Shifting the paradigm: The role of introduced plants in the resiliency of terrestrial ecosystems to climate change
Abstract
Current ecological communities are in a constant state of flux from climate change and from species introductions. Recent discussion has focused on the positive roles introduced species can play in ecological communities and on the importance of conserving resilient ecosystems, but not how these two ideas intersect. There has been insufficient work to define the attributes needed to support ecosystem resilience to climate change in modern communities. Here, I argue that non-invasive, introduced plant species could play an important role in supporting the resilience of terrestrial ecosystems to climate change. Using examples from multiple taxonomic groups and ecosystems, I discuss how introduced plants can contribute to ecosystem resilience via their roles in plant and insect communities, as well as their associated ecosystem functions. I highlight the current and potential contributions of introduced plants and where there are critical knowledge gaps. Determining when and how introduced plants are contributing to the resilience of ecosystems to climate change will contribute to effective conservation strategies.
1 INTRODUCTION
In response to climate change, human-induced ecosystem degradation, and failed efforts to alleviate biodiversity loss, conserving resilient ecosystems (see glossary Box 1)—those that can retain their structure and function despite environmental change—is an increasingly important conservation strategy. Recent international agreements such as the Aichi Biodiversity Targets and the Sustainable Development Goals include conserving resilient ecosystems as a key priority (Willis et al., 2018). Many restoration projects are now required to evaluate the ability of a restored system to withstand impacts from climate change and maintain its resiliency (e.g., Prober et al., 2019). Yet it remains unclear what community attributes (e.g., species composition) are actually needed to confer this resilience (Timpane-Padgham et al., 2017; Willis et al., 2018).
BOX 1. Glossary (terms initially introduced in text in italics)
Ecosystem function—Process related to ecosystem-level transfers of energy and materials. Examples include primary production, water and nutrient regulation, and pollination. For example, insect herbivores can have large effects on ecosystem cycling by changing the quality, quantity, and timing of plant detrital inputs.
Ecosystem structure—Properties related to the numbers and kinds of objects that ecosystems contain (e.g., diversity, composition, and biomass).
Functional composition—Identities and relative abundances of species present in a given place at a given time characterized by their functional traits.
Functional redundancy—The ability of multiple species to perform a similar function.
Functional traits—Characteristics of species influencing organismal performance. Characteristics can include morphology, phenology, physiology, and behavior.
Invasive species—An introduced or non-native species that is, or is likely to become, widespread and has negative impacts on the community or ecosystem. I note that there can be substantial disagreement about the ‘invasive’ classification and that invasiveness can depend on local biotic and abiotic factors.
Introduced species—Species in a given area whose presence there is due to intentional or accidental introduction as a result of human activity.
Non-native species—A species that colonizes an area via natural dispersal during a range shift.
- Resistance—a component of resilience, the amount of change a system can undergo and still retain the same controls on structure and functioning; and
- Recovery—a component of resilience, which is how quickly ecosystem structure and function recover.
Introduced species are part of many landscapes, and their introductions are predicted to increase (Guo et al., 2021; Seebens et al., 2018). In some regions and cities, introduced species already make up more than half of all species and represent large fractions of the regional species-pool (Schlaepfer, 2018). Among introduced species, a small percentage become invasive (Williamson & Fitter, 1996) and threaten native biodiversity and ecosystem function (Guy-Haim et al., 2018; Vilà et al., 2011; Vitousek et al., 1997).
Simultaneously, many introduced plants have positively contributed to local plant species diversity (Sax & Gaines, 2008)—having either increased local diversity or at least resulted in no loss of plant diversity over recent decades (Table 1; Thomas & Palmer, 2015; Vellend et al., 2013, 2017). Further, many plant species introductions have increased or maintained ecosystem function (e.g., productivity; Table 1; Fridley, 2012; Liao et al., 2008; Livingstone et al., 2020; Mascaro et al., 2012; Wilsey et al., 2009). For example, in the deciduous forests of the eastern United States, introduced shrub and liana species are extending the autumn growing season by an average of 4 weeks compared with natives. This has extended the period of carbon assimilation into autumn (Fridley, 2012). There is also increasing evidence that non-native plants can play positive roles in plant–insect communities (Table 1; e.g., Darst et al., 2024; Leuzinger & Rewald, 2021; Schlaepfer et al., 2011).
