Climate warming alters subsoil but not topsoil carbon dynamics in alpine grassland
Abstract
Subsoil contains more than half of soil organic carbon (SOC) globally and is conventionally assumed to be relatively unresponsive to warming compared to the topsoil. Here, we show substantial changes in carbon allocation and dynamics of the subsoil but not topsoil in the Qinghai-Tibetan alpine grasslands over 5 years of warming. Specifically, warming enhanced the accumulation of newly synthesized (14C-enriched) carbon in the subsoil slow-cycling pool (silt-clay fraction) but promoted the decomposition of plant-derived lignin in the fast-cycling pool (macroaggregates). These changes mirrored an accumulation of lipids and sugars at the expense of lignin in the warmed bulk subsoil, likely associated with shortened soil freezing period and a deepening root system. As warming is accompanied by deepening roots in a wide range of ecosystems, root-driven accrual of slow-cycling pool may represent an important and overlooked mechanism for a potential long-term carbon sink at depth. Moreover, given the contrasting sensitivity of SOC dynamics at varied depths, warming studies focusing only on surface soils may vastly misrepresent shifts in ecosystem carbon storage under climate change.
1 INTRODUCTION
The response of soil organic carbon (SOC) cycling to global warming represents a critical feedback of terrestrial ecosystems to climate change (Koven, Hugelius, Lawrence, & Wieder, 2017; Melillo et al., 2017). Until now, most research has focused on topsoil carbon dynamics (Crowther et al., 2016; Koven et al., 2017). In contrast, subsoil (i.e., residing >20 cm below ground) containing more than half of global SOC stocks (Rumpel, Chabbi, & Marschner, 2012) remains poorly investigated in terms of its response to warming. This knowledge gap has emerged as one of the central uncertainties in our understanding of terrestrial carbon storage under climate change (Ahrens & Reichstein, 2017).
Subsoil carbon is conventionally assumed to be relatively stable and unresponsive to air warming due to its long turnover time and good insulation at depth (Harrison, Footen, & Strahm, 2011). However, according to the Intergovernmental Panel on Climate Change (IPCC), subsoils are projected to warm at roughly the same rate as surface soils over the next century (Hicks Pries, Castanha, Porras, & Torn, 2017; IPCC, 2013). More importantly, emerging evidence suggests that subsoils, having varied organic matter sources, microbial communities, and substrate availability compared to the topsoil (Rumpel et al., 2012), may show even stronger response to warming-induced shifts in microbial activities and functioning (Fontaine et al., 2007) in association with altered plant community structure and distribution (Keuper et al., 2017; Liu et al., 2018). For instance, plant roots are expected to grow deeper under warming-induced moisture or nutrient limitation (Johnson, Rygiewicz, Tingey, & Phillips, 2006; Liu et al., 2018; Wang et al., 2017), potentially increasing root inputs (including root litter and exudates) and enhancing carbon accumulation in subsoils that are relatively low in SOC content (Cotrufo et al., 2015). Alternatively, increased fresh carbon input and/or changing soil physical properties may lead to shifts in microbial communities (Cheng et al., 2017) and accelerate the degradation of native SOC (Fontaine et al., 2007; Jia et al., 2017). However, experimental evidence is lacking to demonstrate how the above processes jointly affect SOC dynamics in the subsoil. Detecting changes in subsoil carbon stock is particularly difficult given the long residence time of SOC (Rumpel et al., 2012) and counteracting effects of warming on various carbon pools (Feng, Simpson, Wilson, Williams, & Simpson, 2008). Hence, in-depth investigations into SOC composition and carbon allocation are essential to reveal changing SOC dynamics and to identify SOC pools that are sensitive to warming.
Deep soil carbon dynamics in high-latitude and high-altitude ecosystems warrant particular attention under warming because these soils store the vast majority of the global SOC pool (Tarnocai et al., 2009; Yang et al., 2008). In addition, these colder regions are experiencing a higher-than-average warming trend in recent years (Chen et al., 2013). On the world's highest and largest plateau, Qinghai-Tibetan Plateau (QTP), a natural warming trend in the past decade has led to a significant increase of SOC in the subsurface soil (10–30 cm) while showing no effect on the topmost soil (0–10 cm) in the alpine grasslands (Ding et al., 2017). These results stand in contrast with previous views that warming may induce large carbon losses from soils with a high SOC content (Crowther et al., 2016), especially in the high-latitude and high-altitude areas. Identifying the mechanisms governing these divergent responses of SOC to warming at different depths is critical to minimize uncertainty in future soil carbon feedback projections.
