The functional role of temperate forest understorey vegetation in a changing world
Abstract
Temperate forests cover 16% of the global forest area. Within these forests, the understorey is an important biodiversity reservoir that can influence ecosystem processes and functions in multiple ways. However, we still lack a thorough understanding of the relative importance of the understorey for temperate forest functioning. As a result, understoreys are often ignored during assessments of forest functioning and changes thereof under global change. We here compiled studies that quantify the relative importance of the understorey for temperate forest functioning, focussing on litter production, nutrient cycling, evapotranspiration, tree regeneration, pollination and pathogen dynamics. We describe the mechanisms driving understorey functioning and develop a conceptual framework synthesizing possible effects of multiple global change drivers on understorey-mediated forest ecosystem functioning. Our review illustrates that the understorey's contribution to temperate forest functioning is significant but varies depending on the ecosystem function and the environmental context, and more importantly, the characteristics of the overstorey. To predict changes in understorey functioning and its relative importance for temperate forest functioning under global change, we argue that a simultaneous investigation of both overstorey and understorey functional responses to global change will be crucial. Our review shows that such studies are still very scarce, only available for a limited set of ecosystem functions and limited to quantification, providing little data to forecast functional responses to global change.
1 INTRODUCTION
Temperate forests currently cover around 5.3 million km2 worldwide representing around 16% of global forest area (Hansen, Stehman, & Potapov, 2010). Being located in the most densely populated regions of the globe makes them more altered, fragmented and reduced than most other forest types (Millenium Ecosystem Assessment, 2005). The implications of these changes on the functioning of temperate forests has been a topic of interest since long. This line of research, however, has primarily focussed on the overstorey, often ignoring the functional role of the understorey in these forests.
The understorey layer in temperate forests is the forest stratum composed of vascular plants (woody and non-woody) below a threshold height of ca. 1 m (cf. Gilliam, 2007). This layer is an important biodiversity reservoir of temperate forests that contains on average more than 80% of the vascular plant diversity (Gilliam, 2007). In addition, understorey plants provide food, shelter and habitat, especially for arthropods (Boch et al., 2013) and large herbivores (e.g. Gill & Beardall, 2001; Smolko & Veselovská, 2018). Next to its importance for biodiversity conservation, the understorey can also have an important functional role, regulating ecosystem processes (or functions), for instance via its impact on forest regeneration (e.g. George & Bazzaz, 2014b), water cycling (e.g. Thrippleton, Bugmann, Folini, & Snell, 2018) and nutrient and carbon dynamics (e.g. Elliott, Vose, Knoepp, Clinton, & Kloeppel, 2015; Muller 2014). The number of studies that provide a proper quantification of the importance of the understorey in determining ecosystem functions in temperate forests is, however, still limited (but see Gilliam, 2007 for a review).
The diversity and composition of the understorey vegetation in temperate forests is strongly affected by global change. Over the last decades, evidence has accumulated that changes in land use can leave persistent imprints in understorey community composition and its functional diversity (reviewed by Flinn & Vellend, 2005; Hermy & Verheyen, 2007). Likewise, important impacts of eutrophying and acidifying deposits from the atmosphere have been found (Dirnböck et al., 2014; Perring et al., 2018). More recently, climate warming-induced understorey community changes have come into focus (e.g. Bertrand et al., 2011; De Frenne et al., 2013), next to effects of increased grazing pressure (Rooney & Waller, 2003) and of invasive species (Peebles-Spencer, Gorchov, & Crist, 2017).
There is, in addition, limited understanding of the functional consequences of the abovementioned changes in the understorey vegetation. As outlined in the ‘Hierarchical Response Framework' by Smith et al. (2009), global change will generate immediate plant physiological responses followed by shifts in species' abundances and ultimately in community reordering through colonization and extinction processes. Clearly, all of these changes will impact the functioning of the understorey, but the magnitude and importance of these changes is hard to predict. Particularly since changes in understorey functioning will be contingent upon simultaneous changes occurring in the overstorey. The question further arises whether these changes will increase the importance of the understorey for temperate forest functioning in the future, which would advocate for the inclusion of the understorey in future research on temperate forest functioning.
Here we review the role of temperate forest understoreys for a range of important forest functions. First, we start with a quantification of the relative importance of the understorey for a selection of forest functions. We then develop a conceptual framework synthesizing possible effects of multiple global change drivers on understorey-mediated forest ecosystem functioning based on our understanding of driving mechanisms. Our aim is to propose a generally applicable framework allowing the derivation of testable hypotheses about the understorey's functional responses to global change. These hypotheses can guide future, and urgently needed, research on this topic.
2 SELECTION OF ECOSYSTEM FUNCTIONS AND INDICATORS
Ecosystem functions (or processes) are defined as the fluxes of energy, matter and information among the different compartments of an ecosystem (Meyer, Koch, & Weisser, 2015). These compartments include primary producers, decomposers, dead organic material, consumers and several abiotic compartments including stocks of nutrients and water. The main biogeochemical fluxes in temperate forests include carbon, nutrient and water cycling. The understorey directly contributes to these fluxes via carbon assimilation, nutrient uptake and evapotranspiration (ET) and indirectly by affecting the abundance of other functionally important organism groups, including trees, pollinators, herbivores, pathogens and decomposers.
