Aridity threshold for alpine soil nitrogen isotope signature and ecosystem nitrogen cycling
Abstract
Determination of tipping points in nitrogen (N) isotope (δ15N) natural abundance, especially soil δ15N, with increasing aridity, is critical for estimating N-cycling dynamics and N limitation in terrestrial ecosystems. However, whether there are linear or nonlinear responses of soil δ15N to increases in aridity and if these responses correspond well with soil N cycling remains largely unknown. In this study, we investigated soil δ15N and soil N-cycling characteristics in both topsoil and subsoil layers along a drought gradient across a 3000-km transect of drylands on the Qinghai–Tibetan Plateau. We found that the effect of increasing aridity on soil δ15N values shifted from negative to positive with thresholds at aridity index (AI) = 0.27 and 0.29 for the topsoil and subsoil, respectively, although soil N pools and N transformation rates linearly decreased with increasing aridity in both soil layers. Furthermore, we identified markedly different correlations between soil δ15N and soil N-cycling traits above and below the AI thresholds (0.27 and 0.29 for topsoil and subsoil, respectively). Specifically, in wetter regions, soil δ15N positively correlated with most soil N-cycling traits, suggesting that high soil δ15N may result from the “openness” of soil N cycling. Conversely, in drier regions, soil δ15N showed insignificant relationships with soil N-cycling traits and correlated well with factors, such as soil-available phosphorus and foliage δ15N, demonstrating that pathways other than typical soil N cycling may dominate soil δ15N under drier conditions. Overall, these results highlight that different ecosystem N-cycling processes may drive soil δ15N along the aridity gradient, broadening our understanding of N cycling as indicated by soil δ15N under changing drought regimes. The aridity threshold of soil δ15N should be considered in terrestrial N-cycling models when incorporating 15N isotope signals to predict N cycling and availability under climatic dryness.
1 INTRODUCTION
Nitrogen (N) is one of the most limiting nutrients to terrestrial ecosystem productivity. Understanding N cycle dynamics in ecosystems is crucial to investigate the effects of global change on vegetation and the carbon (C) cycle (Hungate et al., 2003; Kicklighter et al., 2019; Mason et al., 2022). The N isotope ratios (δ15N) of plants and soils provide insights into ecosystem N cycling and is frequently used to indicate terrestrial ecosystem N cycling and explore the response of N cycling to various drivers (Bai et al., 2012; Houlton et al., 2006, 2015; Wang et al., 2023; Xia et al., 2023). Specifically, high soil δ15N values generally indicate the “openness” of soil N cycling, characterized by high soil N availability and N losses (Bai & Houlton, 2009; Harris et al., 2022; Houlton & Bai, 2009). This is because the N lost from ecosystems is preferentially 15N-depleted owing to isotopic fractionation (Högberg, 1997; Robinson, 2001). When N availability is high, pathways such as nitrification and denitrification, which strongly fractionate and prefer isotopically lighter N (14N), become more significant. This leads to the enrichment of heavier 15N in the remaining soil N pool (Craine et al., 2015; Kou et al., 2020; Robinson, 2001). Terrestrial ecosystems with high N availability are typically associated with fast soil N cycling and significant N loss (Aber et al., 1989; Niu et al., 2016). Therefore, soil and plant δ15N values in natural terrestrial ecosystems are known to increase significantly with N availability (Högberg, 1997; Mason et al., 2022). However, the variation in isotopic signatures and the N cycle in the soil–plant system are primarily shaped by climate, especially precipitation (Aranibar et al., 2004; Brunello et al., 2024). Whether 15N isotopes correspond well with soil N cycling under changing drought regimes remains largely unclear.