| Study | Introduced species type | Impacted group | Effects of introduced plants on plants or insectsa | Demonstrated or hypothesized impacts of introduced plants on ecosystem function or resiliency |
|---|---|---|---|---|
| Castro-Diez et al. (2019) | Trees (125 spp.) | Native plants | N/A | Mixed: (1) Increase most regulating ecosystem services; (2) Decrease some provisioning services |
| Castro-Diez et al. (2016) | Succulent chamaephytes genus | Native plants | Mixed: (1) Increase frequency of some functional traits (woodiness and evergreenness); (2) Functional homogenization (richness, divergence, redundancy); (3) None: No impact on some ecosystem properties (e.g., soil organic C) | Potentially minimal impact on function because of weak relationship between functional structure and ecosystem properties. Potentially lower resilience because of lower functional diversity and redundancy |
| Charlebois and Sargent (2017) | Flowering plants | Native plants-indirect effects | None: No pollinator-mediated impacts on native plants | None: No effect on ecosystem service (pollination) |
| Fried et al. (2019) | Single vine species | Native plants-community effects | Landscape scale: Reduced γ-diversity. Minimal change in functional richness; Plot scale: Reduced alpha-diversity and functional richness | Potential decrease in ecosystem resilience with lower functional richness in some traits |
| Native plants-trait effects | Taller communities | Potential increase in productivity | ||
| Insects-pollinators | Shorter flowering duration | Potential decrease in pollination | ||
| Native plants-direct effects | Lower diversity of life forms | Potential increase in river bank erosion | ||
| Harmon-Threatt and Kremen (2015) | Flowering plants | Insects-pollinators | No difference in protein or amino acid composition of pollen used by generalist pollinators | None: No impact on pollination |
| Kaiser-Bunbury et al. (2017) | Woody shrubs | Insects-pollinators | Removal of NN increased pollination (higher flower visitation, more fruit) | Decrease in ecosystem function because of lower functional redundancy and complementarity when NN is present |
| Native plants-direct effects | Removal of NN reduced indirect facilitation of native plants | Potential increase in pollination | ||
| Mandle and Ticktin (2015) | Understory plants in a savanna woodland | Native plants-direct effects | Both taxonomic and functional diversity of recipient plant communities increased | Potential increase in stability and resilience of function |
| Native plants-direct effects | Reduced functional evenness of effect traits | Potential decrease in ecosystem stability through lower maintenance of productivity | ||
| Insects-pollinators | Increased variation in butterfly pollination | Potential increase in pollination | ||
| Ostertag et al. (2015) | Forest plants (15 spp.) | Native plants-direct effects | Introduced forest plants increased seedling recruitment of native species | Potential increase in carbon cycling |
| Pec and Carlton (2014) | Grasses | Native plants-direct effects | Increased total and reproductive biomass because NN inhibits the establishment of woody species | Increase in ecosystem function |
| Petsch et al. (2022) | Multiple species | Multiple species | No effect on β diversity or functional β diversity; decrease taxonomic and phylogenetic diversity | Potentially no negative impact on ecosystem function |
| Salisbury et al. (2015) | Ornamental garden plants | Insects-pollinators | Greater floral resources led to an increase in visits. Larger impact for generalists | Potential increase in pollination |
| Lower abundance of total pollinators | Potential decrease in pollination | |||
| Staab et al. (2020) | Ornamental garden plants | Insects-pollinators | Increases in floral resources. No impact on total flower visits, species richness of flower-visitors | Potential increase in pollination |
| van Hengstum et al. (2014) | Herbaceous and woody plants | Insects-non-pollinators (above-ground arthropods) | Lower arthropod abundance and taxonomic richness | Unclear effects because herbivores and predators similarly affected |
| Williams et al. (2011) | Flowering plants | Insects-pollinators | Greater bee visits in two ‘more disturbed’ habitat types; no difference in natural and semi-natural habitats | Potential no impact or positive increase in pollination. Unclear whether introduced plants are acting as substitutes or additional resources |
| Xu et al. (2022) | Grass, shrub, and tree species | Native plants and soil | Mixed: (1) Enhanced soil microbial activity and soil nutrient content; (2) reduced plant diversity richness and evenness | Mixed: Potential increased soil nutrient cycling. Potential decreases in ecosystem function due to correlations between plant diversity and soil pH and nutrient content |
- a Result is always reported in invaded sites or introduced species relative to non-invaded sites or native species.