Considering both direct and indirect pathways, the understorey has the potential to alter the functioning of temperate forests via three main mechanisms: (a) by directly altering carbon, nutrient and water fluxes as part of the forest's compartment of primary producers; (b) by acting as a filter for overstorey regeneration; and (c) by providing habitat and food for other functionally important species such as pollinators and pathogens. To quantify the importance of the understorey for forest functioning, we selected indicators for each of these functions of the understorey (Table 1). The selection of indicators was based on a trade-off between being representative for the function of interest and the availability of data. To be able to estimate the relative importance of the understorey for forest functioning, paired data needed to be available for both the overstorey and the understorey (for productivity, nutrient cycling and ET) or in the presence or absence of an understorey (for tree regeneration, pollinator and pathogen dynamics).
| Ecosystem function | Indicator | Units | Importance ratio (%) | |
|---|---|---|---|---|
| Formula | Range | |||
| Ecosystem fluxes | ||||
| Productivity | Aboveground litter production (P) | g/m2 | Pund/(Pund + Pov) × 100 | 0 to 100 |
| Nutrient cycling | Foliar nutrient concentration (N) | mg/kg | Nund/Nov × 100 | 0 to +∞ |
| Evapotranspiration | Evapotranspiration (E) | mm/hr | Eund/(Eund + Eov) × 100 | 0 to 100 |
| Understorey–overstorey interactions | ||||
| Tree regeneration | Emergence, establishment, growth and survival of tree seedlings (R) | No./m2; cm/year | (Rund − Rno und)/Rno und × 100 | −∞ to +∞ |
| Habitat provisioning | ||||
| Pollinators | Density of pollinators (Po) | No./ha | (Pound − Pono und)/Pono und × 100 | −100 to +∞ |
| Pathogens | Density of pathogens (Pa) | No./ha | (Paund − Pano und)/Pano und × 100 | −100 to +∞ |
Note
- Subscripts ‘und’ and ‘ov’ refer respectively to the understorey's and the overstorey's contribution to ecosystem fluxes. Subscripts ‘und’ and ‘no und’ refer to functional performance in the presence or absence of understorey plants respectively.
- The represented ranges are mathematical extremes that are not necessarily ecologically meaningful (including, for example, cases with no overstorey).
3 QUANTIFICATION OF THE FUNCTIONAL IMPORTANCE OF THE UNDERSTOREY
To quantify the relative contribution of the understorey to overall forest functioning, we searched the literature for studies that either quantified both understorey as well as overstorey functioning (in the case of productivity, nutrient cycling and ET) or quantified forest functioning in the presence or absence of understorey plants (for the functions tree regeneration and habitat provisioning for pathogens and pollinators). For each selected ecosystem function (Table 1), we did a separate Web of Science topic search based on the search strings provided in Table S1. Search results were subsequently scanned for relevant data, resulting in a subset that was retained for each function (for numbers see Table S1). We complemented the lists by scanning the references of the retained publication. We also used an unpublished dataset on understorey and overstorey characteristics at three European forest sites as an additional source of data to quantify the relative importance of the understorey for forest productivity and nutrient cycling. Below we report our findings for each function separately, providing (a) operational definitions for each function, (b) the values found in the literature and (c) a description of the mechanisms influencing the importance of the understorey.
3.1 Productivity
3.1.1 Definition
We define productivity as the yearly carbon flux to the forest floor. The relative contribution of the understorey to this flux can be estimated by comparing yearly overstorey litter production with yearly understorey litter production. However, as both measures are seldom quantified as such, let alone on the same site, we here quantify the relative contribution of the understorey by comparing the understorey's aboveground biomass to yearly overstorey leaf litter production. Following this definition, the contribution of the understorey to the yearly flux of organic material to the soil can be estimated by harvesting the total aboveground biomass of the understorey at peak biomass, while the contribution of the overstorey can be estimated by the collection of leaf litter via litter traps. We are aware, however, that this definition might result in an overestimation of the understorey's functional importance, especially when dwarf shrubs, tree seedlings or bryophytes are considered as a component of the understorey. As only part of their biomass (including leaves, fruits, senescent woody parts) contribute to the yearly litter production, total harvested biomass might overestimate understorey litter production. The opposite holds for understorey communities that are rich in ephemeral species as most of their living biomass dies off before peak biomass.