Increasing aridity is an important aspect of climate change in global ecosystems (Berdugo et al., 2020), which can affect the availability of essential nutrients, such as N (Delgado-Baquerizo et al., 2016; Wang et al., 2014). Theoretically, drought may affect N cycling because water availability controls substrate diffusion and plant–microbial access to N, suggesting that drought should limit soil N availability (Delgado-Baquerizo et al., 2013). Empirical evidence has shown limited soil N cycling and N availability under drought conditions, with decreasing soil N pools, microbial activity (Borken & Matzner, 2009), and N transformations (Aranibar et al., 2004) under increasing aridity. However, this is not always the case. Recent studies have reported diverse responses of soil N pools (Zhang et al., 2023; Zhao et al., 2023) and N processes (Elrys et al., 2024; Kou et al., 2018; Song et al., 2023) to increased aridity. Additionally, from the perspective of 15N isotopes, soil N cycling under dry conditions is generally considered more open compared with that under wet conditions as many previous studies have demonstrated that soil δ15N values significantly decrease with increasing mean annual precipitation (MAP), both regionally (Brunello et al., 2024; de Freitas et al., 2015) and globally (Amundson et al., 2003; Craine et al., 2009). Conversely, in contrast to the simple linear correlations between 15N isotopes and aridity indicated by previous studies, a few recent studies have found significant nonlinear responses of soil δ15N values to aridity (Lai et al., 2023; Wang et al., 2014), which further increases the uncertainty in evaluating soil N cycling under dry climates using soil 15N isotopes. These inconsistent changes in soil δ15N values and soil N dynamics suggest complex interaction between water and N cycling under aridity conditions, highlighting the urgency of simultaneously studying soil δ15N, N availability, and related N-cycling characteristics, especially in drylands where the impact of climatic variables on N isotopes remains largely unknown.
Drylands cover approximately 41% of the Earth's land surface and are the largest terrestrial biomes on the planet (Schimel, 2010). Climatic controls on N isotopes and their imprint on ecosystem N cycling are particularly evident in dryland ecosystems as their biological activity is mainly driven by soil water availability (Delgado-Baquerizo et al., 2013; Kou et al., 2018). A typical example is a case study in temperate drylands in northern China, where a significant aridity threshold in controlling ecosystem N cycling was observed, as reflected by a nonlinear pattern of soil δ15N values along an aridity gradient (Wang et al., 2014). However, whether the aridity threshold persists in other arid and semiarid regions under different climatic conditions needs to be verified. The Qinghai–Tibetan Plateau (QTP) is a fragile ecosystem with extensive drylands. Owing to the heterogeneous environmental conditions and large geographical ranges, various alpine ecosystems exist under arid conditions in this region, thus providing a great opportunity to investigate dryland N cycling along aridity gradients. Compared with drylands located in other regions, alpine drylands on the QTP are more vulnerable and sensitive to climate change owing to extreme environmental stresses, such as low temperatures, high winds, and poor soil quality (Yu et al., 2012). This may result in dramatically different N-cycling characteristics under climate change in this region (Chen et al., 2022). However, our understanding of alpine drylands soil δ15N patterns and the imprint of soil N cycling on δ15N with increasing aridity is considerably limited. Identifying how the correlations between soil δ15N and soil N dynamics will change with increasing aridity is crucial to improve our understanding of ecosystem N availability and N limitation to productivity under future precipitation changes in this fragile area.
In this study, we investigated δ15N values of both topsoil and subsoil layers along an aridity gradient across a 3000-km transect of drylands on the QTP. Furthermore, to explore the relationships of soil δ15N values with soil N cycling, we quantified soil N-cycling characteristics by measuring soil total N (TN), mineral N content, microbial biomass N (MBN), and biomass C (MBC), as conducted in many previous studies on N cycling (Aranibar et al., 2004; Wu et al., 2022). We also measured the gross rates of soil N mineralization (GMR) and nitrification (GNR), which are key processes that determine soil N availability (Bengtsson et al., 2003). Additionally, soil bacterial abundance (BA) and fungal abundance (FA) were measured as well because bacteria and fungi are the primary drivers of many N transformation processes, and changes in their abundances could have significant implications on N transformation rates (Kuypers et al., 2018). The main objectives of this study were to evaluate: (1) whether soil 15N isotope signals exhibit linear or nonlinear responses to increases in aridity and (2) if the soil δ15N values correspond well with soil N cycling along the aridity gradient. We hypothesized that: (1) there would be a significant linear increase in soil δ15N values along the aridity gradient because of the negative correlation between 15N isotope signals and precipitation, as previously reported; (2) soil 15N isotopes could effectively reflect soil N-cycling characteristics with positive correlations between soil δ15N values and soil N-cycling traits along the aridity gradient as previous studies have demonstrated the close coupling between δ15N values and the “openness” of soil N cycling.
