Why do avian responses to change in Arctic green-up vary?
Abstract
Global climate change has altered the timing of seasonal events (i.e., phenology) for a diverse range of biota. Within and among species, however, the degree to which alterations in phenology match climate variability differ substantially. To better understand factors driving these differences, we evaluated variation in timing of nesting of eight Arctic-breeding shorebird species at 18 sites over a 23-year period. We used the Normalized Difference Vegetation Index as a proxy to determine the start of spring (SOS) growing season and quantified relationships between SOS and nest initiation dates as a measure of phenological responsiveness. Among species, we tested four life history traits (migration distance, seasonal timing of breeding, female body mass, expected female reproductive effort) as species-level predictors of responsiveness. For one species (Semipalmated Sandpiper), we also evaluated whether responsiveness varied across sites. Although no species in our study completely tracked annual variation in SOS, phenological responses were strongest for Western Sandpipers, Pectoral Sandpipers, and Red Phalaropes. Migration distance was the strongest additional predictor of responsiveness, with longer-distance migrant species generally tracking variation in SOS more closely than species that migrate shorter distances. Semipalmated Sandpipers are a widely distributed species, but adjustments in timing of nesting relative to variability in SOS did not vary across sites, suggesting that different breeding populations of this species were equally responsive to climate cues despite differing migration strategies. Our results unexpectedly show that long-distance migrants are more sensitive to local environmental conditions, which may help them to adapt to ongoing changes in climate.
1 INTRODUCTION
Globally, changes in climate are altering the timing of seasonal events (i.e., phenology) for a diverse range of homeotherm organisms (Hammerschlag et al., 2022; Hong et al., 2022; Kiat et al., 2019). Although phenological responses to climate variability are well-documented, responsiveness (i.e., the covariation between climate variability and phenology of life history events) varies within and across taxonomic groups, both in magnitude and direction (Ge et al., 2015; Iler et al., 2013; Zografou et al., 2021). Whereas the factors that influence timing of life history events have been well-studied across multiple taxa and systems (Sutton & Freeman, 2023; Woods et al., 2022), increasingly, phenology is being studied in the specific context of a changing climate. Current efforts are primarily focused on evaluating correlations between phenological responses and species traits and describing geographic trends in relation to environmental cues (Kluen et al., 2017; Song et al., 2020; Woods et al., 2022). However, studies often have conflicting results, such that uncertainty remains about the ecological and environmental components that influence variation in phenological responsiveness (Cohen et al., 2018; Maggini et al., 2020; Tang et al., 2016). Yet this information is critical for developing accurate predictions about wildlife outcomes associated with environmental change (Koppel & Kerr, 2022; Socolar et al., 2017).
Among avifauna, in the Arctic and other seasonal environments, phenological responses to earlier summers can be affected by several species-specific life history traits. For bird species that migrate long distances, onset of spring migration is typically triggered by responses to endogenous cues or to increasing day length at non-breeding areas that aligns with changes in breeding area conditions (Åkesson & Helm, 2020). If correspondence between non-breeding area cues and breeding area conditions is reduced under ongoing climate change, phenological responses will be more constrained for long distance than short distance migrants (Doxa et al., 2012; Senner, 2012; Youngflesh et al., 2021). Several studies have also documented interspecific differences in avian phenological responsiveness related to relative timing of breeding. Compared to species with later average breeding dates, earlier-nesting species may respond to different cues for adjusting timing of breeding and be less time-constrained—therefore being more able to advance nesting dates in relation to climate variability, although the opposite has also been observed (Gurney et al., 2011; Messmer et al., 2021; Saalfeld & Lanctot, 2017). Further, interspecific variation in female body size might affect how birds respond to phenological change. In particular, among larger bodied species, the ability to speed up migration to correspond with advancing phenology may be constrained, thus limiting their ability to adjust timing of arrival to breeding areas and subsequent egg-laying (Bitterlin & Van Buskirk, 2014; Hedenström, 2008). Finally, species for which females have a greater duration of reproductive effort could be less responsive to variable environmental conditions than those with shorter periods of effort, especially when increasing duration of effort corresponds to greater costs of waiting (i.e., reduced breeding opportunity) (Hanssen et al., 2005; Tulp & Schekkerman, 2006).
Along with life history traits, spatial and temporal variability in environmental cues might also be important factors affecting phenological shifts in relation to climate change. In studies of avian migration, some data suggest that species traits are the strongest predictors of phenological shift (Hurlbert & Liang, 2012; Ward et al., 2016), whereas others suggest that environmental factors are more important (Horton et al., 2020; Mayor et al., 2017). Alternatively, both species traits and environmental cues, as well as interactions between the two, can have equally important influences on changes in migration phenology (Horton et al., 2019; Kullberg et al., 2015; Powers et al., 2021). Such studies highlight a growing body of knowledge about why climate-mediated shifts in migration phenology vary (Horton et al., 2019; Kullberg et al., 2015; Powers et al., 2021), but whether similar processes affect variation in phenological shift for other avian life history stages, such as timing of reproduction, is not clear (Chmura et al., 2019; Hällfors et al., 2020; Lameris et al., 2018; Saalfeld & Lanctot, 2017). Specifically, existing evidence regarding linkages between migration distance, relative timing of breeding, and phenological responsiveness is equivocal, and to our knowledge, relationships between female body mass and variation in duration of female reproductive effort and phenological responsiveness are primarily unexamined. A limited understanding of the relative importance of different life history traits and the influence of environmental variability on the responses of bird species to variations in climate remains a key challenge for predicting the impacts of ongoing global change.