3.1.2 Overview of values published in the literature
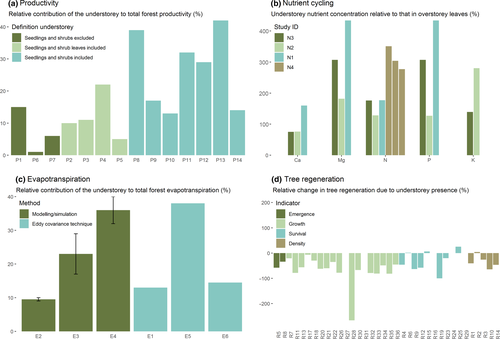
Based on our review of the literature, the contribution of the understorey to the yearly carbon flux to the soil ranges between 1% and 42% (Figure 1a). This estimated range slightly exceeds the one reported by Welch et al. (2007) (0.4%–28.8%). The high variability of values found in the literature can be partially attributed to differences in understorey definitions. While some studies excluded dwarf shrubs and seedlings, others included either their total biomass or their foliar biomass only. When both woody and non-woody parts of dwarf shrubs were included, understorey biomass could reach values that are twice as high compared to studies that only focused on non-woody vegetation. Accounting for this bias in the reviewed studies, we can conclude that the contribution of understorey plants to yearly litter production is probably lower than our full range of values suggests. Selecting only those studies that excluded woody material of seedlings and dwarf shrubs (but included their leaves) results in an understorey contribution ranging between 1% and 22%.
3.1.3 Driving mechanisms
Light, temperature, nutrient and water availability jointly regulate primary production in terrestrial ecosystems. While light is generally not a limiting resource for dominant overstorey trees, it is considered the main limiting factor for understorey growth (e.g. Axmanová et al., 2012), its availability fully controlled by the overstorey. During the growing season, the phenology of the overstorey determines the start, end and hence length of the shaded phase for the understorey, while its structure and composition determine the level of light interception by the canopy and hence light availability at the forest floor. Both the length of the shaded phase and the amount of light available during this phase are considered important factors controlling understorey productivity (Augspruger, Cheeseman, & Salk, 2005; Valladares et al., 2016). Rothstein and Zak (2001) have shown that even for non-spring ephemeral species, more than 60% of the annual understorey production can occur during the high light availability phases in spring and autumn, while other studies have shown that differences in light availability levels during the low light availability phase in summer can also explain variation in understorey productivity among sites (Axmanová, Zelený, Li, & Chytrý, 2011). The latter studies hence suggest a negative relationship between overstorey leaf area index (LAI) and understorey productivity. Although this would translate into a negative relationship between overstorey LAI and our importance ratio (especially since a high LAI also increases our importance ratio's denominator), we do not see this relationship in our literature data (Table S2). Differences in phenology, and hence the duration of the high light availability phase, among sites might potentially explain this finding.
When light is not a limiting factor following natural or anthropogenic disturbances, understorey productivity can be limited by water or nutrient availability on dry and nutrient poor sites respectively. Water availability mainly depends on precipitation amounts, canopy characteristics (Barbier, Balandier, & Gosselin, 2009; Staelens, De Schrijver, Verheyen, & Verhoest, 2006, 2008), landscape topography (Beven & Kirkby, 1979) and soil characteristics such as texture and soil depth (Bréda, Lefevre, & Badeau, 2002). The canopy can affect water availability in two ways: negatively through interception and ET (Barbier et al., 2009), positively by reducing wind speed, irradiation, temperature and vapour pressure deficit (VPD) at the forest floor (Davis, Dobrowski, Holden, Higuera, & Abatzoglou, 2019; Ma, Concilio, Oakley, North, & Chen, 2010). Temperature can also directly influence understorey productivity via increasing photosynthetic rates (Farquhar, von Caemmerer, & Berry, 1980). Among the many nutrients that can affect plant growth, nitrogen (N) and phosphorus (P) generally play a dominant role (Elser et al., 2007). Tree litter, past land use (e.g. litter raking, fertilizer application), soil acidity and atmospheric deposition of N have all been shown to affect nutrient availability in temperate forest soils (Augusto, Dupouey, & Ranger, 2003; Gilliam, 2006; Hinsinger, 2001; Maes et al., 2019; Verheyen, Bossuyt, & Hermy, 1999).
3.2 Nutrient cycling
3.2.1 Definition
Nutrient cycling can be defined as the transfer of nutrients among different forest compartments, after entering the system via atmospheric wet and dry deposition, biological fixation or weathering. The importance of the understorey for nutrient cycling is determined by its biomass, which was reviewed in Section 3.1, and its nutrient concentration. The higher the biomass and/or nutrient concentration, the higher the retention of nutrients in the understorey. Here, we quantify the importance of the understorey for nutrient cycling as the average concentrations of key nutrients (restricted to N, P, K, Ca, Mg) in the herbaceous understorey relative to the average concentrations found in the canopy trees' foliage. Although a comparison of nutrient stocks would be a better indicator for the understorey's nutrient cycling capacity, we here only focus on concentrations as being direct predictors of nutrient cycling rates and to present information that is complementary to that presented in the productivity section (Section 3.1).