Despite this evidence, the removal of introduced species is still included in standard practice guidelines that aim to restore ecosystems to their “native state” (e.g., Canadian Wildlife Federation, 2020; Chazdon et al., 2021; Gann et al., 2019; Society for Ecological Restoration, 2023). This is in part because governments and conservation organizations spend an enormous amount of their time and funding on targeting the control of invasive species (D'Antonio & Meyerson, 2002; Harmon-Threatt & Chin, 2016). However, because conservation practice is complex (e.g., maximizing the utility of herbicide application while minimizing undesirable outcomes) and biases against introduced species remain (Sax et al., 2022, 2023), this guideline can have negative repercussions. For example, the removal of non-invasive introduced plants (e.g., Gornish et al., 2018), non-target effects in the community (e.g., with chemical control; Olszyk et al., 2013), or cause the influx of non-target invasive species (i.e., invasion treadmill; Pearson et al., 2016; Thomas & Reid, 2007). As a result, current practices to remove all introduced species could be detrimental to the conservation of resilient ecosystems.
- The composition of modern communities is in constant flux, largely due to human activities (i.e., climate change and species introductions; Carroll et al., 2023).
- Ecosystems with larger regional species pools have higher resilience (Oliver, Isaac, et al., 2015). In temperate regions, many regional species pools are now much larger than they were historically because of species introductions (Sax & Gaines, 2003).
- Introduced species can tolerate conditions associated with modified landscapes better than native species (Pyšek et al., 2010).
- Introduced species are more likely to respond positively to climate change than natives (Liu et al., 2017; Schlaepfer & Lawler, 2023; Zettlemoyer et al., 2019; but see Sorte et al., 2013).
- As introduced plants often differ in trait space from native plants (Cordell et al., 2016; Strauss et al., 2006; Wolkovich & Cleland, 2011), they can provide “new” functions or “replace” functions formerly served by native species (e.g., shelter and food; Table 1; Schlaepfer et al., 2011).
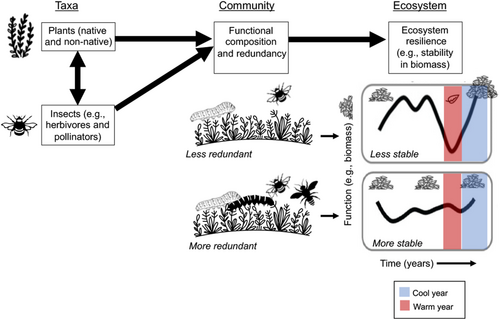
I postulate that introduced plants could contribute to ecosystem resilience to climate change via their roles in plant and insect communities and their associated ecosystem functions (e.g., productivity, pollination; Box 1; Table 1; Figure 1). Below, I describe how the functional composition of communities provides ecosystem resilience to climate change, and then I outline this hypothesis in more detail.
2 GENERAL THESIS: MAINTAINING STABILITY IN ECOSYSTEM FUNCTION RATHER THAN STRUCTURE IS KEY TO PROVIDING ECOSYSTEM RESILIENCE TO CLIMATE CHANGE
Ecosystem resilience is typically defined as the capacity of a system to maintain functioning, structure, and feedback in the face of environmental change. It involves a combination of resistance—the amount of change a system can undergo and maintain structure and function—and recovery—the ability of system functioning to return to pre-perturbation levels.