To address questions concerning the broader influences of species traits and environmental cues on phenological responsiveness in avian fauna, we modeled variation in timing of nesting in relation to fluctuations in the Normalized Difference Vegetation Index (NDVI), a proxy for the start of spring (SOS) growing season (i.e., green-up), for eight common and broadly distributed species of Arctic-breeding shorebirds at 18 circumpolar sites across 23 years (Doiron et al., 2013; Myers-Smith et al., 2020). The eight species were selected to encompass a range of life history strategies, breeding distributions, and habitat preferences (see Liebezeit et al., 2014; Saalfeld & Lanctot, 2015; Saunders et al., 2022) and included American Golden-Plover (Pluvialis dominica), Sanderling (Calidris alba), Dunlin (C. alpina), Pectoral Sandpiper (C. melanotos), Semipalmated Sandpiper (C. pusilla), Western Sandpiper (C. mauri), Red-necked Phalarope (Phalaropus lobatus), and Red Phalarope (P. fulicarius). Specifically, our objectives were to (i) describe variability in the start of the spring growing season and measure phenological responsiveness across species by quantifying interspecific variation in adjustments to timing of nesting in response to variable onset of spring and (ii) test if phenological responsiveness covaries with four species-level traits (migration distance, relative timing of breeding, female body mass, and expected female reproductive effort). We also aimed to better understand the combined roles of life history traits and environmental cues in phenological responsiveness by evaluating spatial variation in responsiveness among populations of Semipalmated Sandpipers. Across its range, this widely distributed species uses distinct strategies during spring migration, with populations breeding in the western Arctic primarily making small jumps along the Mississippi and Pacific flyways, and eastern-breeding populations mostly using the East Atlantic flyway, making intercontinental non-stop jumps that require large stores of extra fuel to stay in the air for thousands of uninterrupted kilometers (Brown et al., 2017; Hicklin & Gratto-Trevor, 2020).
Shorebirds, which rely primarily on exogenous nutrients for production of eggs, are likely to be strongly affected by environmental constraints during the breeding season (Hobson & Jehl, 2010; Klaassen et al., 2001). We therefore anticipated long distance migrants, like Pectoral Sandpiper, would be less phenologically responsive than short-distance migrants like Dunlin. Similarly, we expected reduced phenological responsiveness for earlier-nesting species, which may be unable to further shorten the interval between arrival and egg-laying (Saalfeld & Lanctot, 2017). We also predicted decreasing responsiveness among large-bodied shorebird species, and for species with parental care that extends through chick rearing. Among breeding populations of Semipalmated Sandpipers, we reasoned that eastern populations would be more dissociated from climate cues on breeding areas (Ely et al., 2018; Kwon et al., 2019) and therefore less responsive to variations in SOS than western populations.
2 MATERIALS AND METHODS
2.1 Study sites and data collection
Field data on nesting shorebirds were collected in 23 non-consecutive years over a 33-year period (1983 to 2016) at 18 sites across the Arctic, encompassing ~16° of latitude (74.5° N–58.7° N) and ~336° of longitude (164.9° W to −170.6° E) (Table 1; Figure 1). Sites included 15 locations in low Arctic or subarctic tundra habitats, dominated mainly by sedges, grasses, and moss combined with small ponds (Saalfeld & Lanctot, 2017), and three high Arctic tundra locations with large expanses of mesic Cassiope and Dryas heather and wet fens (Meltofte & Rasch, 2008). Thirteen sites were part of the Arctic Shorebird Demographics Network (ASDN), an ad-hoc research group that has monitored sites along the Arctic coasts of Alaska, Canada, and Russia (Weiser et al., 2018). The other five sites were initially established as part of earlier nesting studies (Gratto, 1988; Liebezeit et al., 2014) or were part of ongoing biological monitoring programs (Meltofte et al., 2021). For our final data set, we included only site, year, and species combinations with greater than 30 observations for timing of nest initiation (n = 8489 nests).
| Site name (abbreviation) | Location (latitude, longitude) | Region | Years (range) (n) | Start of spring (range) | Nests (n) |
|---|---|---|---|---|---|
| Nome (NOME) | 64.4° N, 164.9° W | Alaska, USA | 1993–1996, 1998–1999, 2008–2014 (13) | May 21 to June 15 | 1343 |
| Cape Krusenstern (CAKR) | 67.1° N, 163.5° W | Alaska, USA | 2011–2014 (4) | May 27 to June 8 | 303 |
| Utqiaġvik (formerly Barrow) (BARR) | 71.3° N, 156.6° W | Alaska, USA | 2003–2014 (12) | June 17 to July 1 | 3155 |
| Ikpikpuk (IKPI) | 70.6° N, 154.7° W | Alaska, USA | 2010–2014 (5) | June 21 to July 1 | 288 |
| Teshekpuk (TESH) | 70.3° N, 153.1° W | Alaska, USA | 2005, 2006 (2) | June 20 to July 1 | 66 |
| Colville River Delta (COLV) | 70.4° N, 150.7° W | Alaska, USA | 2011–2014 (4) | June 27 to July 1 | 390 |
| Kuparuk (KUPA) | 70.2° N, 150.0° W | Alaska, USA | 2002–2004 (3) | June 17 to July 2 | 149 |