Here, we utilize a manipulative soil warming experiment in the QTP alpine grassland (Liu et al., 2018) to compare warming effects on the composition and sourcing of SOC pools at different depths. Consistent with field observations over QTP alpine grasslands for the past decades, 5 years of continuous warming increased grasses with deep roots and decreased sedges and forbs with shallow roots in our experiment, resulting in elevated belowground net primary productivity (BNPP) in the subsoil (30–50 cm; Liu et al., 2018). Compared to aboveground biomass (59.7 g/m2), roots are the primary carbon inputs to the QTP grassland soils (330.5 g/m2; Yang, Fang, Ji, & Han, 2009). With an enhanced BNPP in the subsoil, we anticipate a stronger warming impact on subsoil carbon dynamics compared to the topsoil (0–10 cm) in terms of new carbon accrual and labile-carbon-stimulated degradation of native SOC. Furthermore, the altered plant community composition (Liu et al., 2018) may have cascading effects on the quantity and quality of plant-derived carbon inputs into soils and consequently the chemical composition of soil organic matter. To reveal the detailed mechanisms, we use radiocarbon analysis coupled with soil fractionation to disentangle the allocation of new carbon among SOC pools of varied turnover times. Complementary molecular-level analyses, that is, biomarkers, nuclear magnetic resonance (NMR), and high-resolution mass spectrometry, are further employed to investigate the fate and degradation of various plant- and microbial-derived components in the soil. These state-of-the-art analyses of SOC components, complemented by in situ monitoring of ecosystem carbon fluxes, help to deliver a detailed understanding of how soil carbon dynamics respond to warming in this alpine grassland.
2 MATERIALS AND METHODS
2.1 Experiment design and soil sampling
The field warming experiment is located at the Haibei Alpine Grassland Ecosystem Research Station (101°19′E, 37°36′N, 3,215 m a.s.l.). The site has a continental monsoon climate with a mean annual temperature of −1.2°C and a mean annual precipitation of 489 mm. Soils at the site are Mat-Gryic Cambisol with a clay loam texture and a mean pH of 8.01. The surface layers of the soil are seasonally frozen with freezing initiating from the top in the late fall. The native plant community is dominated by Kobresia humilis, Carex przewalskii, Helictotrichon tibeticum, Stipa aliena, Saussurea pulchra, and S. pulchra. A full factorial design with two temperature levels, that is, control and year-round warming (W1; warmed by 1.5–1.7°C), and three precipitation levels (ambient, +50% precipitation, and −50% precipitation) were set up in July 2011 (details in Figure S1). Each treatment had six randomly distributed plots (1.8 m × 2.2 m each) on a homogeneous landscape. As warming rates are reported to be significantly higher in winter than the other seasons on the QTP (Chen et al., 2013), the experiment was expanded to include a winter warming (W2) treatment in January 2012. On an annual average basis, surface soil temperature in the W2 treatment increased by the same magnitude as that in the W1 plots, but with varied scenarios of seasonality (W2 increased by ~3°C during late-October to late-April and by 0.5–1°C for the rest of the year). Warming plots were heated by infrared heaters installed 1.6 m above ground while dummy heaters were installed above the control plots. Heating started immediately after installation and soil temperature was monitored continuously at the depths of 5, 10, and 20 cm.
Three soil cores (diameter of 3 cm, depth of 70 cm) were randomly collected from each plot of the control and W1 treatments in July 2011 (before warming) and from all control, W1 and W2 plots in the August of both 2013 and 2015 (after 3 and 5 years of W1 warming, respectively). Soils were separated into various depths and those from the same plot and same depth were homogenized in situ. For this study, we selected the topsoil (0–10 cm) and subsoil (30–50 cm for 2011; 30–40 cm for 2013 and 2015) from four plots of each temperature treatments (i.e., n = 4). A fraction of fresh soils was stored at −80°C immediately after sampling for glycerol dialkyl glycerol tetraether (GDGT) analysis. The rest was passed through 2 mm sieves and freeze-dried immediately with stones and visible roots removed.
Assuming that the warming treatments did not change the chemical composition of dominant species during our experimental period, we took four additional soil cores (40 cm in depth) from adjacent areas outside the control plots in August 2015 to analyze lignin phenols in plant roots. Root materials were picked out from the entire soil cores, immediately washed under water and grouped into six species, namely grasses (H. tibeticum and S. aliena), sedges (K. humilis and C. przewalskii), and forbs (S. pulchra and S. pulchra), which totally contributed to ~56% of aboveground net primary productivity at the site. All plant materials were kept at −20°C, freeze-dried, and ground into fine powder before analysis.