One attribute that is hypothesized to increase the resilience of ecosystems is the functional composition of communities (i.e., the types of species present) because species vary in their capacity to persist in the face of environmental perturbations (Hodgson et al., 2015; Willis et al., 2018). Species in the community with traits conferring reduced sensitivity to the type of environmental change occurring will increase the resistance of the ecosystem to that change by maintaining ecosystem function (Figure 1; Cadotte et al., 2011; Díaz et al., 2013; Oliver, Heard, et al., 2015). For example, drought-tolerant trees will continue to photosynthesize in drought years and thus contribute to temporal stability (i.e., resilience) in productivity, a key ecosystem function.
Another attribute thought to lead to resilience is functional redundancy—multiple species that perform similar functions. It increases the likelihood that species can compensate for one another if some are lost following disturbance or environmental change (Bernhardt & Leslie, 2013; Oliver, Heard, et al., 2015)—more drought-tolerant tree species are better than fewer drought-tolerant tree species.
Although the concept of resilience has traditionally involved the maintenance of ecosystem structure (e.g., diversity and composition) over time (Holling, 1973), the temporal stability of species composition itself should not be considered an attribute of resilience in modern landscapes (Carroll et al., 2023; Oliver, Heard, et al., 2015). Given shifts in community structure due climate change and species introductions, we should not expect species composition or species interaction networks to remain stable (CaraDonna et al., 2021; Carroll et al., 2023). In fact, species turnover can contribute to the stability of ecosystem function over time (i.e., resilience; Oliver, Heard, et al., 2015; Zhang et al., 2018). For example, van der Plas et al. (2016) showed that landscapes with lower turnover in tree species composition typically had fewer functions (e.g., timber production and litter decomposition) compared to landscapes with higher turnover. If species with important functional roles are lost due to climate change (e.g., drought-intolerant trees), then ecosystem function will decline unless new species with similar functional roles replace them (e.g., drought-tolerant trees; Oliver, Heard, et al., 2015). Therefore, maintaining ecosystem function in the face of climate change is likely to be a more effective strategy to achieve resilience than maintaining ecosystem structure.
I now discuss how introduced plants are contributing to ecosystem resilience to climate change through their roles in plant and insect communities and their associated ecosystem functions.
2.1 Plant communities
As climate changes, species with traits that reduce their sensitivity to the way climate is changing in an area (e.g., hotter and drier) will be favored and thus contribute to the resilience of the ecosystem to that change. Plant traits thought to confer an advantage under climate change in a general manner include: high growth rate, wide climatic tolerance, stress tolerant and high dispersal ability (Schweiger et al., 2010). Many introduced plant species possess these “favorable” traits (e.g., Dukes & Mooney, 1999; Theoharides & Dukes, 2007; Vilà et al., 2007): relatively strong dispersal abilities (Rejmanek & Richardson, 1996), minimal reliance on specialized mutualists (Van Kleunen et al., 2008), rapid growth rates (Grotkopp et al., 2010), tolerance of water stress (Alpert et al., 2000; but see Liu et al., 2017) and high phenotypic plasticity (e.g., Daehler, 2003). Therefore, introduced plants are likely already contributing disproportionately to the functional composition and redundancy of communities.
Introduced plants can maintain ecosystem function in the context of climate change, and thus, the resilience of ecosystems to climate change. This could occur if introduced plants (1) fulfill a similar functional role as native plant species (i.e., contributing to functional redundancy) that have been lost and/or (2) provide a critical role that cannot (yet) be performed by ‘local’ native plant species (i.e., contributing to functional composition; Leuzinger & Rewald, 2021). Here, I provide an example of each scenario.
Water scarcity due to climate change and other global change drivers has led to the decline of drought-sensitive native plants and the replacement of drought-tolerant introduced plants in the Northern Hemisphere (Kominoski et al., 2013; Perry et al., 2012). For example, Ulmus pumila, an introduced elm to North America, persists in riparian areas that are now too xeric for native Salicaceae. While it is considered to be invasive in some jurisdictions in the United States (Zalapa et al., 2010), little is actually known about the negative ecological impacts of its invasion (Reynolds et al., 2022). Ulmus pumila has similar structural traits to Populus species and has been shown to perform similar functional roles in the community (e.g., photosynthesis and wildlife habitat; Kominoski et al., 2013). Effectively, U. pumila is mitigating trait shifts in drought tolerance in the community and contributing to ecosystem resilience by providing stability in ecosystem function.