| Prudhoe Bay (PRBA) | 70.0° N, 149.0° W | Alaska, USA | 2004–2006, 2011 (4) | June 21 to June 25 | 170 |
| Canning River (CARI) | 70.1° N, 145.8° W | Alaska, USA | 2003–2007, 2010–2014 (10) | June 11 to June 24 | 1506 |
| Mackenzie River Delta (MADE) | 69.4° N, 135.0° W | Northwest Territories, Canada | 2013, 2014 (2) | June 21 to June 27 | 80 |
| Churchill (CHUR) | 58.7° N, 93.8° W | Manitoba, Canada | 2011, 2013 (2) | June 4 to June 14 | 65 |
| La Pérouse Bay (LAPB) | 58.7° N, 93.5° W | Manitoba, Canada | 1983, 1984 (2) | June 17 to June 24 | 79 |
| Coats Island (COAT) | 62.9° N, 82.5° W | Nunavut, Canada | 2004, 2006 (2) | June 13 to June 30 | 69 |
| Igloolik (IGLO) | 69.4° N, 81.6° W | Nunavut, Canada | 2016 (1) | June 27 | 40 |
| Bylot Island (BYLO) | 73.2° N, 80.0° W | Nunavut, Canada | 2010–2014 (5) | June 25 to July 6 | 329 |
| Zackenberg (ZACK) | 74.5° N, 20.6° W | Northeast Greenland | 2007–2009; 2012, 2013, 2015, 2016 (7) | June 9 to July 24 | 276 |
| Lower Khatanga River (LKRI) | 72.9° N, 106.1° E | Krasnoyarsk, Russia | 2012, 2014 (2) | June 19 to June 22 | 148 |
| Chaun River Delta (CHAU) | 68.8° N, 170.6° E | Chukotka, Russia | 2013 (1) | June 18 | 33 |
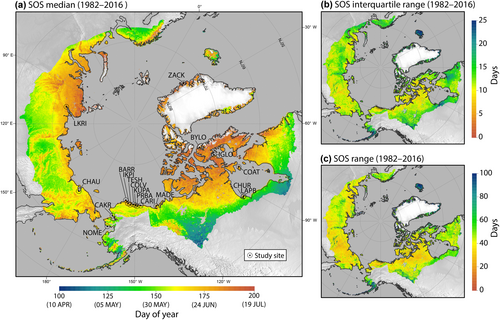
Data on shorebird nesting were collected during the pre-laying and nesting period from late May until late June at all sites, except for Zackenberg, Greenland, where data collection began during the first days of June. Field work at ASDN sites followed standardized protocols, with specific configuration of study plots dependent on nest density and habitat (Brown et al., 2014). Across sites, nests were located using single-person area searches, rope dragging to flush incubating birds, opportunistically while monitoring previously discovered nests, or by observing distraction displays of attending parents (Brown et al., 2014). The typical clutch size of Arctic shorebirds is four eggs, with one egg laid every 1–2 days (Colwell, 2006; Sandercock, 1998). For nests found with fewer than four eggs, we estimated nest initiation date (NID) for the day the first egg was laid by subtracting 1 day for each egg initially found in the nest from the date the nest was found (Kwon et al., 2019; Saalfeld & Lanctot, 2015). For nests found during incubation, NID values were estimated by subtracting the species' average incubation period from the hatch date, and if this was not possible, by back-calculating from the number of days of embryo development determined by floating eggs (Liebezeit et al., 2007). To test the accuracy of our float data and whether estimation methods influenced our results, for a subset of nests (n = 3754), we calculated the difference between observed hatch date and predicted hatch dates, based on float data. Data and results for this assessment are available in the data release and in Supporting Information (Figure S2).
2.2 Variable preparation
All data used for the analyses in this study are openly available in Zenodo at (https://doi.org/10.5281/zenodo.11095196; Tavera et al., 2024). We used satellite remote sensing data which documents vernal greening in seasonal areas of the Earth and is related to warming temperatures (Körner & Basler, 2010; Park et al., 2019; Yao et al., 2021), to estimate the annual timing of early season vegetation growth at each site, hereafter called ‘Start of Spring’ (SOS). NDVI data provide a consistent measure of annual vegetative growth and are strongly related to Arctic tundra biomass as well as timing of snowmelt, a key environmental metric used in other Arctic bird phenology studies (Bison et al., 2020; Epstein et al., 2012; Liebezeit et al., 2014). We constructed a 35-year time series (1982–2016) of SOS, based on NDVI, from two data sources, each comprised of daily global NDVI mosaics on a 0.05-degree pixel-resolution grid: (1) the Long Term Data Record Version 3 collected by the Advanced Very High Resolution Radiometer (AVHRR), 1982–1999, (https://ltdr.nascom.nasa.gov, accessed October 2010); and (2) the Earth Science Data Record of preprocessed Version 4 NDVI collected by the Moderate Resolution Imaging Spectroradiometer (MODIS), 2000–2016, (https://vip.arizona.edu, accessed November 2017).
NDVI values were scaled to attain continuity with MODIS NDVI using satellite-specific top-down equations documented at the vip.arizona.edu website (Miura et al., 2006). Maximum-value NDVI composites were produced for ~10-day periods (3 per month; days 01–10, 11–20, and 21+) (Holben, 1986). Pixels were excluded from the composite estimates if their solar zenith angle was >75° due to weak illumination, or if their satellite view angle was >42° due to degraded spatial resolution and greater atmospheric interference. Date of the maximum NDVI for each pixel in each composite period was retained. Entirely missing data for AVHRR (n = 10 of 648, 1.5%) or MODIS (n = 14 of 612, 2.3%) composite periods were filled by averaging the preceding and subsequent years. Only four of the 24 missing composite periods occurred during the principal months of Northern Hemisphere green-up, March through August. Analogous multi-decadal NDVI time series were used by Brook et al. (2015) and Ross et al. (2017, 2018) to study the implications of phenological mismatch on gosling growth and survival of tundra nesting geese.