2 MATERIALS AND METHODS
2.1 Study sites and sampling
The study was conducted along a 3000-km transect in alpine grasslands across the Tibetan Plateau (79°49′39″–100°15′33″ E, 31°06′37″–32°43′09″ N), with an average altitude of 4424 m, ranging from 3544 to 5016 m. Along this transect, 23 sites were randomly selected with a spatial distance of approximately 120 km between adjacent sampling sites to determine soil δ15N (Table S1; Figure S1). Longitude, latitude, and altitude were recorded by an outdoor handheld instrument navigator locator with a global positioning system (GPS) at each site. MAP and mean annual temperature (MAT) data were obtained from the China Meteorological Data Service Centre (CMDC; http://data.cma.cn) based on the location information of each site. Climatic results showed that our study sites covered a broad precipitation gradient, varying between 72 and 648 mm, and the mean annual temperature (MAT) ranged from −3.9 to 5.8°C. Furthermore, the aridity index (AI; the ratio of annual precipitation to potential evapotranspiration) (Delgado-Baquerizo et al., 2013) at each site was extracted from the Global-Aridity and Global Potential Evapotranspiration Climate database (Hu et al., 2021), which ranged from 0.06 to 0.73 along the aridity gradient.
Field sampling was conducted between July 30 and August 28, 2020 (each site was visited once over this period). Briefly, at each site, four 1 m × 1 m sampling plots were randomly selected to measure aboveground and belowground biomass from July to August 2020. Specifically, aboveground plants were clipped and belowground roots were collected from three soil samples in each plot with a 7.5 cm diameter auger. All aboveground clipped plants were dried at 60°C to a constant weight and weighed to determine plant aboveground biomass. Living roots were obtained from soil samples and washed thoroughly with deionized water, and then oven-dried at 60°C for 72 h to weigh to estimate belowground biomass of community.
In each plot, three soil cores from 0 to 10, 10 to 20, and 20 to 30 cm were collected using an auger (7.5 cm in diameter). As the majority of roots are located within the 0–30 cm soil depths (Yang et al., 2009), soil samples from 0 to 10 and 20 to 30 cm were categorized as topsoil and subsoil, respectively. The soil cores at each soil depth were combined into one soil sample, sieved through a 2-mm mesh to remove any non-soil materials and then divided into three subsamples. One subsample was air-dried to determine the soil 15N abundance and soil properties; the second subsample was stored at 4°C for analyzing the inorganic N (NH4+ and NO3−) concentrations, N transformation rates, and microbial biomass; the third subsample was stored at −80°C for measuring soil bacteria, fungi and denitrifying gene abundances. We also sampled the two dominant grass species, Leontopodium nanum, and Stipa purpurea, to determine foliar δ15N. These two species were selected based on their vast distribution along the aridity gradient (Li et al., 2023).
2.2 Laboratory treatment and chemical analysis
The isotopic dilution method was used to measure the gross N transformation rate (Hart et al., 1994). In brief, four subsamples of fresh soil (equivalent to 20 g dry weight) were labeled with 15N, to which two were labeled by the (15NH4)2SO4 solution (5.2 atom%) and the other two were labeled by the K15NO3 solution (5.14 atom%). The soils were incubated at 20°C after being adjusted to 60% water-holding capacity. One subsample of each 15N labeled portion was extracted with 2 M KCl solution after labeling for 0.5 and 24 h. The NH4+ and NO3− concentrations in the extracts were determined using a continuous flow analyzer. The N isotope compositions of NH4+ and NO3− were measured by an isotope ratio mass spectrometer after distilling the NH4+ and NO3− using the diffusion method. Gross N mineralization and nitrification rates were calculated following previous studies (Davidson et al., 2006; Kirkham & Bartholomew, 1954).