3.2.2 Overview of values published in the literature
The concentrations of all nutrients in all four studies were higher in herbaceous vegetation compared to tree leaves (except for Ca concentration in one study performed by Gosz et al., 1972). After omitting one outlier (around 30 times higher concentration of K in understorey leaves compared to overstorey leaves; Welch et al., 2007), nutrient concentrations in the understorey were on average between 1.5 and 5 times higher than those found in overstorey leaves, depending on the nutrient considered. Average nutrient specific understorey:overstorey concentration ratios were 103% for Ca, 236% for N, 289% for P, 308% for Mg and 210% for K. The overall mean ratio was 231% across all nutrients (Figure 1b; Table S3).
We acknowledge, however, that the way nutrient concentrations were generally measured, being based on fallen litter for overstorey trees (post nutrient resorption) and standing biomass for understorey vegetation (prior to nutrient resorption), might bias our findings towards comparatively higher nutrient concentrations in the understorey due to nutrient resorption. However, the study of Gosz et al. (1972), the only study that did account for resorption by only sampling senescent understorey biomass, did not yield ratios that were consistently lower than those found by the other studies (Figure 1b [study N3]; Table S3).
Although the numerical values above show that understorey vegetation contains on average more nutrients on a mass basis than overstorey litter, they do not provide a complete picture of the understorey's importance for nutrient cycling. Due to differences in timing of nutrient uptake and release between the understorey and the overstorey, the understorey might be more important for nutrient cycling than the abovementioned values suggest. As hypothesized by the vernal dam theory (proposed by Muller & Bormann, 1976), understorey herbs take up a significant amount of nutrients early in the growing season when temperatures start to warm but trees are still dormant before canopy flush. If these nutrients would not be captured temporarily in spring-emergent herb biomass, they would mostly be lost due to leaching and other hydrological processes (Mabry, Gerken, & Thompson, 2008). Empirical evidence for this early season storage of nutrients is, however, still weak (Rothstein, 2000).
3.2.3 Driving mechanisms
Differences between overstorey and understorey species, in terms of growing strategies, largely determine the higher nutrient concentrations found in the understorey and hence the importance of the understorey for nutrient cycling in temperate forests. Herbaceous species have both a higher nutrient assimilation efficiency than canopy trees (Buchmann, Gebauer, & Schulze, 1996) and can take up nutrients more easily as their fine roots are concentrated in the topsoil (Bakker, Augusto, & Achat, 2006), which generally contains more nutrients than the deeper soil layers (Jobbágy & Jackson, 2001). Moreover, more than woody species, herbaceous species tend to position themselves along the leaf economics spectrum towards resource acquisitive leaves with high leaf area to mass ratio, high N concentration and low leaf longevity (Díaz et al., 2016).
Apart from species-specific differences, soil nutrient availability is a key factor determining foliar concentrations. Although soil nutrient availability is largely driven by inherent soil fertility, also past land use, deposition of nutrients, climate change and the understorey itself can affect nutrient concentrations in the soil. Legacies of prior agricultural land use can, for example, persist via an increased soil N and P availability for at least decades, which has been shown to lead to higher foliar P concentrations and biomass of the understorey (Baeten et al., 2011). Under very intensive N enrichment, Fraterrigo et al. (2009) found that foliar N concentrations of typical forest herbs were elevated regardless of the forest land-use history. Soil nutrient availability may also vary due to precipitation and temperature changes, affecting soil microbial activity (Rustad et al., 2001).
Despite the importance of soil nutrient availability in determining foliar nutrient concentrations, light and CO2 availability can also influence foliar nutrient concentrations. Nutrient dilution in plant tissue can occur when plants increase their C acquisition under elevated CO2 concentrations or light availability, while nutrient uptake cannot increase at a similar rate (e.g. when soil nutrient levels are low; Woodin, Graham, Killick, Skiba, & Cresser, 1992). In the opposite direction, when light availability decreases, compensatory responses in an attempt to maintain previous rates of photosynthesis (by increasing leaf-level chlorophyll concentrations), can decrease foliar C:N ratios (Niinemets, 1997).
Studies reporting changes in foliar base cation (K, Ca, Mg) concentrations are limited to studies focussing on acidifying depositions (Lucas et al., 2011), which decreases those nutrients in foliage of canopy trees but little is known on how the herbaceous understorey responds (Van Diepen et al., 2015).
3.3 Evapotranspiration
3.3.1 Definition
Understorey ET consists of three components: (a) interception by, and evaporation from, the surface of the understorey vegetation; (b) transpiration by the understorey vegetation; and (c) forest floor evaporation. Here, we were mostly interested in (a) and (b), but in practice soil evaporation is hard to separate from the two other components. Therefore we use the sum of the three components relative to the total above-canopy forest ET as an indicator for the importance of the understorey in this part of the water cycle.