NDVI values were extracted from the 10-day composites for each pixel within each study-site polygon encompassing the nesting area and surrounding areas of similar habitat to bolster sample size. Polygons averaged 52 pixels in size (standard deviation, 36; range, 8–133, which equates to ~550 km2 on average at 70° N). For each pixel, periods of implausible drops in NDVI commonly caused by persistent cloud cover were smoothed by linearly interpolating between the NDVI values of adjacent periods. NDVI values <0.05 were assigned 0.05 to disregard inconsequential noise accompanying very low NDVI estimates. At each pixel, daily NDVI estimates were linearly interpolated between the NDVI acquisition dates during each composite period, after which a time-series of daily median NDVI among all pixels at each respective study area (except one) was calculated to construct a seasonal NDVI phenology curve for each study site and year of the 35-year time series. At one site (Zackenberg), we calculated daily NDVI based on the 75th percentile because the median NDVI (50th percentile) was weak and unstable owing to the sparseness of vegetation cover at this high-latitude study area. Last, the date (day of year) when 50% of the annual NDVI amplitude was attained was extracted as a metric describing SOS for each year and study site (Figure 1). We chose 50% because lower thresholds risked sensitivity to weak and less stable NDVI signals. Due to proximity and resolution of the remote measurements, the same NDVI data were used for Churchill and La Pérouse Bay, thus we had NDVI data for 17 sites in total.
To determine an average migration distance for each species, we mapped latitudes and longitudes of breeding and non-breeding ranges at the four outermost locations on distribution maps (east, north, west, and south) using Birds of the World online database (Billerman et al., 2020; Koleček et al., 2020), and updated information provided by Reneerkens et al. (2020) for Greenland. We then calculated the difference in degrees latitude and degrees longitude between the midpoint of the breeding range estimated from the most northerly and most southerly breeding latitude and the most westerly and easterly longitude and the midpoint of the non-breeding range estimated from the most northerly and most southerly breeding latitude and the most westerly and easterly longitude (Thomas et al., 2006). Coordinates were transferred to Google Maps to approximate distance for the complete migration route. To estimate species-level values for seasonal timing of breeding (senso latu), we calculated the mean NID for each species across sites and years (Raquel et al., 2016) (Table 2).
| Species | Migration distancea (km) | Seasonal timing of breeding (NID) (mean; range)b | Body massa (g) | Expected female reproductive effort (days)c |
|---|---|---|---|---|
| Western Sandpiper | 10,772 | May 30; May 12 to June 27 | 31 | 26.99 |
| Semipalmated Sandpiper | 7995 | June 8; May 15 to July 6 | 27 | 25.36 |
| Dunlin | 5912 | June 11; May 30 to July 4 | 45.1 | 28.86 |
| Red-necked Phalarope | 7618 | June 12; May 16 to July 6 | 37.4 | 11.2 |
| Pectoral Sandpiper | 12,071 | June 15; May 27 to July 5 | 65.1 | 46.6 |
| Red Phalarope | 10,564 | June 15; May 31 to July 1 | 57.2 | 12 |
| Sanderlingd | 8473 | June 16; June 1 to July 4 | 55.4 | 30.2 |
| American Golden-Plover | 11,926 | June 19; June 6 to July 6 | 146.0 | 33.6 |
Average body mass estimates for females of each species were compiled from the Birds of the World online database (Billerman et al., 2020). For expected female reproductive effort, we calculated a value for the expected average duration of reproductive effort by summing the number of days invested in egg-laying, incubation, and the brood rearing stages and adjusting for the expected number of clutches laid per year. Due to sex role-reversal in phalaropes, where the males are responsible for all incubation and parental care duties, we only considered parental effort during the laying period for successfully pairing and laying females, ignoring females that did not mate (Rubega et al., 2020; Tracy et al., 2020). For other species, the laying stage assumed females had only one successful clutch per year with a low probability of potential renesting occurring following 0.3 days of the initial incubation period. Detailed values, sources, and calculation of the duration of reproductive effort index are given in Supporting Information, Methods. Trait variables used in the analyses were not correlated (Table S2) and represent global values that do not incorporate variation at the potential sub-species level (Dunlin, Semipalmated Sandpiper), as our research questions are more related to broad life history characteristics, rather than finer resolution drivers of responsiveness.
2.3 Data analyses
All analyses were implemented in program R ver. 4.0.5 (Shake & Throw; R Core Team, 2021). We used general linear mixed models (library “lme4”, Version 1.1-34) and compared models based on an information-theoretic approach (library “lmerTest”, Version 3.1-3), with models ranked according to 2nd-order Akaike's information criterion (Bates et al., 2015; Burnham & Anderson, 2002; Kuznetsova et al., 2017). Inference concerning fixed effects was based on precision (95% confidence intervals, CI) of regression coefficients (β) (Arnold, 2010). All model statements are provided in Supporting Information, Methods.
To evaluate differences in phenological responsiveness across species, we created an a priori set of candidate models, which represented NID as a function of species (with sub-species grouped together for Dunlin), SOS, and the interaction of these factors (Species*SOS). To account for covariance in NID related to non-independence of data points, we included site and a year-site interaction as random effects on the intercept (Harrison et al., 2018; Schielzeth & Nakagawa, 2013).
We developed a second set of candidate models to test for effects of species traits on phenological responsiveness. The species trait models included NID as the response variable and SOS as a predictor (fixed effect), with four species traits as additional predictors: migration distance, relative timing of breeding, female body mass, and expected female reproductive effort. As our key goal was to evaluate variation in adjustments in NID related to variation in SOS as a function of species-level traits, we also included interaction terms between SOS and each trait as fixed effects in these model sets. The random effect structure for species trait models included site, species, and year-site interaction effects on the intercept. To test for potentially confounding effects of phylogenetic relationships among species that were unaccounted for by the random species term, we calculated the phylogenetic signal (Pagel's λ), using library “phytools”, Version 2.1-1 for both NIDs and residual errors from our full model (Kwon et al., 2022; Revell, 2024), see Supporting Information, Methods. To further assess effects of species traits on phenological responsiveness, we used a factorial model to compare slope parameters from the regression of NID and SOS among species. For species trait candidate models, all predictor variables were standardized with a z-transformation (over one standard deviation) to address potential scaling issues (Zuur et al., 2010).