2.2 Ecosystem CO2 exchange measurement
To complement our SOC measurement related to warming effect on soil carbon stock changes, growing-season net ecosystem CO2 exchange (NEE) and ecosystem respiration (Reco) were measured with a transparent chamber (40 cm × 40 cm × 60 cm) attached to an infrared gas analyzer (LI-6400; LiCor) during 2013 and 2015. We measured NEE and Reco 2 days per month from May to October on sunny days during 9:00–11:00 (local time) for all treatments. For each measurement, six consecutive recordings of CO2 and water vapor concentrations were taken at 10 s intervals over a period of 60 s with a small fan running continuously inside the chamber during NEE measurement. The flux rate of CO2 was calculated based on the slope of the linear regression for the six concentration records in the time series. Reco was measured immediately after NEE measurement using a shade cloth to cover the transparent chamber. To calculate daily NEE, diurnal patterns of NEE at 2 hr intervals (over 24 hr) were monitored and a calibration coefficient of 0.17 (ratios of daily average values based on diurnal patterns to values measured during 9:00–11:00) was used. The calibrated daily values were then used to estimate growing-season NEE. Gross ecosystem productivity (GEP) was calculated as the difference between NEE and Reco. Positive and negative values of NEE indicate CO2 release and fixation, respectively. During the non-growing season (late-October to late-April), diurnal changes in Reco were small (Kato et al., 2006; Wang et al., 2014) and respiration of non-growing season was measured once a month using the LI-8100 Automated Soil CO2 Flux System with LI-8100-103 short-term chamber (Li-Cor Inc.). Reco represents NEE during the non-growing season due to the absence of photosynthesis.
2.3 Soil size fractionation and radiocarbon analysis
Soils were fractionated using a method modified from Wilson, Rice, Rillig, Springer, and Hartnett (2009) to examine the allocation of new carbon and biomarkers among macroaggregates (>250 µm), microaggregates (250–63 µm), and silt-clay fractions (<63 µm; details in Text S1.1). The organic carbon (OC) and 14C contents of all size fractions and bulk soil were measured after removal of inorganic carbon (Text S1.1). Radiocarbon contents were reported as ∆14C (‰) with field replicates (n = 4). As the subsoil showed varying Δ14C values among fractions (see Section 3.3), we further estimated the turnover time of subsoil (but not topsoil) OC fractions. Soil fractions were assumed to be in a steady state over the modeled period (~1 × 104 years) and their turnover time was estimated based on ∆14C accounting for both radioactive decay and incorporation of the bomb-derived 14C produced in the 1950s and 1960s (Torn, Swanston, Castanha, & Trumbore, 2009).
2.4 Biomarker analyses
A range of biomarkers (Table S1) was selected to examine changes in the source and degradation of SOC components that represent major plant and microbial contributions. Freeze-dried bulk soils (<2 mm) collected before (2011) and after warming (2013 and 2015) as well as soil size fractions in 2015 were first solvent extracted to remove extractable lipids and then sequentially subject to base hydrolysis and copper oxidation to isolate hydrolysable lipids (including suberin and microbial-derived lipids) and lignin phenols, respectively (Otto & Simpson, 2007). Root materials of the six plant species were also analyzed for lignin phenols. Bulk soils collected from 2015 (but not size fractions due to limited availability) were further analyzed for non-cellulosic sugars (hereafter referred to as “sugars”; Eder, Spielvogel, Kӧlbl, Albert, & Kӧgel-Knabner, 2010) and core GDGTs (Tierney, Schouten, Pitcher, Hopmans, & Sinninghe Damsté, 2012). The macroaggregates and silt-clay fractions of the subsoils in 2015 were solvent-extracted using dichloromethane and methanol to isolate core GDGTs for comparison with the bulk soil. Details regarding extraction procedures can be found in Text S1.2.
Biomarkers of interest were quantified after derivatization on a Trace 1310 gas chromatograph coupled to an ISQ mass spectrometer (MS; Thermo Fisher Scientific) or an Agilent 1200 liquid chromatograph and triple quadrupole MS using internal standards (Text S1.2). To better illustrate warming-induced changes to biomarker concentrations after removing inter-annual variabilities, relative changes of major molecular components were calculated as the concentration offset between warming (W1 and W2) and control plots relative to the concentration in the control plots of the respective sampling year and expressed as percentages.
Other than lignin phenols, hydroxy phenols, potentially derived from proteins, tannins, and/or lignin degradation products in the soil (Zaccone, Said-Pullicino, Gigliotti, & Miano, 2008), were also detected in substantial amounts in the roots of dominant plant species at the Haibei Station (Figure S2). In particular, sedges (including K. humilis and C. przewalskii) with shallow roots (<25 cm, with 95% of the root biomass distributed in the upper 10 cm) were most concentrated with hydroxy phenols while grasses (including S. aliena and H. tibeticum) with the deepest roots (down to 85 cm; Liu et al., 2018) were most enriched with lignin phenols in the root mass. Given the high volume of root mass, both hydroxy and lignin phenols are therefore considered to mainly derive from plants at our site.