In the second scenario, introduced plants that play similar roles to native plants could contribute to ecosystem resilience until native plants colonize. There is strong evidence that many native plants are shifting their distributions to higher elevations (Gottfried et al., 2012; Grabherr et al., 1995; Lenoir et al., 2008). However, a colonization credit (i.e., delayed colonization) exists for a large proportion of warmth-demanding mountain native plants with lower dispersal ability (Rumpf et al., 2019). When directly compared, introduced plants are spreading upwards faster than natives (Dainese et al., 2017). Thus, introduced species could provide a functional buffer until low-dispersing native species colonize. Work is needed to determine how ecosystem function is changing along the elevational gradient (Payne et al., 2017). At low elevations, evidence suggests that introduced species are filling similar roles in the community (Dainese & Bragazza, 2012). If this is also the case at high elevations, introduced plants could be maintaining ecosystem function in the short- to near-term until low-dispersing native plants arrive.
Nurse plants are another example of the functional role introduced plants can play in the context of climate change. They improve the above-ground microclimate under their canopies by reducing climatic harshness (e.g., excessive solar radiation and extreme temperatures; Tapella et al., 2021). Thus, they can facilitate the regeneration of other species in areas where drought or extreme heat conditions are likely to become more common, thus increasing the stability of the plant community. Management of microclimates is increasingly considered an important conservation strategy to help buffer species from climate change (e.g., Suggitt et al., 2018).
Since introduced species have been shown to be more heat tolerant than related native species collected from the same habitats (Liu et al., 2017), they have the potential to act as nurse plants. For example, the European legume gorse (Ulex europaeus) acts as a nurse plant for native forest regeneration in areas of New Zealand (Norton, 2009). It shades out invasive grass and creates suitable microsites for the regeneration of native woody species (Norton, 2009). In Hawaii, two native shade-tolerant understory tree species have persisted under the canopies of introduced trees (Mascaro, 2011). It is unclear how generalizable these examples are. In the highland woodlands of central Argentina, an introduced shrub (Cotoneaster franchetii) was shown not to facilitate the regeneration of two native trees (Tapella et al., 2021). Additional work is needed to understand the functional roles of introduced plants in the context of climate change.
2.2 Insect communities
Many aspects of plant-insect interactions are being disrupted by climate change. These include phenological asynchrony, spatial mismatching, disrupted energetic matching, and changes in plant and insect community structure (e.g., Gómez-Ruiz & Lacher Jr, 2019; Harvey et al., 2023; Schweiger et al., 2010; Yang et al., 2021). These disruptions could lead to declines in ecosystem function provided by insects such as pollination and nutrient cycling (Burkle et al., 2013; de Manincor et al., 2023; Hamann et al., 2021; Schweiger et al., 2010). For example, species' phenological shifts in response to climate change can reshuffle the timing and overlap among interacting organisms, influencing the probability of interaction formation and network structure (CaraDonna et al., 2021). Over time, this can lead to the degradation of network structure and loss of ecosystem function (e.g., Burkle et al., 2013).
In cases where plant-insect interactions are sensitive to climate change, introduced plants could contribute to ecosystem resilience by providing new resources to insects (i.e., functional composition) and/or by strengthening existing networks and complimenting current native resources (i.e., functional redundancy). There is increasing evidence that native insects can quickly exploit newly available plants (Forister et al., 2013; Keeler & Chew, 2008; Singer et al., 1993). This suggests a plasticity in resource acquisition by native insects and that novel interactions with introduced species will continue to occur (Braga, 2023; Kleijn & Raemakers, 2008; Pearse & Altermatt, 2013). If so, introduced plants could help to buffer insects from negative impacts of climate change and therefore maintain the functional roles of insects (Schweiger et al., 2010). Here, I provide examples of each scenario.
As an example of how introduced plants can provide new resources to insects, the leaf phenology of many introduced species is advancing more with warming temperatures than native species (e.g., Wolkovich & Cleland, 2011; Zettlemoyer et al., 2019; but see Zettlemoyer et al., 2019). Extended leaf phenology or longer growing season relative to native species could extend the length of time food is available for native insect herbivores (Meineke et al., 2021), as it was for mammalian herbivores in an experimental California plant community (Waterton & Cleland, 2016).