3.3.2 Overview of values published in the literature
The contribution of the understorey to the total forest ET was found to be variable, but non-negligible (Figure 1c). The understorey contributes 10%–15% of ET in forests with a dense canopy and/or a sparse understorey vegetation, but this contribution can rise to 40% in more open forests (LAI around 3 or less; Table S4). Oshi et al. (2018) showed that the understorey contribution to total ET varies throughout the year and is particularly high just before the leafing out of the canopy (up to 76%). The results from our review seem in line with Roberts' (1983) hypothesis. He suggested that the contribution of the understorey vegetation will lead to similar annual transpiration among stands with differing densities. In that sense, forest ET can be considered to be a conservative process with a shifting role of the overstorey versus understorey contribution. The thinned versus control stands of Vincke et al. (2005) indeed show a similar total ET, but a variable contribution of the understorey (Table S4).
3.3.3 Driving mechanisms
Black and Kelliher (1989) and Wilson et al. (2000) provide insightful reviews on the factors controlling understorey ET. These controlling factors can be grouped into three categories: (a) the micrometeorological conditions in the understorey; (b) the composition and abundance of the understorey vegetation; and (c) the forest floor and soil characteristics. The net radiation reaching the forest understorey, together with the VPD and the wind speed at the understorey level are the most important micrometeorological forcing variables. Net radiation is strongly influenced by the phenology and density of the forest canopy. In temperate deciduous forests, the net radiation under the canopy is generally highest in spring, just before the leafing out of the trees. Wilson et al. (2000), for example, found that approximately one-third of the annual radiation was received during a 40 day period prior to leaf emergence. The same authors also demonstrated that the coupling between above and below canopy conditions was much stronger for VPD than for net radiation, due to the overriding canopy impact on net radiation. This implies that VPD is a more important driver for understory ET during the leaf-on period than net radiation.
Understorey vegetation abundance, often quantified by its LAI or foliar biomass, is another important factor controlling understorey ET (Thrippleton et al., 2018). Understorey species' identity also plays an important, but less well-studied role. Transpiration is controlled by stomatal conductance, which is modulated in a species-specific way by the above-mentioned micrometeorological variables and by soil water availability (Black & Kelliher, 1989). For instance, Gobin et al. (2015) found that Calluna vulgaris showed little or no regulation of transpiration in response to soil water depletion or air VPD, whereas Pteridium aquilinum showed a low transpiration rate regardless of the conditions. Rubus sect. Fruticosi gradually decreased transpiration during soil water depletion and increased VPD, whereas Molinia caerulea responded strongly to soil water depletion but only moderately to VPD.
Finally, litter layer and soil layer characteristics will also influence understorey ET, by altering forest floor evaporation rates and understorey transpiration rates respectively. Changes in the wetness of the litter layer, which can take place on a time scale of several hours when the atmospheric demand is large, can have an important influence on forest floor evaporation rates (Wilson et al., 2000). Litter wetness depends on the water-holding capacity of the litter layer, which in turn is affected by the origin of the organic matter accumulated in this layer (cf. Ilek, Kucza, & Szostek, 2015). Soil water availability, in contrast, mainly controls understorey transpiration rates, with understorey vegetation assumed to be able to better compete for topsoil water than tree seedlings (Thrippleton et al., 2018).
3.4 Tree regeneration
3.4.1 Definition
Tree regeneration is a crucial process in forest ecosystems as it provides the next generation of overstorey trees. The functional role of the understorey can be regarded as a filter for regeneration (sensu George & Bazzaz, 1999a, 1999b) that can affect the recruitment of new overstorey trees, by affecting emergence (e.g. Dolling, 1996; George & Bazzaz, 1999a, 1999b; Provendier & Balandier, 2008; Royo & Carson, 2008), growth and survival of tree seedlings (e.g. George & Bazzaz, 1999b; Provendier & Balandier, 2008; Royo & Carson, 2008). We define the importance of the understorey for tree regeneration as its role as a filter. We quantify this importance as the relative change in tree regeneration (expressed in terms of number of seedlings, growth rate or survival percentage) in contrasting vegetative conditions, i.e. in the presence or absence of understorey plants (see also Table 1).
3.4.2 Overview of values published in the literature
Literature data on the effects of the understorey on regeneration generally originated from regeneration experiments that considered multiple treatments (e.g. regeneration in overstorey gaps, in enclosures, with or without understorey vegetation and/or seed predation) and multiple tree species. To isolate the effects of the understorey, we compared regeneration in plots with versus without understorey vegetation presence under closed canopies, and preferably fenced against large herbivores and unfenced against seed predators (see Table S5 for more details on this selection procedure). When multiple tree species were considered, values were averaged across tree species. We mainly found a negative impact on all stages of tree regeneration induced by the presence of an understorey (Figure 1d; Table S5 for a more detailed overview of our findings). Only three studies reported no effect, or a small insignificant positive effect. Based on the findings across studies, we found a mean reduction of 46%, 20%, 35% and 55% in emergence, survival, density and growth of tree seedlings in the presence of understorey plants respectively.