2.5 Molecular characterization of bulk organic matter
To further characterize warming-induced changes in organic matter composition in the subsoil, water-extractable organic matter (WEOM) and bulk soil were examined by Fourier transform-ion cyclotron resonance mass spectrometry (FT-ICR MS) and NMR, respectively. Briefly, WEOM was extracted from freeze-dried, mixed subsoils using diluted HCl (0.1 M), and concentrated by solid-phase extraction with commercially available Bond Elut cartridges with styrene divinyl benzene polymer (PPL, Agilent; Text S1.3). FT-ICR MS analysis was performed on a solariX FT-ICR MS (Bruker Daltonic GmBH) equipped with an electrospray source and a 15 Tesla magnet at Oldenburg University (Text S1.3). Identified compounds were aligned according to their aromaticity index (AImod), ratios of oxygen-to-carbon and hydrogen-to-carbon in their formula (Koch & Dittmar, 2006) and divided into five groups: (a) polycyclic aromatics, (b) polyphenols, (c) highly unsaturated compounds, includes lignin degradation products, (d) unsaturated aliphatics and peptides, and (e) saturated compounds, including lipids and carbohydrates.
The HCl-extracted soil residues were further treated with hydrofluoric acid (10%) 15 times to remove paramagnetic materials and to concentrate SOC. The treated residue was rinsed repeatedly with deionized water, freeze-dried, and ground into fine powder for solid-state 13C NMR analysis. The 13C Cross Polarization/Magic Angle Spinning NMR spectra were acquired on a Bruker BioSpin Avance III 500 MHz NMR spectrometer with a 4 mm probe using spin rate of 13 kHz, contact time of 1 ms, recycle delay of 1 s, acquisition time of 0.0135 s, line broadening of 75 Hz, and with 2,048 time domain points. Structures were represented by alkyl (0–45 ppm), O-alkyl (45–110 ppm), aromatic and phenolic (110–165 ppm), and carboxylic and carbonyl (165–215 ppm) carbon. Alkyl/O-alkyl ratios, increasing with increasing degradation, were calculated by dividing the areas of the alkyl and the O-alkyl regions of the spectra (Simpson, Otto, & Feng, 2008).
2.6 Statistical analysis
All statistical analyses were performed using SPSS 18.0 (SPSS). Homogeneity of variances and normal distribution of the data were tested before applying parametric methods and log transformation was performed where necessary. Nonparametric tests were conducted if normal distribution of the transformed data or homogeneity of variances was not achieved. The t test was used to examine differences in the concentrations of SOC and biomarkers as well as acid-to-aldehyde (Ad/Al) ratios between control and W1 plots before warming. One-way ANOVA was performed to test the effects of warming on the Reco, GEP, NEE, SOC contents, biomarker concentrations, and ratios in the bulk soil and size fractions. Two-way ANOVA was used to examine the main and interactive effects of warming and soil size fractions on SOC content, Δ14C, and biomarker concentrations in 2015. Differences were considered to be significant at the level of p < .05. Principal component analysis (PCA) was performed for biomarkers in soils of 2015 using CANOCO 4.5. Min–max normalization of original data into the range of [0, 1] was conducted before PCA analysis.
3 RESULTS
3.1 Warming effects on ecosystem carbon fluxes and soil bulk properties
Compared with the control, both W1 and W2 treatments significantly increased soil temperature at the depths of 5, 10, and 20 cm across 2013 and 2015 (p < .05, Figure S3a–c). Soil temperature at the depth of 30–40 cm was not monitored but estimated based on GDGTs (Weijers, Schouten, van den Donker, Hopmans, & Sinninghe Damsté, 2007), which showed an increasing trend (0.26 ± 0.08, 0.48 ± 0.21, and 0.69 ± 0.14°C for control, W1, and W2, respectively; details are given in the Supporting Information). Warming increased the length of thawing period, decreased soil freezing depth (38.3, 35.4, and 32.6 cm for control, W1, and W2, respectively) and the duration of freezing period (Lin, Wang, Zhang, & He, 2017). Soil moisture decreased at the depths of 5, 10, and 20 cm under W1 and at 5 cm under W2 compared with the control in both years (p < .05; Figure S3d–f).