Differences in phenology between native and introduced plants have also had implications for pollinators. A recent study found that introduced plants flowered later than natives in an endangered savanna ecosystem, likely because of the increased drought-tolerance of the introduced relative to the natives (Rivest et al., 2023). As a result, the native butterflies were entirely dependent on the nectar provided by introduced plants at the end of the season (Rivest et al., 2023). Given a lack of historical data, it is unclear whether the introduced plants have extended (i.e., provided new resources) or maintained the length of the season (i.e., replacing native resources) in this ecosystem (Rivest et al., 2023). There have been similar reports from other ecosystems where introduced plants have extended the season where nectar is available for pollinators (Frankie et al., 2019; Staab et al., 2020; Table 1). By providing late-season nectar, introduced plants could be buffering native butterflies from changing patterns of drought at the end of the season due to climate change.
Beyond these phenological effects, introduced plants could play a role in insect communities via the effects of climate change on resource quality. For example, increasing temperatures have the potential to change the quality of nectar (e.g., production and composition) and pollen (e.g., lipid concentrations) resources for pollinators (McCombs et al., 2022; Scaven & Rafferty, 2013). Warming has been shown to increase (McCombs et al., 2022) and decrease (e.g., Mu et al., 2015; Takkis et al., 2015) the quality or production of nectar and pollen (Hodge & Prasad, 2013; Russo et al., 2020) in native species. However, to my knowledge, only one study has investigated how warming has changed the pollen quality of introduced plant species (Russo et al., 2019) and not even in comparison to native species.
The effects of warming on the host plant quality of introduced species and, consequently, insect herbivory have also been poorly studied. In native plant species, warming has been shown to have many impacts on leaf quality with varying impacts on insect herbivores (e.g., Hamann et al., 2021; Jactel et al., 2019). To my knowledge, only a couple of studies have compared rates of insect herbivory on introduced versus native plant species under warming conditions (Lu et al., 2015; Welshofer et al., 2018). In these couple of cases, rates of herbivory were similar on the native and introduced plants, suggesting that host plant quality in introduced species may not have been affected by warming (Lu et al., 2015; Welshofer et al., 2018). If these results apply more broadly, introduced plants could compliment native food resources for insects under warming conditions.
In all these cases, the contribution of introduced plants to the persistence of these insect populations increases the likelihood that the functional roles of the insects (e.g., pollination and nutrient cycling; Merckx et al., 2013) and thus their contributions to ecosystem functioning are conserved. However, this contribution remains to be empirically demonstrated. With the significant loss of insect abundance and diversity over recent decades (Harvey et al., 2023; Seymour et al., 2020), ecosystem resilience is likely already threatened. As such, these potential positive effects of introduced plants on insect communities could prove vital to conserving ecosystem function and resilience.
3 MOVING FORWARD
Here, I argue two points: (1) we need to rethink the way we consider introduced species in the resiliency of ecosystems to climate change and (2) we should prioritize the maintenance of ecosystem function over ecosystem structure. I am not arguing that introduced species do not have negative impacts; rather, we need a clearer picture of the complex roles they play in our ecosystems and how they respond to climate change. Further, establishing that some introduced species have benefits is not evidence against the value of actively restoring native species. I now discuss where we need the development of knowledge from theoretical, subjective, and practical perspectives.
3.1 Theoretical perspective
The literature on the impacts of introduced plants on native biodiversity is restricted and biased (Charlebois & Sargent, 2017; Hulme et al., 2013; Parker et al., 2016). Research has traditionally focused on identifying the negative consequences of introduced species which has stifled our understanding of their overall impacts and how to effectively guide management decisions (Sax et al., 2022). Many studies that quantify these impacts have resulted in a publication bias in which species with known impacts are selected for investigation far more often than benign species (Guerin et al., 2018). Most research on the dynamics and impacts of plant invasions has evaluated patterns and effects over brief time periods (i.e., <4 years; Table 1; Flory et al., 2017). As such, little is known about the persistence of invasions and their long-term impacts on native species (Flory et al., 2017). Recent experimental evidence suggests that the impacts of plant invasions can decline over time (over 8 years; Flory et al., 2017). Finally, few studies have measured impacts on functional diversity (Petsch et al., 2022). Learning from past lessons (e.g., introducing Monterrey pines to New Zealand forestry, introducing smooth brome to feed North American cattle) about predictive invasive species traits and applying these lessons to constrain candidate introduced species will increase the success of this paradigm shift.