We further determined the copy numbers of bacteria and fungi using quantitative real-time polymerase chain reaction (qPCR) (Borneman & Hartin, 2000; Caporaso et al., 2011). The abundances of bacteria and fungi were used to calculate the bacteria-to-fungi ratios. The copy numbers of denitrifying genes (nirK, nirS) were simultaneously measured as gaseous N losses during soil denitrification have strong discrimination against heavier isotopes (15N), which can strongly affect the 15N isotope patterns (Feng et al., 2023; Högberg, 1997). Detailed descriptions of the primers used for PCR amplification are presented in Table S2. Basic soil properties were also measured. Soil total organic carbon (TOC) and TN were measured using a LECO macro-CN analyzer (LECO, St. Joseph, MI, USA). The chloroform fumigation-extraction method was applied to measure MBC and MBN with a conversion factor of 0.45 (Vance et al., 1987). Soil-available phosphorus (AP) was first extracted by the NaHCO3 solution (0.5 M) and then analyzed using the molybdenum-antimony anti-spectrophotometric method. Soil pH was assessed at a water ratio of 2.5 using a pH meter.
2.3 Statistical analyses
All data were carefully checked to ensure quality before the statistical analyses. First, we examined potential linear or quadratic (nonlinear) relationships of soil δ15N with increasing aridity. The model goodness-of-fit was evaluated using the Akaike information criterion (AIC), where a lower AIC value represents a superior model fit (Cavanaugh & Neath, 2019). Thresholds were detected when the quadratic regressions fit the data better. We fit segmented regressions using the “segmented” package of R (Muggeo, 2008) to identify the tipping point where the soil δ15N values abruptly changed along the aridity gradient, with the tipping point detected as the aridity threshold as many previous studies (Feng et al., 2019; Hao et al., 2021). To validate the robustness of the threshold, the relationship between aridity and soil δ15N values on either side of the threshold was analyzed by linear regression (Berdugo et al., 2020). Furthermore, to better distinguish the relative importance of predictors controlling soil δ15N below and above the aridity thresholds, we performed two separate random forest models using the “randomForest” (Liaw & Wiener, 2002) package in R, and the “rfPermute” package was also used to assess the significance of each predictor's importance (Archer, 2016). To assess the model performance and mitigate overfitting, we conducted a tenfold cross-validation, with 80% of the data for training and 20% for validation following another study (Hu et al., 2024). The estimations of soil δ15N in wetter and drier regions by random forest regression showed determination coefficients (R2) of .79 and .92 in predicted versus observed regressions, respectively, indicating that the models exhibited reasonable goodness-of-fit. The relationships of soil N-cycling traits with increasing aridity were also examined by linear or quadratic regressions. All analyses were performed in R4.1.3 (R Core Team, 2022), and figures were created by “ggplot2” package with significance at p < .05.
3 RESULTS
3.1 Soil δ15N patterns and soil N dynamics along the aridity gradient
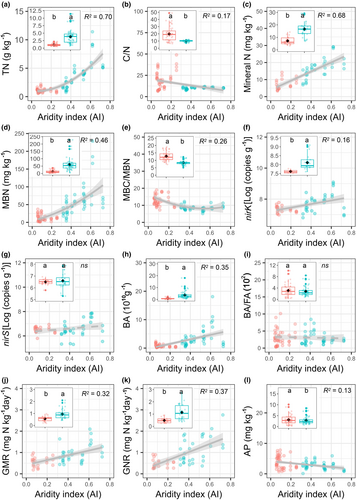
We found that the topsoil δ15N values ranged from 3.48 to 9.78‰ and exhibited a quadratic (upward-concave) pattern along the aridity gradient, with an aridity index (AI) threshold of 0.27 (Figure 1a). Similar to topsoil, the quadratic pattern of soil δ15N was also detected in the subsoil layer (with an AI threshold of 0.29) (Figure 1b). In contrast to soil δ15N, in both soil layers, soil N pools (TN and mineral N content), N-cycling processes (GMR and GNR), and microbial properties (MBN, BA, and nirK gene) significantly decreased with increasing aridity (Figure 2; Figure S2), whereas the soil C:N and MBC:MBN ratios significantly increased with increasing aridity (Figure 2; Figure S2).