Although these particular studies all point in the same direction, results may not be generalizable to all understorey contexts. The studies that met our selection criteria tended to focus on competitive species (e.g. the grass M. caerulea or the fern Dennstaedtia punctilobula) with a high cover. In these contexts, competition for resources is most likely the primary mechanism driving these negative understorey effects. Consequently, the presented values potentially overestimate the negative effects of the understorey on tree regeneration, especially for sparse understorey layers that are composed of less competitive species. Moreover, the negative effects reported by the reviewed studies do not necessarily persist over time. Thrippleton et al. (2016), for example, showed, by using model simulations, that understorey competition alone might not be enough to put a forest ecosystem into a state of arrested succession; it might appear so, but it is more a delayed state. Taking into account alternative regeneration performance indicators might also reveal positive effects. Jensen and Löf (2017), for example, showed that the herbaceous and shrub understorey facilitated the development of tall straight monopodial oaks by strengthening the inherent apical dominance and promoting height growth.
3.4.3 Driving mechanisms
The balance of negative (competition) and positive (facilitation) interactions between the understorey and seedlings will determine the net effects on tree regeneration (Callaway & Walker, 1997). Royo and Carson (2006) provided a framework with five mechanisms outlining how understoreys can interfere with different stages of tree regeneration: (a) competition for resources; (b) allelopathy; (c) interference with seed(ling) predation; (d) formation of a mechanical barrier through litter accumulation; or (e) mechanical damage.
Asymmetric competition for light is considered to be the primary mechanism of how understorey vegetation affects tree regeneration (e.g. George & Bazzaz, 1999b; Horsley, 1993). The higher understorey biomass and the more acquisitive plant species in the understorey, the higher the competition for light (Balandier, Collet, Miller, Reynolds, & Zedaker, 2006; George & Bazzaz, 2014a; Grime, 2001). Although competition for light is generally considered as the most important mechanism, belowground competition for nutrients and water also has the potential to impede regeneration (Balandier et al., 2006). In general, understorey competitiveness is reported to increase with increasing resource availability, including light, soil nutrients and water (Honnay et al., 2002; Laurent, Mårell, Korboulewsky, Saïd, & Balandier, 2017; Willoughby, Balandier, Bentsen, Mac Carthy, & Claridge, 2009). Hence, similar mechanisms as those driving understorey productivity (see Section 3.1) are driving the strength of the understorey filter for tree regeneration. This relationship between understorey productivity and tree regeneration was, however, not visible in our data due to a lack of detailed understorey biomass data and a bias towards more acquisitive and highly productive understorey species.
Under more stressful conditions, facilitation is expected to become more frequent and important (i.e. the ‘Stress-gradient hypothesis’; sensu Bertness & Callaway, 1994). The role of facilitation is often identified as more important in southern Europe, where tree seedlings are often exposed to high temperature and drought, leading to water stress (Gómez-Aparicio et al., 2004; Smit, Vandenberghe, Den Ouden, & Müller-schärer, 2007). In such conditions, a high understorey vegetation cover may help to improve the prevailing soil conditions and create a more suitable microclimate for seedlings to grow. However, even in temperate forests, where conditions are regarded as less environmentally extreme, facilitation may occur. Temperate forest tree seedlings are generally less adapted to drought and can thus experience high levels of stress even when environmental conditions are not extreme (Berkowitz, Canham, & Kelly, 1995; Holmgren & Scheffer, 2010; Putnam & Reich, 2017). Such positive interactions can, however, be overruled by the negative effects of competition (Wright, Schnitzer, & Reich, 2014). This might explain why we did not find evidence for facilitation in the reviewed studies.
While browsing by large herbivores (e.g. by deer) can suppress tree regeneration directly (Harmer, Kerr, & Boswell, 1997; Tilghman, 1989), browsing can also alter the influence of understorey communities on tree regeneration (Royo & Carson, 2006). Overbrowsing may lead to depauperate understoreys containing only plant species that are unpalatable (due to mechanical or chemical defences [e.g. Rubus fruticosus or P. aquilinum]) or tolerant (species able to quickly regrow [e.g. Deschampsia flexuosa]) against browsing (Bergquist, Örlander, & Nilsson, 1999; den Ouden, 2000; Horsley, Stout, & DeCalesta, 2003; Tilghman, 1989). Under favourable growing conditions, when nutrients, water and light are abundantly available, this may lead to a very dense understorey that has strong negative impacts on tree regeneration (Royo & Carson, 2006). Under certain conditions, however, browsing can induce facilitation as understoreys can protect tree seedlings from browsing, either by acting as a shelter or by providing an alternate food source (Diwold, Dullinger, & Dirnböck, 2010; Harmer et al., 1997; Perea & Gil, 2014).
Finally, the strength of the understorey filter also depends on the tree species under investigation. Depending on a tree seedling's traits, e.g. shade or drought tolerance, it may be able to better tolerate competition from the understorey and therefore establish more successfully than others (George & Bazzaz, 1999a, 1999b; Pagès, Pache, Joud, Magnan, & Michalet, 2003). Even though the overall average effect found in the selected studies was negative, the studies in our data with multiple seedling species report varying magnitudes and even directions in effects per species (George & Bazzaz, 1999a, 1999b; Pagès et al., 2003; Walters, Farinosi, Willis, & Gottschalk, 2016).