We need to move beyond assigning simple functions to species and consider the multitude of effects that introduced species can have—both positive and negative (Table 1; Figure 2). While not a comprehensive list, the examples in Table 1 demonstrate that most species have a variety of impacts, some of which might increase resiliency to climate change and others that might not. For example, in riparian habitats in southern France, an introduced vine species, Humulus japonicus, has had both negative and positive impacts on the native community and ecosystem function (Table 1; Figure 2). In another example, introduced shrub and liana species in the deciduous forests of the eastern United States have had a positive impact on carbon sequestration (Fridley, 2012) while simultaneously negatively affecting spring ephemeral forb abundance (Miller & Gorchov, 2004). Evaluating the net effects of introduced plants on ecological communities and ecosystem functioning would provide a clearer picture of their role in ecosystem resiliency in the context of climate change.
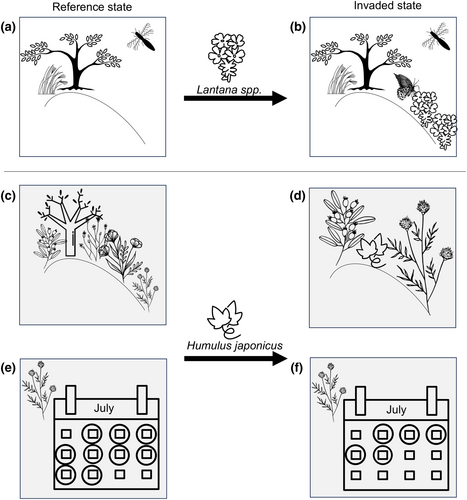
As evidence continues to accumulate of introduced plants providing alternative hosts and refuge for insects, determining when these shifts are maladaptive is a crucial next step (Braga, 2023; Rivest et al., 2023; Steward et al., 2022; Yoon & Read, 2016). One of the best-known examples where introduced plants provided a new resource to insects is from populations and sub-species of Edith's checkerspot butterfly (Euphydryas editha; Severns & Warren, 2008; Singer & Parmesan, 2018). At a site in Nevada, Plantago lanceolata, an introduced perennial, compensated for historical phenological asynchrony between E. editha and its native host plant. Euphydryas editha butterflies achieved higher fitness on P. lanceolata because P. lanceolata was available much longer than its short-lived annual native host, thus freeing the butterflies from a life history trade-off imposed by time constraints (Singer & Parmesan, 2018). While initially beneficial, the introduced host (P. lanceolata) ended up being an eco-evolutionary trap and contributed to the population extirpation of E. editha (Singer & Parmesan, 2018). However, this ‘trap’ has not been documented for any of the other E. editha populations feeding on P. lanceolata.
Testing the hypotheses I have outlined here and in Box 2 will require more field-based and experimental studies. One particularly fruitful avenue would be to create counterfactual scenarios (i.e., states that currently do not occur but could under different conditions; Carroll et al., 2023) that test the specific impact of introduced species on ecosystem functioning. For example, experimentally assessing the effects of vegetation restoration on ecosystem resilience (e.g., Kaiser-Bunbury et al., 2017) would test whether the removal of introduced plants leads to a decrease in resiliency. Alternatively, experimentally increasing the abundance of an introduced species should increase its impact on ecosystem function and would test whether the role of introduced species is context-dependent (e.g., based on its abundance; Ramus et al., 2017). Testing the impacts of introduced plants on insects and, consequently, ecosystem functioning is especially needed.
BOX 2. Outstanding questions
- In the provisioning of ecosystem functions, is there a point of saturation or threshold in the abundance of a single introduced plant species in a community, beyond which the introduced species provides no additional value or decreases ecosystem function?