Finally, to test for intraspecific spatial variation in phenological responsiveness, we used a subset of the data from Semipalmated Sandpipers as a widely distributed species (n = 2605 nests; n = 10 sites). In this assessment, our candidate models for NID included SOS, site, and an SOS by site interaction (SOS*Site) as fixed effects, with a year-site interaction as a random effect on the intercept, to account for dependence among nests at the year and site levels.
3 RESULTS
3.1 Variability in SOS and timing of nest initiation
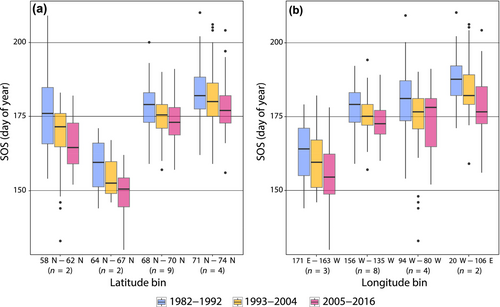
Between 1982 and 2016, the range of SOS varied strongly across sites, being generally earlier at lower latitudes and in more western locations (Figure 2). Over the past three decades, all sites trended towards earlier springs, however, annual variation in SOS was substantial across all sites. The range in SOS (across all years) was greatest at Zackenberg, spanning 54 days, with the earliest estimated spring in the first week of June (1994, 2013) and the latest SOS values at the end of July, in 1985 and 1987. In contrast, for the site at Kuparuk (in Alaska), variability in climate phenology between 1982 and 2016 (as indexed by SOS) was relatively low, ranging from early springs in mid-June (1990, 1998, 2015) to later springs in early July during most of the 1980s. Other more westerly sites (with some exceptions) also tended to show less variation in SOS across our study period (Figure 2b). When considering only the SOS data included in our analyses, similar patterns were observed, with the earliest estimated spring at Nome (May 21, 2014) and the latest at Zackenberg (July 24, 2015). Ranges for each site, based on the data we analyzed, are summarized in Table 1.
Similarly, across our eight focal shorebird species, mean annual NIDs showed considerable variation across sites and years, although site-level variation in NIDs was less for some species (Dunlin, Pectoral Sandpiper) than for others (Semipalmated Sandpiper, Western Sandpiper, Red-necked Phalarope) (Figure S1). Estimates of mean annual nest initiation day ranged from May 21 (Western Sandpiper, 1994) to June 23, (Sanderling, 2015), with the annual span of nest initiation ranging from 10 days (Semipalmated Sandpiper, 2002, n = 34 nests) up to 45 days (Red-necked Phalarope, 2011, n = 81 nests).
3.2 Phenological responsiveness across species and effects of species traits
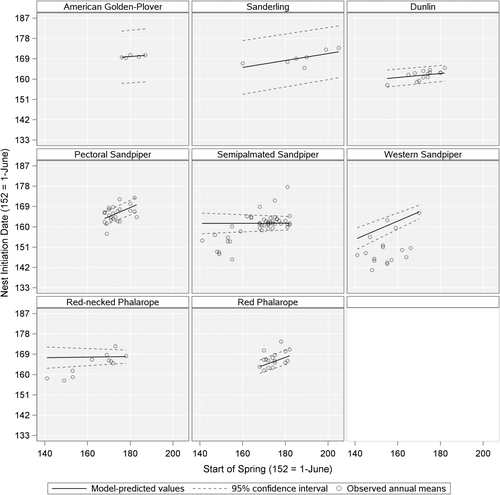
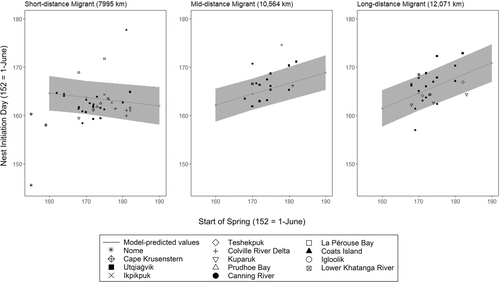
Responses to changes in the SOS varied across our focal species (Table 3; Figure 3). No species completely tracked variation in the SOS, however, Western Sandpipers (βSOS = .404, 95% CI: .265 to .543), Pectoral Sandpipers (βSOS = .405, 95% CI: .246 to .564), and Red Phalaropes (βSOS = .344, 95% CI: .182 to .507) showed the strongest responsiveness (i.e., slopes of NID as function of SOS; Figure 3). Values of zero for Pagel's λ indicated that phylogenetic relationships (i.e., common descent) were not important determinants of NID (see Supporting Information). Rather, across the species in our study, the most parsimonious model for nest initiation day included migration distance and its interaction with the SOS, with the effects of the other three species traits not being supported by our data (Table 4). Contrary to our prediction, the shorebird species classified as long-distance migrants (>10,000 km, e.g., American Golden-Plover, Pectoral Sandpiper, Western Sandpiper, and Red Phalarope) showed greater phenological responsiveness than medium and short-distance migrants, like Dunlin, Red-necked Phalarope, and Semipalmated Sandpiper (Figure 4).
| Model structurea | K b | −2logL | ΔAIC | w i |
|---|---|---|---|---|
| SOS * Species | 19 | 53,126.1 | 0.0 | 1.0 |
| SOS + Species | 12 | 53,276.4 | 113.7 | 0.0 |
| Species | 11 | 53,291.9 | 125.7 | 0.0 |
| SOS | 5 | 54,190.5 | 1005.0 | 0.0 |
| Intercept | 4 | 54,204.9 | 1016.8 | 0.0 |
- Abbreviations: −2logL, deviance; SOS, start of spring; wi, Akaike weight.
- a The + between variables indicates an additive effect, the * denotes interaction; where interactions are listed, main effects were also included.