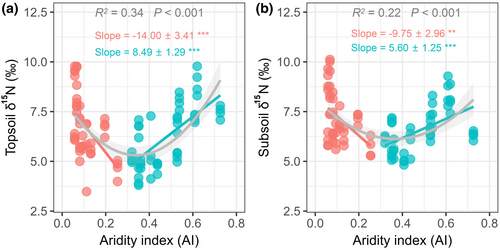
3.2 Correlations between soil δ15N values and soil N dynamics along the aridity threshold
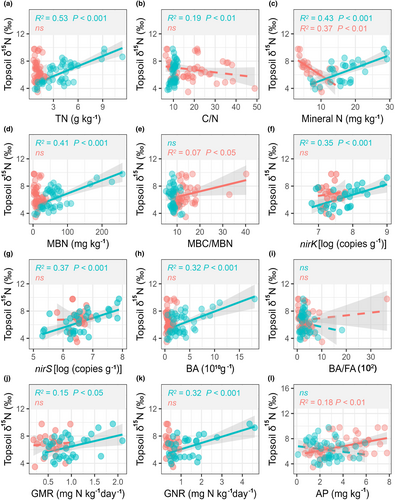
Soil δ15N showed dramatically different correlations with soil N-cycling traits above and below the AI threshold (Figure 3; Figure S3). Specifically, topsoil δ15N had significantly positive relationships with TN, mineral N content, MBN, nirK and nirS abundances, BA, GMR, and GNR in the wetter sites (AI >0.27), whereas these positive correlations disappeared in the drier sites (AI <0.27) (Figure 3). Similar to topsoil, subsoil δ15N also showed significantly positive correlations with certain soil N-cycling traits (e.g., TN, MBN, nirK, and nirS abundances, BA, and N transformations) in wetter sites, whereas no significant relationships or negative correlations (such as mineral N content and MBN) were observed in drier sites (Figure S3).
3.3 Regulation factors in soil δ15N under different aridity levels
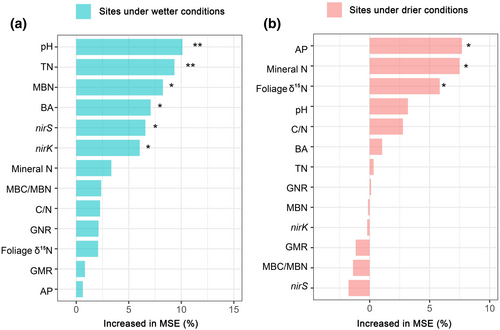
Significant effects of soil N-cycling traits and microbial properties and plant δ15N on soil δ15N were explained by the random forest model, but the main regulatory factors were different under wetter and drier conditions. Results showed that soil pH, TN, and microbial properties (MBN and bacteria and denitrifying gene abundances) were the factors significantly affecting soil δ15N in wetter sites. In contrast, in drier sites, most soil N-cycling traits were not significantly correlated with soil δ15N. Soil AP, mineral N, and foliage δ15N were significant contributors to the variation of soil 15N (Figure 4; Figure S4).
4 DISCUSSION
By conducting a systematic survey along a 3000-km transect in alpine grasslands across the Tibetan Plateau, we found upward-concave patterns of soil δ15N in both topsoil and subsoil layers along the aridity gradient, with AI thresholds of 0.27 and 0.29, respectively. In contrast to soil δ15N, soil N pool, and N process traits significantly decreased along the aridity gradient, suggesting that soil N availability decreased with increasing aridity. Furthermore, we identified different mechanisms driving soil δ15N variation above and below the threshold. Specifically, under wetter conditions, soil δ15N values were significantly correlated with multiple factors, including soil pH, N-cycling processes, and N-associated microbial properties, with positive relationships between soil δ15N and soil N-cycling traits. Conversely, under drier conditions, soil δ15N values were significantly associated with soil-available P, foliage δ15N, and mineral N. Our findings highlight that soil δ15N closely mirrors soil N dynamics in regions with low aridity, but becomes decoupled from soil N dynamics in regions with high aridity. Considering that the estimates of changes in soil N cycling and availability based on soil δ15N values often ignore the role of aridity levels, these findings underscore the importance of the aridity threshold in nitrogen-water interactions under changing drought regimes.