3.5 Pollinator dynamics
3.5.1 Definition
Although most tree species in temperate forests are wind-pollinated, some families and genera, such as Sapindaceae (Acer, Aesculus), Malvaceae (Tilia), Rosaceae (Prunus, Sorbus) and Fabaceae (Robinia), rely on insects for pollination (San-Miguel-Ayanz, de Rigo, Caudullo, Durrant, & Mauri, 2016). Pollinators can hence play an important role for the regeneration of these tree species. The understorey can influence the process of insect pollination by providing habitat for pollinators and its importance can be quantified as the relative difference between pollinator abundance or richness when understoreys are present compared to when not present (Table 1).
3.5.2 Overview of the literature
Based on current literature, we were not able to quantify the importance of the understorey for pollinator dynamics. However, qualitative evidence is available that the understorey can influence pollinator dynamics (with a focus on bees and hoverflies). Multiple studies have, for example, shown that an increase in understorey cover can increase the abundance and species richness of hoverflies and bees (Fayt et al., 2006; Fuller et al., 2018; Proesmans, Bonte, Smagghe, Meeus, & Verheyen, 2019). Vertical stratification of pollinators (as found by Ulyshen et al., 2010 and De Smedt et al., 2019 for bees and moths respectively), however, suggests that this positive understorey effect does not necessarily promote overstorey pollination, but only the overall species richness and abundance of these pollinators in forests. Other studies indicated a correlation between reduction in shrub layer cover and an increase in herb layer cover and species richness, leading to an increase in pollinator abundance and diversity (Campbell, Vigueira, Viguiera, & Greenberg, 2018; Hanula, Horn, & O'Brien, 2015). While most studies show a positive correlation between herb layer cover and pollinator abundance and diversity, the effects may differ, depending on pollinator taxonomy and time of the year, as most insect-pollinated herbs flower in spring (Proesmans et al., 2019).
3.5.3 Driving mechanisms
The presence, in the understorey, of insect-pollinated plants, which can serve as pollen and nectar sources for pollinators, largely determines the importance of the understorey for pollinator dynamics (see, for example, Proctor, Nol, Burke, & Crins, 2012). Light is considered one of the main factors influencing the understorey's importance for pollinator dynamics. Light does not only increase pollinator abundance (McKinney & Goodell, 2010), but also the abundance of flowering plants in the understorey that can attract pollinators (Proctor et al., 2012). The study of Mckinney and Goodell (2010) additionally shows that shade alone can be enough to decrease pollinator abundance in the understorey. This suggests that, in closed stands, the understorey might be less important for pollinator dynamics, regardless of the amount of flowering plants present in the understorey. While many other mechanisms might determine the importance of the understorey for pollinator dynamics, most of them, however, remain understudied.
3.6 Pathogen dynamics
3.6.1 Definition
Plants are subject to pathogen attacks leading to declines in their fitness and possibly mortality. The understorey may play a pivotal role in determining overstorey pathogen dynamics as this layer could function as a reservoir for pathogens fostering high disease risk, while a diverse understorey could dilute disease transmission risk by reducing host availability (Mitchell, Tilman, & Groth, 2002). The importance of the understorey for pathogen dynamics can be quantified as the relative difference between the abundance of pathogens (or overstorey infection rate) when understoreys are present compared to when not present.
3.6.2 Overview of the literature
Although some studies exist that report upon understorey–overstorey linkages in pathogen dynamics, we were not able to calculate an importance ratio here due to a lack of quantitative studies. The bulk of studies that we reviewed investigated how certain pathogens affected mortality or growth rates in specific understorey host species (Bayandala, Fakasawa, & Seiwa, 2016; Bayandala, Masaka, & Seiwa, 2017; Boyce, 2018), rather than investigating the role of the understorey for pathogen occurrence in general. Some of these species-specific studies focused on tree seedlings (Bayandala et al., 2016, 2017; Reinhart, Royo, Kageyama, & Clay, 2010), while others focused on herbaceous understorey species (Boyce, 2018; Elliott, Vose, & Rankin, 2014; Jefferson, 2008; Meeus, Brys, Honnay, & Jacquemyn, 2013; Warren & Mordecai, 2010). Several of these studies additionally address whether overstorey gaps influenced pathogen effects on understorey species (Bayandala et al., 2016, 2017; Boyce, 2018; O'Hanlon-Manners & Kotanen, 2004, 2006; Reinhart et al., 2010). Bayandala et al. (2016), for example, found greater tree seedling mortality caused by soil-borne damping-off pathogens in closed forests than in forest gaps. Reinhart et al. (2010) suggested that canopy gaps, due to the higher soil temperatures and lower soil moisture levels from greater light levels, may create unfavourable growing conditions for pathogens, thereby creating safe refugia for susceptible tree species. Current research, however, has not yet provided any evidence on whether understorey communities can play a role as well in promoting or suppressing pathogens.
