β-glucosidase (BG), NAG, and LAP, induced by nutrient addition (i.e., N and P), accelerated the decomposition of microbial necromass and hindered its accumulation in both an alpine grassland of the Tibetan Plateau (Ma et al., 2023) and in tropical forest ecosystems (Yuan et al., 2021). Warming-induced increases in soil EEA have been observed in numerous field experiments and in meta-analysis studies (Cai et al., 2023; Chen et al., 2018; Fanin et al., 2022). This result is supported by previous research conducted at the same site and during the same soil sampling campaigns (Tian, Schindlbacher, et al., 2023). We found a negative relationship between LAP and NAG activities and MNC (Figures 3h,i and 4), as well as with its components BNC and FNC (Figures S1h,i and S2). This result suggests an increased microbial effort toward decomposing microbial necromass under long-term soil warming. The stimulation of EEA can, in part, be attributed to soil microbes facing increased limitations in C and/or P in the warming plots compared to the ambient plots, as discussed earlier (Shi et al., 2023; Tian, Schindlbacher, et al., 2023). Consequently, microbes allocate more C to enzyme production for mining or recycling microbial necromass because microbial necromass exhibits lower C:nutrient ratios and higher nutrient contents compared to plant tissue, a phenomenon particularly evident under conditions of nutrient scarcity (Buckeridge, Creamer, & Whitaker, 2022; Cui et al., 2020).
Furthermore, changes in soil pH may also play a role in reducing MNC stabilization (Buckeridge, Creamer, & Whitaker, 2022; Cai et al., 2023; Ma et al., 2023). Lower pH levels can cause minerals to dissolve, diminishing their capacity to stabilize MNC (Buckeridge, Creamer, & Whitaker, 2022; Kästner et al., 2021). This, in turn, leads to the presence of more unprotected MNC that can be more readily decomposed and utilized by microbes than mineral-bound MNC. The positive correlation between MNC and pH supports this perspective, though the soil pH values varied across a small range only (pH 6.6–7.1) (Figure 3c). Thus, whether lower pH levels in the warming plots (Tian, Schindlbacher, et al., 2023) can potentially enhance the desorption of mineral-associated MNC remains to be demonstrated. In addition, increased root biomass triggered by soil warming may enhance MNC decomposition through greater root activity and a 30% rise in root exudation, especially organic acids that can release carbon from mineral-bound MNC (Heinzle et al., 2023; Keiluweit et al., 2015; Kengdo et al., 2022, 2023). Organic acid exudation potentially accelerates MNC breakdown by stimulating rhizosphere priming processes (Buckeridge, Creamer, & Whitaker, 2022; Kästner et al., 2021; Keiluweit et al., 2015).
4.3 Warming reduced the microbial necromass carbon contribution to SOC
In our study, the MNC contribution to SOC was found to decrease from 34%–37% in control plots to 23%–33% in warmed plots, with a more significant decline in the 10–20 cm layer compared to the 0–10 cm layer (Figure 2). This suggests that warming accelerated the decomposition of MNC more strongly than that of bulk soil organic matter. This phenomenon is likely influenced by multiple factors such as enhanced microbial mining of MNC for nutrients coupled with increased turnover rates of amino sugars and increased plant C inputs via root turnover leading to more plant-derived organic matter, which partly offset SOC losses. Firstly, as mentioned above, soil microbes in control plots were limited by C, which shifted toward C–P co-limitation after 14 years of soil warming (Shi et al., 2023). In the context of nutrient scarcity, living microbes accelerate the mining of microbial necromass as a source of C and N (and P), due to its high proportions of lipids and proteins (Cui et al., 2020; Kögel-Knabner, 2002), and of nucleic acids (Makino et al., 2003). This process may consequently increase the turnover rate of microbial necromass biomarkers, such as amino sugars. For example, the turnover rate of the amino sugars was found to be faster under elevated CO2 (Glaser et al., 2006). Secondly, our previous studies have confirmed enhanced plant root C input in warming plots, due to increased root growth/turnover and root exudation (Heinzle et al., 2023; Kengdo et al., 2022, 2023), indicating a shift in the balance of plant- versus microbial-derived SOM under climate warming. Complementing this, Feng et al. (2008) reported an increased accumulation of cutin monomers in a mixed temperate forest in southern Ontario, Canada, due to enhanced litter degradation under warming. Consequently, the assumption that MNC belongs to the stable SOM pool under climate warming is challenged, despite its contribution of more than 30% to SOC in our study (Figure 2). Regardless, these findings align with the concept that the stability of SOC is not an inherent trait, but rather depends on ecosystem properties that vary with soil depth. This study offers essential insights into understanding the long-term fate of MNC relevant to C sequestration strategies.
There is evidence that warming preferentially increased the decomposition of BNC fractions in SOC compared to FNC at 0–10 cm depth. This can be attributed to the following reasons: First, in comparison with fungal necromass, which typically has an average C:N ratio of 10, microbes preferentially use bacterial necromass (average C:N of 4) (Paul, 2014). Bacterial necromass thus constitutes a more nutrient-rich resource (including N and P), making it nutritionally favorable for microbial growth (Paul, 2014; Sinsabaugh et al., 2016). Secondly, fungal cell walls are made from chitin and often have relatively high contents of melanin (Fernandez & Koide, 2014), a relatively recalcitrant organic compound class (Buckeridge, Creamer, & Whitaker, 2022; Joergensen, 2018). These components can make fungal cell walls more resistant to decomposition in soils compared with bacterial cell walls. BNC accounted for approximately 30% of MNC in this study (Figure 2). As a result, a decrease in BNC content, such as the observed 32% decline in the warming plots, would have a significant impact on its contribution to SOC.
5 CONCLUSION
Collectively, our results indicate that 14 years of soil warming at +4°C in a temperate forest has significantly reduced the MNC content and its contribution to SOC, which is linked to changes in microbial traits, soil P availability, and EEA. Specifically, our results suggest that the reduction in MNC due to long-term soil warming is primarily attributed to a decrease in its production and an increase in its decomposition, driven by reduced soil-available P limiting microbial biomass biosynthesis and CUE, coupled with enhanced soil EEA indicating accelerated MNC decomposition. This demonstrates a decoupling of MNC production and decomposition, which represents a potential mechanism for reduced accumulation of MNC in temperate forest soil in a warming world. Overall, this is the first evidence of a causal link between microbial physiology, soil nutrient availability, soil enzymes, and MNC dynamics in the context of climate warming.
AUTHOR CONTRIBUTIONS
Xiaofei Liu: Investigation; methodology; data curtation; visualization; writing – review and editing. Ye Tian: Investigation; methodology; writing – review and editing. Jakob Heinzle: Investigation; writing – review and editing. Erika Salas: Methodology; writing – review and editing. Steve Kwatcho-Kengdo: Investigation; writing – review and editing. Werner Borken: Conceptualization; investigation; writing – review and editing. Andreas Schindlbacher: Conceptualization; investigation; project administration; writing – review and editing. Wolfgang Wanek: Conceptualization; data curation; investigation; methodology; supervision; visualization; writing – review and editing.
ACKNOWLEDGMENTS
We sincerely thank Christian Holtermann for his invaluable contributions to maintaining the soil warming system in the field site.
FUNDING INFORMATION
This study was funded by the Austrian Science Fund (FWF; project I 3745) and China Scholarship Council (CSC) Grant (202008350128).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
All data used in this study are available at the Figshare https://doi.org/10.6084/m9.figshare.24566494.v1.