- b Number of parameters estimated.
| Model structurea | K b | −2logL | ΔAIC | w i |
|---|---|---|---|---|
| SOS * Migration Distance | 8 | 53,166.2 | 0.0 | 1.0 |
| SOS * Body Mass | 8 | 53,268.7 | 94.4 | 0.0 |
| SOS * Seasonal Timing of Breeding | 8 | 53,277.2 | 102.9 | 0.0 |
| SOS * Expected Female Reproductive Effort | 8 | 53,308.1 | 133.8 | 0.0 |
| SOS + Migration Distance | 7 | 53,313.1 | 135.8 | 0.0 |
| SOS | 6 | 53,315.5 | 135.3 | 0.0 |
| Intercept | 5 | 53,332.2 | 149.3 | 0.0 |
- Abbreviations: −2logL, deviance; SOS, start of spring; wi, Akaike weight.
- a The + between variables indicates an additive effect, the * denotes interaction; where interactions are listed, main effects were also included.
- b Number of parameters estimated.
3.3 Intraspecific spatial variation in responsiveness
As expected, timing in nest initiation in Semipalmated Sandpiper varied strongly across sites, with earliest nesting at westerly sites (Nome, least-square means estimate, lsm = 151, 95% CI = 149 to 153; Cape Krusenstern, lsm = 157, 95% CI = 154 to 160) and latest nesting in the east (La Pérouse Bay, lsm = 170, 95% CI = 166 to 174; Coats Island, lsm = 171, 95% CI = 167 to 175). Akaike weights (wi), however, provide an evidence ratio of only 20% in favour of including an SOS by site interaction (Table 5), suggesting that responsiveness of this species to variations in spring phenology does not differ across sites.
| Model structurea | K b | −2logL | ΔAIC | w i |
|---|---|---|---|---|
| Site | 12 | 15,727.3 | 0.0 | 0.8 |
| Site + SOS * Site | 21 | 15,711.7 | 2.4 | 0.2 |
| SOS | 4 | 15,803.8 | 60.5 | 0.0 |
| Intercept | 3 | 15,830.6 | 92.0 | 0.0 |
- Abbreviations: −2logL, deviance; AIC, Akaike's information criterion; SOS, start of spring; SOS * Site, start of spring by site; wi, Akaike weight.
- a The + between variables indicates an additive effect, the * denotes interaction; where interactions are listed, main effects were also included.
- b Number of parameters estimated.
4 DISCUSSION
By analyzing breeding data for eight species of Arctic-nesting shorebirds that were collected over large spatial and temporal scales (18 sites and 23 years), our study identified advances in NID ranging from 0 to 0.4 days earlier per day of advancing SOS (Figure 3). This finding is consistent with previous studies that suggest over time, nesting phenology is advancing at variable rates among some Arctic-breeding shorebird species (Leung et al., 2018; Liebezeit et al., 2014; Saalfeld & Lanctot, 2017). Controlling for the SOS, we further assessed potential effects of four life history traits and looked for site-specific effects for the most widely studied species, Semipalmated Sandpipers. Species with longer migration distances showed a stronger relationship with the SOS than those with shorter migrations. Additionally, we did not detect site-specific variation in responsiveness of Semipalmated Sandpipers. Taken together, these findings improve our understanding of factors influencing the adaptability of avian fauna to ongoing environmental change, as maintaining synchrony with variable timing of green-up may allow certain species to better track concurrent annual fluctuations in resource availability for offspring, thus reducing potential trophic mismatch and fitness declines (Kentie et al., 2018; Reneerkens et al., 2016; Saalfeld et al., 2021).
Life history strategies of shorebirds that nest at northern latitudes are adapted to highly seasonal and variable environments. Unfavorable weather can make daily energy expenditure high and create unpredictable breeding and feeding conditions for parents and chicks and can also impact fitness in subsequent seasons or life stages (Piersma et al., 2003; Vézina et al., 2012). Flexibility in the timing of nesting in response to annual variability in weather conditions is expected (Doxa et al., 2012; Hällfors et al., 2020; Messmer et al., 2021), but responses of our focal species to changes in SOS were variable. As illustrated recently, some shorebird species might be reaching the limits of phenological responsiveness, particularly if changes in climatic events are becoming more variable, rather than undergoing a directional shift (Schmidt et al., 2023). Although our long-term data on SOS do suggest a negative linear trend in timing of spring green-up, they also indicate high variability (Figure 2).
Further studies to evaluate the directionality of Arctic spring green-up across broad spatial and temporal scales will be particularly important in the context of selection for earlier laying dates. In Pied Flycatchers (Ficedula hypoleuca), for example, directional increases in spring temperature have led to changes in spring arrival and reproduction for both captive and wild birds that indicate an evolutionary response to climate change (Helm et al., 2019; Visser et al., 2015). Across species, differences in the rate of such responses are potentially related to variation in life span or generation time (Berteaux et al., 2004; Thackeray et al., 2010). Among birds, however, evidence that phenological responsiveness is greater for longer-lived species is equivocal (Sandvik & Einar Erikstad, 2008; Vegvari et al., 2010). Conflicting results may be related to the fact that life span is correlated with other life history traits, but the degree of environmental variability that different species experience is likely an important consideration in terms of selective pressure on plasticity in nest initiation (Chmura et al., 2019; Gienapp et al., 2014). To more broadly understand the capacity for evolutionary adaptations to climate change in Arctic-nesting shorebirds, longitudinal studies that span multiple generations and that consider systematic differences in the environment over space and time are needed.