Ecosystem carbon fluxes were monitored in 2013 and 2015, exhibiting slight inter-annual variabilities (Figure 1a) and yielding similar results (i.e., NEE and Reco) in the control treatments to those measured by eddy covariance techniques (Kato et al., 2006) or automated continuous measurement in the same grassland nearby (Wang et al., 2014). Compared to the control, GEP did not change under warming in the growing season (late-April to late-October) of 2013 but increased in W1 and not W2 in 2015 (p < .05). Reco increased in both W2 and W1, relative to the control during the growing season of 2013 and 2015, respectively (p < .05). Respiration rates also increased under warming (except W1 in 2015) during the non-growing season of both years (p < .05). As a result, NEE was slightly less negative in W2 than the control in 2013, indicating increased carbon release (instead of fixation) under W2, while NEE was similar across all treatments in 2015. Overall, the above results suggest that warming had minimal impacts on carbon fluxes of these alpine grasslands during the study period.
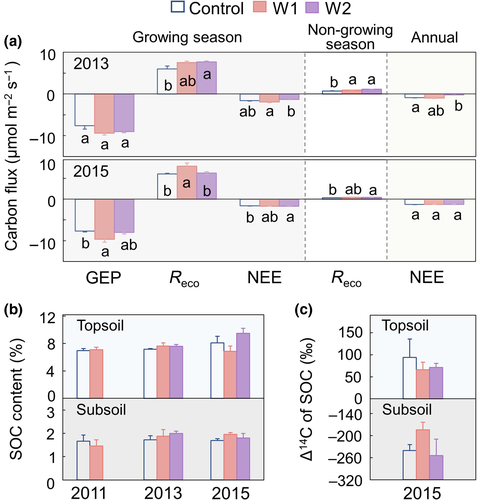
Changes in NEE were too small to be detected in the SOC stocks in that SOC contents remained similar among all treatments at both depths before (for W1 and control in 2011) and after warming (in 2013 and 2015; p > .05; Figure 1b). As W2 was established 1 year later, initial soils were not collected for comparison. However, given the homogeneous landscape and vegetation coverage at the study site, we assume similar SOC contents for the control and W2 plots before warming as well. The Δ14C values of SOC, being more depleted in the subsoil (−224 ± 17‰; mean ± SEM; n = 4) than topsoil (77 ± 14‰) during 2015, showed no difference among treatments, either (p > .05; Figure 1c).
3.2 Changing dynamics of major SOC molecular components under warming
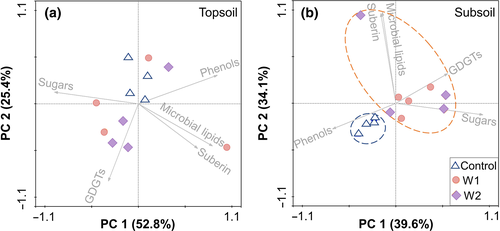
Despite unaltered bulk SOC properties, considerable changes were observed in major plant- and microbial-derived molecular components. Importantly, these effects were detected in the subsoil only, with no changes in the topsoil. Specifically, biomarkers of various origins had comparable abundances in the control and W1 plots at both depths at the start of the experiment (p > .05; Figure 2). Neither warming treatment affected biomarker abundances in the topsoil in 2013 or 2015 (p > .05). However, subsoils in both W1 and W2 plots displayed a decrease of plant-derived phenols (Figure 2a) relative to the control in 2013 and 2015 (p < .05) despite enhanced growth of deep grass roots (Liu et al., 2018) that are relatively abundant in lignin (Figure S2). In contrast, microbial-derived hydrolysable lipids (referred to as “microbial lipids” hereafter) and sugars of both microbial and plant origins increased in the W1 plots in 2015 (p < .05). The latter two groups of biomarkers also showed an increasing trend relative to the control in the W2 subsoils in 2015, but the difference was not statistically significant, probably due to large spatial heterogeneity that obscured these effects. GDGTs derived from archaeal and bacterial membrane lipids also increased in the W2 subsoils in 2015 (p < .05). By comparison, root-derived suberin lipids remained similar among treatments (p > .05).

Changes in the entire SOC molecular profile were identified using PCA of biomarkers in the warmed and control soils, which explained 78.2% and 73.7% of variance in the top- and subsoil of 2015, respectively (Figure 3). While the topsoil samples did not cluster according to treatments, subsoils from both warming treatments were separated from those under control with phenols weighing in the opposite direction of sugars and GDGTs on the x-axis and microbial lipids and suberin weighing on the y-axis. These results collectively suggest an accumulation of lipid and sugar components derived from both plants and microbes at the expense of lignin in the warmed subsoil.