4.1 Soil N availability along the aridity gradient
Previous studies have demonstrated that aridity has a negative effect on the soil N supply, likely exacerbating the limitation of soil N availability (Delgado-Baquerizo et al., 2016; Tang et al., 2021). Based on a systematic survey, we found a significant decrease in soil N pools and N processes in both the topsoil and subsoil layers along the aridity gradient, indicating a corresponding decrease in soil N availability with increasing aridity. Decreased soil N availability at arid sites is unsurprising as drought limits the activity of soil microorganisms (Fuchslueger et al., 2014), which play a vital role in soil N cycling. For example, 16S rRNA gene abundance suggests that the nitrifying activity of soil bacteria and archaea is reduced under drought conditions, resulting in a lower N nitrification rate (Hartmann et al., 2013). Similarly, in this study, the decreased MBN and bacterial abundance along the aridity gradient indicated that drought restricted N-associated microorganisms, leading to a decrease in soil N mineralization and nitrification rates (Figure 2; Figure S2), and consequently reduced soil N availability.
4.2 Soil δ15N patterns along the aridity gradient
Soil δ15N values, especially in the topsoil layer, are generally used as an indicator of soil N availability (Kou et al., 2020; Robinson, 2001). Considering the reduced soil N availability with increasing aridity, soil δ15N values are expected to decrease along the aridity gradient. However, contrary to our first hypothesis, we found that δ15N values in both topsoil and subsoil exhibited an upward-concave pattern along the aridity gradient. The unexpected soil 15N patterns under different drought conditions challenge the traditional view of a simple linear correlation between 15N signals and precipitation (or aridity) (Craine et al., 2009). Furthermore, the quadratic pattern of soil δ15N values also contradicts the decreased soil N pools and processes along the aridity gradient, given the positive correlation between soil δ15N and N availability in the traditional view owing to significant 15N isotope fractionation during open N-cycling processes under conditions of high N availability (Högberg, 1997; Mason et al., 2022). These results suggest that under different aridity conditions, different mechanisms underlying ecosystem N cycling may control soil δ15N values.
Soil δ15N values are determined only by the input and output pathways of 15N to and from soils (Robinson, 2001). As the δ15N value of N input (mainly by N deposition and N2 fixation) is approximately equal to 0‰ (Bai & Houlton, 2009; Houlton et al., 2007), the enrichment of soil δ15N values indicates the occurrence of output processes leaving more 15N in the soil (Liu et al., 2016). Gaseous N loss, net plant N accumulation, and leaching N loss are output processes, wherein gaseous N loss exhibit the most pronounced isotope fractionation effects (εG ≈ 16‰–30‰), followed by net plant N accumulation (εP ≈ 5‰–10‰) and leaching N loss (εL ≈ 1‰) (Feuerstein et al., 1997). In regions with low aridity, we speculated that the pattern of soil δ15N is likely influenced by gaseous N loss derived from N transformations, as indicated by significantly positive correlations between soil δ15N values and GMR or GNR (Figure 3; Figure S3). Additionally, the significantly positive correlations between soil δ15N and denitrifying genes (nirK and nirS) in the wetter sites but not in the drier sites also highlight the role of denitrification in soil δ15N values under wetter conditions (Figure 3; Figure S3). These positive correlations between soil δ15N and soil N transformations and associated functional genes indicate that in wetter regions, high soil microbe-driven N cycling with a significant 15N fractionation significantly contributes to the enrichment pattern of soil δ15N.