In contrast to our expectation that species migrating over shorter distances would show a stronger relationship between timing of nesting and green-up, we found that the relationship between reproductive timing and SOS was stronger for long distance migrants. This finding contradicts the idea that long-distance migrants have limited behavioral or evolutionary capacity to respond to changing environments. It also differs from other findings that report greater phenological responsiveness for short-distance migrants or no effect of migration distance, particularly with respect to spring arrival dates (Barton & Sandercock, 2018; Pulido & Widmer, 2005; Travers et al., 2015; Zaifman et al., 2017; Zalakevicius et al., 2006). Our results join a growing number of studies that have identified greater phenological responsiveness in long-distance migrants and suggest that their assumed lesser ability to adapt timing of life history events to changes in climate may be overestimated (Haest et al., 2020; Helm et al., 2019; Jonzén et al., 2006).
Our study further highlights that factors affecting shifting arrival dates and timing of nesting are not necessarily directly linked, and that studies that simultaneously investigate phenological shifts in multiple aspects of life histories will be necessary to obtain a more complete understanding of phenological responsiveness. Among Barnacle Goose (Branta leucopsis) populations breeding at different latitudes, for example, responsiveness during nesting was constrained by timing of arrival relative to snowmelt (Lameris et al., 2019). A similar finding was shown for Northern Wheatears (Oenanthe oenanthe), where responsiveness to early green-up was limited by the interval between arrival and breeding (Sander et al., 2021). Taken together, such studies indicate the importance of considering responses to climate change in the context of the entire annual cycle—and specifically that carry-over effects (conditions during migrating affecting arrival time) likely influence phenological responsiveness (Finch et al., 2014). Data on timing of arrival for Arctic-breeding shorebirds is not broadly available due in part to logistic constraints, but we suggest that such information will be particularly valuable in the context of understanding their ability to track variations in spring phenology (Meltofte et al., 2021). More generally, such findings suggest that ongoing studies—which use more precise estimates of life history traits—will allow for a more nuanced understanding of the factors affecting phenological responsiveness at finer spatial scales.
Explanations for the stronger SOS responsiveness of longer-distance migrants in our study may be reflecting phenological advancements or individual plasticity associated with the environmental conditions encountered at non-breeding or stopover locations (Conklin et al., 2021; Ely et al., 2018; Stutzman & Fontaine, 2015). It is also possible that our time series was insufficient to detect the dynamic nature of climate-phenology relationships, which in turn might have dampened differences in climate sensitivity between short- and long-distance migrants (Kolářová et al., 2017). In either circumstance, our findings highlight that understanding the underlying mechanisms by which migratory species adjust phenology is a key information need. If long-distance migrants are adjusting breeding phenology in response to non-breeding ground conditions, their responsiveness under future climate change scenarios is likely to be constrained as climatic correlations between non-breeding and breeding become less predictable or if there is a threshold beyond which they can no longer adjust (Garonna et al., 2016; Lawrence et al., 2022; Senner, 2012). Further, it remains important to assess phenological responses to climate change over the context of the entire life cycle, and to understand how adjustments in one stage influence subsequent stages (Layton-Matthews et al., 2020; Meltofte et al., 2018).
We did not find evidence for effects of other life history traits, such as seasonal timing of breeding, female body mass, or expected female reproductive effort, on phenological responsiveness in our study species. The cues used to determine timing of breeding, in general, are poorly understood and such factors warrant continued consideration at broader taxonomic, spatial, and temporal scales (Bründl et al., 2020; Cohen et al., 2018; Messmer et al., 2021). For example, settlement strategies vary among Arctic shorebirds from conservative, with high site fidelity and relatively constant population densities, to opportunistic, with low site fidelity with high annual variation in nest densities (Saalfeld & Lanctot, 2015, 2017). Settlement patterns could also be an important factor affecting reproductive phenology in shorebird species, but the evidence remains equivocal. Consistent with McGuire et al. (2020), who reported that NID responses to snow melt did not correspond with variation in settlement strategy, our most responsive species included a range restricted species that selects breeding locations conservatively (Western Sandpiper) and two highly vagile species that are opportunistic in site settlement (Pectoral Sandpiper, Red Phalarope).
Our intraspecific comparison of sensitivity to climate variability among populations of Semipalmated Sandpipers did not detect an interaction between breeding site and changes in spring phenology, suggesting that populations of this species are similarly responsive across their range. Although we did not test the effects of latitude or longitude directly, our results suggest that Semipalmated Sandpiper are somewhat distinct—despite being exposed to a broad range of SOS values across their breeding distributions, their responses to site-level variation in SOS is remarkably consistent. In apparent contrast, Purple Martin (Progne subis) modify laying date more strongly in response to climate change with increasing breeding latitude, and Hudsonian Godwit (Limosa haemastica) have different responses to climate between western (Alaska) and eastern (Churchill, Manitoba) breeding populations, likely related to use of different phenological cues among populations (Senner, 2012; Senner et al., 2017; Shave et al., 2019). Instead, Semipalmated Sandpiper populations appear to be using similar cues (or a set of cues with high spatiotemporal correlation) to time nest initiation across their breeding range, like American Golden-Plover, where timing of migration is driven by snow melt at breeding sites across disparate breeding populations (Lamarre et al., 2021).
Available evidence suggests that timing of snow melt also influences nesting phenology for Semipalmated Sandpipers, possibly through effects on availability of key arthropod food resources (Liebezeit et al., 2014; Mortensen et al., 2016). Under this scenario, geographic variation in phenological mismatch may be linked to the decoupling of snow melt from peak abundance of arthropods at some breeding sites but not others, due to spatially heterogeneous changes in climate, or possibly due to variation in re-nesting potential across sites (Grabowski et al., 2013; Kwon et al., 2019). Spatial variation in the demographic characteristics of breeding populations of Semipalmated Sandpipers related to annual life cycle effects such as over-summering could also contribute to geographic variation in mismatch (Ydenberg et al., 2022). Future studies investigating mechanisms that determine annual schedules across multiple spatial and taxonomic scales will be critical for understanding the consequences of apparent spatial uniformity in phenological responsiveness (Briedis et al., 2016). In addition, we suggest that ongoing studies continue to monitor the responses of birds to climate variability, while also testing for cues that explain observed responses (Chmura et al., 2019; Gutiérrez & Wilson, 2021).