Enhanced lignin degradation in the warmed subsoil was further evidenced by the Ad/Al ratios of vanillyl (V) and syringyl (S) phenols, which typically increase with elevated degradation (Otto & Simpson, 2006). Before the warming treatment initiated in 2011, both ratios were higher in the top- than subsoils (p < .05; Figure S4a), but became similar between depths in 2015 (p > .05; Figure S4b), indicating convergence of lignin oxidation state between depths under warming. Moreover, in contrast to the common observation of increasing Ad/Al ratios with soil depths (Otto & Simpson, 2006), the lower (Ad/Al)S ratio in the subsoil at this site indicated a relatively low oxidation stage of lignin likely due to substrate and/or temperature limitations at depth. By comparison, the higher (Ad/Al)V ratio in the top, relative to subsoils of 2011, can also be partly attributed to the influence of the shallow-rooted, dominant vegetation (K. humilis) with a very high (Ad/Al)V ratio in its roots (Figure S2d).
3.3 Shifting carbon allocation in soil physical fractions
Different size fractions had similar OC and 14C contents in the topsoil in 2015 (p > .05), but the OC contents and Δ14C values decreased with decreasing size of fractions in the subsoil (p < .05; Figure 4a,b). In the control subsoil, the macroaggregates and silt-clay fractions had a turnover time of ~1,500 and ~3,100 years, respectively, confirming the slow-cycling nature of OC associated with fine particles. Warming did not change the mass proportion of size fractions at either depth (p > .05; Figure S5a). The OC and 14C contents did not change for any topsoil fraction under warming, either. However, warming had significant effects on the subsoil silt-clay fraction by increasing the OC content of W2 (by ~56%) and increasing the Δ14C values of W1 relative to the control (p < .05). The silt-clay fraction of W2 subsoils also showed an increasing trend in Δ14C, but the change was not significant due to a high spatial heterogeneity (p > .05). These results suggest an accumulation of recently synthesized carbon in the fine fraction of subsoils under warming. Warming did not produce interactive effects with fraction size on the mass proportion, OC, or 14C contents of soil fractions at either depth (p > .05).
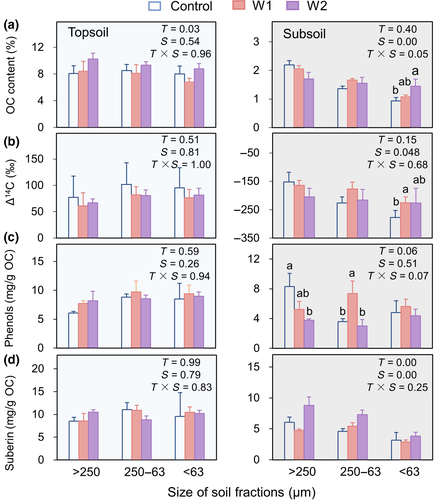
Phenols and lipids were further examined in various soil fractions to explain their changes in the bulk soil (Figure 4; Figures S5 and S6). In the topsoil, neither concentrations nor proportions of biomarkers were affected by warming in any fraction. By contrast, suberin concentrations increased in the W2 relative to control and W1 subsoils with all fractions considered (p = .00) while GDGT concentrations decreased in the silt-clay fraction of W2 than control and W1 subsoils (p < .05). Warming decreased phenols in the subsoil macroaggregates, with significantly lower values in the W2 than control plots (p < .05). As a result, warming decreased the percentage of phenols in the macroaggregates of W2 subsoils compared with control (p < .05) without affecting the allocation of other biomarkers (Figure S6).
Lignin Ad/Al ratios did not differ among soil size fractions or warming treatment in the topsoil in 2015 (p > .05; Figure S7). However, macroaggregates had lower (Ad/Al)S ratios relative to other soil fractions in the control subsoils (p < .05). Moreover, the W1 treatment increased the (Ad/Al)S ratio in the macroaggregates but decreased the (Ad/Al)V ratio in the silt-clay fraction of subsoils relative to the control (p < .05). A similar albeit nonsignificant pattern was observed for the W2 subsoil, indicating increased lignin oxidation in the macroaggregates and a decreased oxidation stage in the fine fraction of subsoils likely due to fresh lignin inputs.
3.4 Composition of bulk organic matter in the subsoil
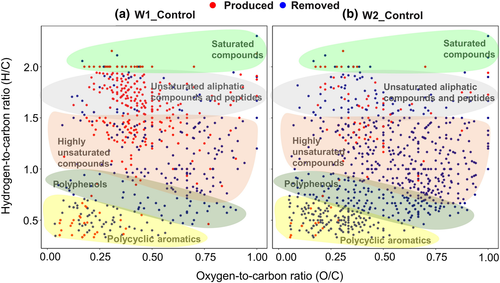
Warming-induced changes to bulk organic matter composition were further investigated for the subsoil in 2015. Unsaturated aliphatics and peptides (e.g., proteins) were more abundant in the WEOM from both warmed subsoils (especially in W1) relative to the control (Figure 5). Meanwhile, polycyclic aromatics, polyphenols, and highly unsaturated compounds were less abundant (especially in W2), suggesting a decrease of soluble aromatic compounds (including tannins and lignin degradation products) under warming. These results are consistent with the decrease of phenolic biomarkers and increase of lipids in the bulk subsoil. By comparison, solid-state 13C NMR analysis of bulk SOC showed increased ratios of alkyl/O-alkyl in the subsoils of W1 (1.09) and W2 (1.11) compared with control (0.96; Table S3), again suggesting enhanced degradation of subsoil SOC under warming, despite negligible changes in the topsoil.