In agreement with this, the random forest result showed that among multiple factors, soil N-cycling traits (e.g., TN, BA, MBN, and denitrifying genes) significantly explained soil δ15N changes in wetter sites, which provides robust evidence that 15N enrichment under relative drought conditions may largely result from strong soil N losses through microbe-driven N transformations under high N availability conditions. Additionally, this microbe-driven mechanism of soil 15N enrichment is further supported by the pronounced contribution of soil pH to soil δ15N. As a key factor shaping microbial structure and function, soil pH strongly affects soil N-cycling microbial communities (Ontman et al., 2023; Rousk et al., 2010; Scarlett et al., 2021). Our results showed that pH was the most pronounced driver of soil δ15N in wetter sites, with dramatically different pH values under different aridity conditions (Figure S5), indirectly highlighting the contribution of microbe-driven N cycling to soil δ15N. Overall, these findings show that in wetter regions, soil 15N closely tracked with soil N dynamics, suggesting that soil 15N isotopes can accurately reflect soil N cycling and N availability in low aridity environments (Figure 5).
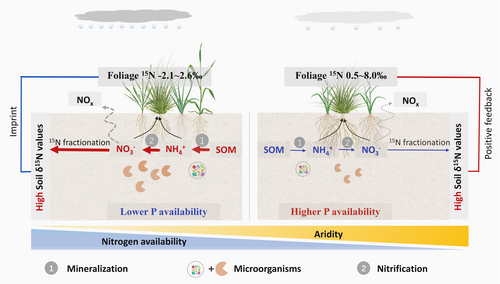
Conversely, under extremely dry conditions, microbe-driven soil N-cycling processes should not be the main reason for the increase in soil δ15N owing to the insignificant correlations between soil δ15N and soil N dynamics in the drier sites (Figure 3; Figure S3), which has not been detected in previous studies. More intense and prolonged droughts owing to climate change are expected to promote open N cycling in drylands through sustained microbial N processing but low plant N uptake (Evans & Burke, 2012; Wu et al., 2022). Microbial processes have been postulated to predominantly drive N gas evasion in arid systems, thereby significantly affecting soil δ15N (Homyak et al., 2017). Contrary to expectations, our study spanning an aridity transect revealed that the “open” N cycling accompanied by microbe-driven soil N losses and 15N enrichment was only observed under low aridity conditions. In high aridity sites, pathways other than microbial mechanisms may significantly contribute to 15N enrichment. This discrepancy highlights an unclear mechanism controlling soil δ15N with increasing aridity. We propose the following reasons that may be responsible for the 15N patterns in drier regions.
On the one hand, according to the aforementioned N isotope input and output model, we speculated that increased plant net N accumulation may explain the increased soil δ15N values under extreme drought conditions. Previous studies have demonstrated that increasing aridity significantly decreases N return from plants to the soil (Wu et al., 2022; Yuan & Chen, 2008). This may be attributed to the fact that under high aridity conditions and low soil N availability, plants are able to alleviate N limitation by enhancing N retention (e.g., by increasing N resorption) (Luo et al., 2021; Yuan & Chen, 2008). This process may result in an increase in plant net N accumulation, thereby increasing the contribution of 15N fractionation to soil 15N enrichment during plant net N accumulation. The result of greater correlation between soil and foliage δ15N values in drier than wetter regions also highlight different N feedback mechanisms between the plant and soil under different aridity conditions (Figure 4).
On the other hand, previous studies have demonstrated that abiotic N loss processes, such as ammonia (NH3) volatilization (Liu et al., 2020; Soper et al., 2016; Wang et al., 2014) and the loss of oxidized gaseous N (McCulley et al., 2009), are prevalent under high aridity conditions. We speculated that NH3 volatilization, which has a significant isotope fractionation factor and typically occurs in high pH soils (Heaton, 1986), may be another process attributed to the observed isotopic pattern in extremely dry regions, where soil pH values are significantly higher than those in less dry regions (Figure S5). Overall, our findings highlight different ecosystem N-cycling processes driving the quadratic pattern of soil 15N isotopes along the aridity gradient, which may help explain 15N enrichment under severe aridity conditions.