AUTHOR CONTRIBUTIONS
Eveling A. Tavera: Conceptualization; formal analysis; writing – original draft; writing – review and editing. David B. Lank: Conceptualization; data curation; writing – original draft; writing – review and editing. David C. Douglas: Conceptualization; data curation; formal analysis; methodology; visualization; writing – original draft; writing – review and editing. Brett K. Sandercock: Data curation; funding acquisition; writing – review and editing. Richard B. Lanctot: Data curation; funding acquisition; methodology; writing – review and editing. Niels M. Schmidt: Data curation; funding acquisition; writing – review and editing. Jeroen Reneerkens: Data curation; investigation; writing – review and editing. David H. Ward: Conceptualization; data curation; funding acquisition; methodology; writing – review and editing. Joël Bêty: Data curation; funding acquisition; writing – review and editing. Eunbi Kwon: Data curation; investigation; methodology; writing – review and editing. Nicolas Lecomte: Data curation; funding acquisition; writing – review and editing. Cheri Gratto-Trevor: Data curation; funding acquisition; writing – review and editing. Paul A. Smith: Data curation; funding acquisition; writing – review and editing. Willow B. English: Data curation; investigation; writing – review and editing. Sarah T. Saalfeld: Data curation; investigation; methodology; writing – review and editing. Stephen C. Brown: Data curation; funding acquisition; writing – review and editing. H. River Gates: Data curation; investigation; writing – review and editing. Erica Nol: Data curation; funding acquisition; writing – review and editing. Joseph R. Liebezeit: Data curation; funding acquisition; investigation; writing – review and editing. Rebecca L. McGuire: Data curation; investigation; writing – review and editing. Laura McKinnon: Data curation; funding acquisition; writing – review and editing. Steve Kendall: Data curation; funding acquisition; writing – review and editing. Martin Robards: Data curation; funding acquisition; writing – review and editing. Megan Boldenow: Data curation; investigation; writing – review and editing. David C. Payer: Data curation; funding acquisition; writing – review and editing. Jennie Rausch: Data curation; funding acquisition; investigation; writing – review and editing. Diana V. Solovyeva: Data curation; funding acquisition; writing – review and editing. Jordyn A. Stalwick: Data curation; formal analysis; visualization; writing – review and editing. Kirsty E. B. Gurney: Conceptualization; data curation; formal analysis; funding acquisition; methodology; project administration; supervision; validation; visualization; writing – original draft; writing – review and editing.
ACKNOWLEDGEMENTS
We thank all the field assistants, field crew leaders, grad students and researchers who helped collect the field data for this project throughout the years. Thanks to local communities and landowners, including the Ukpeaġvik Iñupiat Corporation, the people of the Inuvialuit Settlement Region, Sitnasuak Native Corporation at Nome, Alaska, the Kuukpik Corporation at Nuiqsut, Alaska, the North Slope Borough of Alaska, and the community of Coral Harbour, Nunavut for permitting us to conduct research on their lands. The funding agencies that made this study possible included Alaska Department of Fish and Game Partner Program, Bureau of Land Management, Disney Conservation Awards, Kresge Foundation, Liz Claiborne/Art Ortenberg Foundation, U.S. Fish and Wildlife Avian Influenza Surveillance Grants, WCS Private Donors, and the U.S. Geological Survey's (USGS) Changing Arctic Ecosystem Initiative that is supported by funding from the Wildlife Program of the USGS Ecosystem Mission Area. U.S. Fish and Wildlife Service (Region 7 Migratory Bird Management Division), National Fish and Wildlife Foundation, Bureau of Land Management (Fairbanks District Office), University of Alaska Fairbanks, University of Colorado Denver, Kansas State University, and the University of Missouri Columbia. Core support for the Arctic Shorebird Demographics Network was provided by the Arctic Landscape Conservation Cooperative, National Fish and Wildlife Foundation (grants 2010-0061-015, 2011-0032-014, 0801.12.032731, and 0801.13.041129) and the Neotropical Migratory Bird Conservation Act (grants F11AP01040, F12AP00734, and F13APO535). Additional funding to participating sites was provided by: Arctic Goose Joint Venture, Arctic National Wildlife Refuge, BP Exploration (Alaska), Canada Fund for Innovation, Canada Research Chairs, Cape Krusenstern National Monument Grant, Centre for Wildlife Ecology at Simon Fraser University, Churchill Northern Studies Centre, Cornell University Graduate School Mellon Grant, Ducks Unlimited Canada, Environment and Climate Change Canada, FQRNT (Quebec), Government of Nunavut, Indigenous and Northern Affairs Canada, Kresge Foundation, Manomet Inc., Mississippi Flyway Council, Murie Science and Learning Center Grants, National Science Foundation (Office of Polar Programs grant ARC-1023396 and Doctoral Dissertation Improvement grant 1110444), Natural Resources Canada (Polar Continental Shelf Program), Natural Sciences and Engineering Research Council of Canada (Discovery Grant and Northern Supplement), Northern Studies Training Program, Selawik National Wildlife Refuge, Trust for Mutual Understanding, Université du Québec à Rimouski, Université of Moncton, and the Garfield Weston Foundation. Logistical support was provided by the Barrow Arctic Science Consortium, BP Exploration (Alaska), Kinross Gold Corporation, the Umiaq, LLC, Selawik National Wildlife Refuge (USFWS), ConocoPhillips Alaska, and Sirmilik National Park (Parks Canada). Thanks to Zackenberg Research Station in Greenland. And thanks to Churchill Northern Studies Centre, Canada, and the Canadian Wildlife Service for logistic support in La Perouse Bay. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data used for the analyses in this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.11095196.