4 DISCUSSION
Five years of warming (for W1) did not alter SOC concentrations in the studied alpine grassland due to relatively small changes in NEE compared with soil carbon stocks. However, given the complex composition and varying turnover of SOC pools, shifts in carbon allocation and dynamics among various soil fractions may impart contrasting warming feedbacks in the long term, even though changes in bulk soil carbon stocks are not detected at the current (initial warming) stage. Using a combination of sensitive measurements on SOC characteristics, we reveal divergent responses of different SOC components, providing evidence for shifting carbon dynamics (reflected in both molecular and 14C composition) in the subsoil. These changes may indicate future shifts in the carbon storage potential of soils under warming. Importantly, none of these effects were detected in the topsoil, highlighting that the vast subsoil carbon stocks may be the most sensitive components of SOC pools in this study region (Figures 2 and 3).
In contrast to lignin accrual in the macroaggregates of a tallgrass prairie soil under elevated litter inputs (Cotrufo et al., 2015), plant-derived phenols decreased in the warmed subsoils (especially in macroaggregates) relative to the control (Figure 2a) despite enhanced inputs of lignin-enriched grass roots (Figure S2). Our results point to warming-enhanced lignin degradation at depth in this alpine grassland. This finding is corroborated by the relative change of lignin Ad/Al ratios with progressive warming (Figure S4), in line with increased alkyl/O-alkyl ratios (Feng et al., 2008; Pengerud et al., 2017) in the warmed subsoils and decrease of soluble aromatics derived from lignin and tannin, etc., in the WEOM (Figure 5; Table S3). The decrease of phenols in bulk subsoils was largely caused by their declining concentrations and proportions in the macroaggregates (Figure 4c; Figure S6a), which are enriched with fungal communities (and hyphae) as the primary lignin degrader (Gleixner, Czimczik, Kramer, Lühker, & Schmidt, 2001) and poor in minerals conferring physiochemical protection for carbon substrates (Wilson et al., 2009). Enhanced lignin oxidation in the macroaggregates is also evidenced by the higher (Ad/Al)S ratio in the warmed than control subsoil fractions (Figure S7a).
The above changes collectively suggest that lignin degradation is more sensitive to warming in the sub- than topsoil of this alpine grassland. This may be linked to several factors. First, underdegraded lignin in the subsoil (indicated by lignin Ad/Al ratios) may be more susceptible to degradation in the warmed soils with an extended period of microbial activity (Lin et al., 2017) and a potentially increased size of enzyme pools (Alvarez et al., 2018), highlighting the vulnerability of relatively fresh lignin partially “cryo-locked” in the deep horizons of alpine soils. Second, microbial degradation of millennia-old SOC was found to be fueled by increased inputs of labile carbon via root penetration to the deep layers of a temperate grassland (Shahzad et al., 2018). Similarity, lignin decomposition could have been stimulated in our alpine subsoil due to warming-induced root deepening. This explanation is supported by a stronger cometabolic decay of lignin in the glucose-amended incubation of subsoils from the warming compared to control plots (Jia et al., 2017). Third, microbial genes involved in degrading complex organics such as lignin are shown to increase under warming in tundra soils (Sistla et al., 2013; Xue et al., 2016), and microbial communities can shift in favor of fungi that dominate lignin decomposition (Gleixner et al., 2001). In a QTP alpine meadow, warming has been reported to induce microbial community shifts toward bacteria at 0–10 cm but toward fungi in the deeper layers, highlighting that functional shifts in soil communities could underpin these subsoil changes in SOC dynamics (10–50 cm; Zhang et al., 2015).