4.3 Implications and limitations
To the best of our knowledge, this is the first study revealing the quadratic pattern of δ15N values in both topsoil and subsoil layers across an aridity gradient in alpine grasslands. The markedly different correlations between soil δ15N and soil N dynamics under different aridity conditions in the current study broadens our traditional understanding that enriched soil 15N signals indicate the “openness” of soil N cycling and high N availability. Numerous studies use natural nitrogen isotopic benchmarks, particularly topsoil δ15N, to improve the accuracy of nitrogen-based climate change projections (Feng et al., 2023; Kou et al., 2020). Our results highlight the need for caution when estimating soil N availability through δ15N of soils or plant organs, particularly in drylands where resources, such as water and nitrogen, are extremely limited. These findings advance our understanding of ecosystem N cycling and N limitation of ecosystem productivity with increasing aridity. However, there are still some limitations.
It is important to note that despite we found the different mechanisms driving the quadratic pattern of soil δ15N along the aridity gradient, a significant portion of the variability in soil δ15N change in drier regions remains unexplained. This indicates that further studies are required to obtain a more comprehensive understanding of ecosystem N cycling and δ15N in alpine drylands with severe droughts. Furthermore, although we focused on the correspondence between soil δ15N and soil N cycling, foliage δ15N should also be included to explore the 15N imprint between soils and plants. We only selected the two most widely distributed dominant species on the aridity gradient to explore foliage δ15N patterns, which may be insufficient to represent all the plant isotope signatures due to significant differences in foliage δ15N values among different functional types (Craine et al., 2009; Wang et al., 2023). Future studies should pay more attention to the diversification of plant isotopic niches along climatic gradients to better explore the coupling between leaf and soil δ15N under different drought conditions. Additionally, differences in potential evapotranspiration methods in AI analysis may increase result uncertainties (Tegos et al., 2023). Although the method we used to collect AI data has been widely employed in many related studies (Hou et al., 2020; Wang et al., 2014), our results need validation in other dryland ecosystems to verify the mechanisms underlying these responses. Overall, to better reveal the characteristics of soil δ15N values and N cycling and their responses to climatic dryness, further observational and experimental studies spanning larger climatic gradients are urgently required across ecosystems beyond alpine drylands.
5 CONCLUSIONS
By analyzing soil δ15N and N-cycling traits along an aridity gradient, we found a quadratic pattern of soil δ15N values, contradicting the expected decrease in soil N availability with increasing aridity. We further found different mechanisms driving soil 15N isotopes in different aridity regions. In wetter regions, soil δ15N values were positively related to soil N-cycling traits, demonstrating that high soil δ15N values may result from the “openness” of soil N cycling under conditions of high soil N availability. However, in drier regions, soil δ15N was significantly related to other factors (such as soil P availability and foliage δ15N) but not to soil N-cycling traits, indicating that pathways other than typical soil N cycling may result in high soil δ15N values under high aridity conditions. In contrast to previous estimates of changes in soil N cycling and availability inferred from soil and tissue δ15N values that are independent of drought, our results provide new insights into ecosystem N cycling with aridity change from the perspective of the aridity threshold of soil 15N isotopes. Our results also highlight the importance of considering the aridity threshold in nitrogen–water interactions in biogeochemical models to improve the predictions of soil N availability under increasing N deposition rates and intensifying droughts.
AUTHOR CONTRIBUTIONS
Jinhua Mao: Conceptualization; data curation; formal analysis; investigation; methodology; writing – original draft; writing – review and editing. Junxiao Pan: Investigation. Lei Song: Investigation. Ruiyang Zhang: Writing – review and editing. Jinsong Wang: Writing – review and editing. Dashuan Tian: Conceptualization. Quancheng Wang: Conceptualization. Jiaqiang Liao: Writing – review and editing. Jinlong Peng: Conceptualization. Shuli Niu: Conceptualization; funding acquisition; methodology; project administration; resources; supervision; validation; writing – review and editing.
ACKNOWLEDGMENTS
We thank the anonymous reviewers for their valuable comments and suggestions on the manuscript. This work was financially supported by the National Natural Science Foundation of China (31988102, 32301394), the National Key Research and Development Program of China (2022YFF0802102), and the China Postdoctoral Science Foundation (2023M743457).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in Figshare at: https://figshare.com/articles/dataset/Data_Mao_et_al_GCB_xls/25770057.