- What is the role of introduced plants in mitigating the effects of climate change on food quality for herbivorous insects? Are introduced plants currently providing high-quality pollen and/or nectar for native pollinators, and how will this change with climate change?
- What are the evolutionary consequences of introduced-native interactions on native species?
- Does the beneficial role of introduced plants to insects depend on the overlap in vegetative or reproductive phenology of introduced and native plants in the community?
- How do we weigh the relative importance of competing dimensions of ecosystem function when an introduced species improves some but degrades others?
3.2 Subjective perspective
A lot of the literature, including this paper, has focused on whether introduced species have conservation value. Moving forward, it will be more efficient to focus on cases where introduced plants have the potential to increase resiliency and less on whether negative impacts are likely to be the most common outcome of introduced species. We need to figure out how to weigh the relative importance of competing dimensions of ecosystem function when an introduced species improves some functions but degrades others (e.g., U. pumila; Figure 2).
I join previous calls that we need to acknowledge that most species considered to be invasive probably do have some positive impacts (e.g., Figure 2; abundance of some butterflies—Shapiro, 2002; bees—Tepedino et al., 2008) and move away from dichotomies like “native is good, alien is bad” (Goodenough, 2010; Slobodkin, 2001). The reality is that the role of most introduced species in communities is uncertain or complex (Figure 2; Gilbert & Levine, 2013; Harmon-Threatt & Kremen, 2015; Sax et al., 2022).
3.3 Practical perspective
For restoration to be a more effective conservation strategy in maintaining ecosystem function over time, we need a better understanding of how introduced plant removal affects plant-insect communities and how it impacts the resiliency of those ecosystems to climate change. Getting a clear understanding of how a given introduced species affects the long-term resilience and function of a particular ecosystem is a daunting research challenge in the best of circumstances, and, like most things in ecology, it is likely to be context-dependent. The reality is that in most cases, the science will be far too uncertain to do a complete cost–benefit analysis of an introduced species' impacts on ecosystem functioning.
Regardless of scientific uncertainties about the exact role of introduced plants in ecosystems, restoration plans should better consider the potential ramifications of introduced species removal on the community. For example, while herbicide treatments decreased the cover of an introduced grass species in an Oregon grassland, they also resulted in many negative effects, namely, multiple secondary invasions and the suppression of growth of a butterfly's host plant (Bennion et al., 2020). In restored tallgrass prairies, often the introduced plants removed are legumes (Harmon-Threatt & Chin, 2016), which are a source of nitrogen and promotor of plant productivity. Further, legumes tend to offer high-quality pollen that is nutritionally important for bee reproduction (Harmon-Threatt & Chin, 2016; Winfree, 2010). In this case, the removal of introduced legumes could reduce ecosystem function and, consequently, resilience to climate change.
4 CONCLUDING REMARKS
Ultimately, the role of introduced plants in the resiliency of ecosystems to climate change will depend on many factors such as the relative tolerance of native and introduced plants to climate change, and how climate change will influence species interactions such as competition and facilitation. Our incomplete understanding of introduced species roles in the community could lead to an underestimate of the negative (e.g., extinction debt; Gilbert & Levine, 2013) and positive impacts of introduced species, which will only be known with time and monitoring. Given the ability of introduced species to tolerate and adapt to a broad range of environmental conditions, they are likely to play an increasingly important role in ecosystems, especially in places where native species can no longer persist.
AUTHOR CONTRIBUTIONS
Heather M. Kharouba: Conceptualization; writing – original draft; writing – review and editing.
ACKNOWLEDGEMENTS
Thanks to Dov Sax and two anonymous reviewers for their constructive feedback on this manuscript. I thank the University of British Columbia's Department of Geography for providing the space to write this manuscript, S. Rivest for many engaging discussions on the topic, and R. Sargent and M. Vellend for their time and constructive feedback on this manuscript.
CONFLICT OF INTEREST STATEMENT
The author declares that there are no conflicts of interest.
APPENDIX 1: LITERATURE CITED IN TABLE 1
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