In contrast to phenols, root- and microbe-derived biomarkers suggest that bulk SOC received increased inputs of lipids and sugars from both plants and microbes in the warmed subsoil. In particular, accompanied by an increased grass root redistribution into the deeper soil (Liu et al., 2018), the silt-clay fraction of warmed subsoils showed an increase of OC content (Figure 4a) and an accumulation of newly synthesized carbon (Figure 4b) as well as undegraded (fresh) lignin indicated by lower (Ad/Al)V ratios relative to the control (Figure S7b). These results agree with an increased silt-clay fraction in a warmed subarctic grassland (Poeplau, Katterer, Leblans, & Sigurdsson, 2017) and with favorable sequestration of new carbon in the fine-sized soil particles under elevated root growth (Desjardins, Barros, Sarrazin, Girardin, & Mariotti, 2004). It is also notable that in contrast to their increase in the bulk subsoil (Figure 2d), archaea- and bacteria-derived GDGTs declined in the silt-clay fraction of W2 relative to control subsoils (Figure S5c), likely due to dilution by fresh plant-derived OC. Therefore, newly sequestered carbon in the fine fraction appeared to be mainly plant (root) derived. Taken together, our results indicate that while warming-enhanced degradation (of phenols) mainly occurred in the fast-cycling pool (i.e., macroaggregates), sequestration of newly synthesized carbon was elevated in the slow-cycling pool (i.e., silt-clay fraction; Figure 6). As fine-sized or mineral-associated fraction is considered to be responsible for the long-term stabilization of SOC (Cotrufo et al., 2015), the shifting allocation of carbon from fast- to slow-cycling pools may benefit long-term SOC storage and explain the reported increase of subsurface carbon stocks with warming on the QTP (Ding et al., 2017).
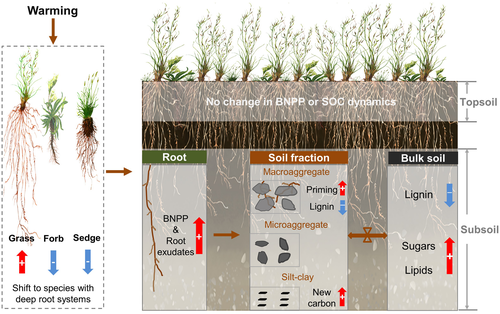
In summary, our study provides compelling evidence for shifting soil carbon dynamics on the QTP under warming (Figure 6). Given that these effects were observed in the subsoil and not topsoil, our results highlight the high sensitivity of deep SOC cycling to warming in alpine grasslands (Dorrepaal et al., 2009; Hicks Pries et al., 2017). Until now, studies have focused primarily on the warming response of topsoil. Our results suggest that these studies may misrepresent or underestimate total changes in SOC dynamics or terrestrial carbon cycling under warming. The specific ecological mechanisms identified can help to provide insights into the vulnerability of high-altitude and high-latitude regions under warming. Moreover, as warming is reported to induce deeper root distribution in a wide range of ecosystems (Arndal, Tolver, Larsen, Beier, & Schmidt, 2018; Johnson et al., 2006; Keuper et al., 2017; Leppälammi-Kujansuu et al., 2013; Lim et al., 2018; Liu et al., 2018; Shi et al., 2017; Wang et al., 2017), the observed root-driven acceleration of subsoil carbon cycling and new carbon sequestration may also occur in non-alpine systems. It is important to assess whether, where, and when new carbon accrual in the fine-sized fraction may outweigh the decay of native SOC at depth. It is also necessary to examine if the outcome is related to the nature of organic matter preserved (such as the freshness of lignin components) and/or the status of nutrient availability in the soil, given that both microbial activity and plant growth are strongly regulated by nutrient cycling under global changes (Fontaine et al., 2011; Perveen et al., 2014). Given the tremendous carbon storage in subsoils, addressing these questions will be critical to improve confidence in future projections of soil carbon dynamics under climate change. This could also help us to identify potential “hot spots” for new carbon sequestration versus old carbon preservation under warming.
ACKNOWLEDGEMENTS
This study was supported financially by the Chinese National Key Development Program for Basic Research (2017YFC0503902, 2015CB954201), the National Natural Science Foundation of China (41422304, 31630009, 31370491, 41773067), and the International Partnership Program of Chinese Academy of Sciences (Grant no. 151111KYSB20160014). J. Jia thanks China Scholarship Council for supporting her visit to ETH Zürich. We are grateful to Xinying Zhang, Ting Liu, and Zhiyuan Ma for help in sampling and data analyses. The data used are listed in the tables, figures, and Supporting Information of the paper.
CONFLICT OF INTEREST
The authors have no competing interests that might be perceived to influence the results and discussion reported in this paper.
AUTHOR CONTRIBUTIONS
X.F. and J.J. designed the study. J.S.H., L.L., and Z.Z. designed and carried out the warming experiment. J.J., Z.C., C.L., and Y.W. carried out sample analyses with help from N.H., L.W., and T.I.E. for 14C, H.B. and T.D. for FT-ICR MS, M.J.S. for NMR, and H.Y. for GDGTs. J.J., X.F., and T.W.C. wrote the paper with inputs from all co-authors.