3.6.3 Driving mechanisms
The understorey can have a direct impact on disease transmission if it can host pathogens that can affect tree species. For instance, rust fungi of the family Cronartium have two alternate hosts: a coniferous as well as an angiosperm host which could be a shrub or a herb species. In this case, the understorey could act as a reservoir for pathogens. When the understorey becomes more species-rich, dilution effects can again reduce the fitness of such pathogens (Johnson, Ostfeld, & Keesing, 2015).
Indirect understorey effects are possible as well. Understoreys can influence the environmental conditions at the forest floor where pathogens might depend upon during one or more of their life stages. For vector-transmitted pathogens, the understorey could affect the fitness of the vector (typically insects) which would in turn affect pathogen transmission efficiency. Pierce's disease (caused by the bacterium Xylella fastidiosa), for example, causes damage on many different tree species in the United States and is transmitted by generalist leafhoppers that may be affected by the understorey (Redak et al., 2004).
4 RESPONSE TO GLOBAL CHANGE
Major global change drivers that will affect future temperate forest ecosystems include climate change, altered disturbance regimes, invasive species, land-use change, forest-management changes and changes in N deposition (Gilliam, 2016). Most of these global change drivers have the potential to alter understorey functioning by altering resource availability and growing conditions at the forest floor that will drive understorey productivity and the functions that largely depend on this productivity, including nutrient cycling, ET and tree regeneration. Global change, however, will also affect the overstorey, which is a second important driver for the functioning of the understorey (mainly by regulating light availability; Section 3.1). Hence, indirect global change effects via changes in the overstorey will be important as well. It is this combination of direct and indirect effects that will mainly determine functional responses to global change in the understorey (Figure 2). The dark-coloured pathways in Figure 2 are likely the most dominant pathways that will determine short-term global-change effects. However, in the longer term, when initial physiological responses to global change are succeeded by species reordering in the overstorey and the understorey, other pathways (represented by dashed lines) will become important as well.
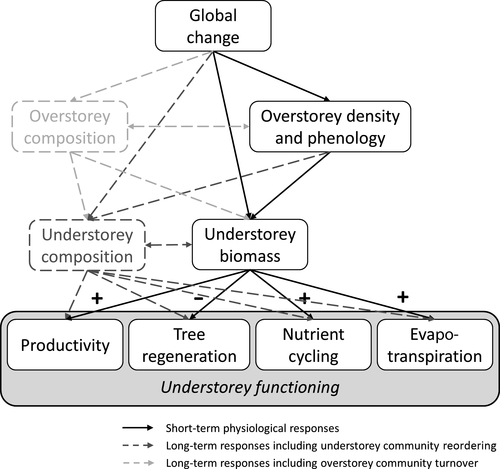
Global change drivers with a pronounced negative effect on overstorey density, such as changes in forest management and overstorey disturbance events, will alter understorey functioning mainly via the indirect pathway discussed above. If understorey–overstorey competition decreases, this will promote understorey productivity and, as a consequence, also its nutrient cycling capacity and transpiration rates. Whether these opposite trends in functional responses of the overstorey and the understorey will result in no net change of total forest functioning, as suggested for ET in Section 3.2, remains to be investigated. For the understorey's influence on tree regeneration, these indirect effects will be more complex. As detailed in Section 3.4, tree regeneration generally decreases following an increase of understorey biomass. However, in case of severe disturbances or harvest events, light will become abundantly available, reducing the negative effects of the understorey on tree regeneration (Pages & Michalet, 2003; Pagès et al., 2003). In some cases, the understorey might even act as a facilitator for tree regeneration by establishing more suitable moisture levels for tree regeneration compared to bare soil conditions (Gómez-Aparicio et al., 2004). Although indirect effects of overstorey disturbance on understorey functioning, as discussed above, are probably the most important, direct effects on understorey functioning might be important as well. Harvest activities can, for example, damage understorey plants but also lead to soil compaction, which can have long-lasting effects on the understorey (Zenner & Berger, 2008) and likely also its functioning. Similar direct effects might occur under storm or pest-induced disturbances. Unfortunately, research assessing the impacts of these events often focusses on the overstorey, ignoring the potential direct effects on the understorey (e.g. Seidl, Schelhaas, Rammer, & Verkerk, 2014).
Next to changes in overstorey density, changes in overstorey phenology (e.g. due to climate change; De Frenne et al., 2018) can also alter understorey functioning via the indirect pathway discussed above. Depending on whether phenological shifts in the overstorey deviate from those in the understorey, both decreases and increases of understorey productivity and associated functioning can be expected. Given that for many understorey communities the majority of biomass is produced prior to canopy closure, understorey communities are likely more sensitive to phenological shifts compared to the overstorey. As simulated by Jolly et al. (2004), an extension of the understorey's growing season may have a strong effect on understorey productivity, stronger than those expected in the overstorey for a similar increase in growing season length. Moreover, as overstorey phenology is expected to respond more quickly to climate change than understorey phenology, a decrease in understorey productivity can be expected as a result of phenological shifts in temperate forests (Heberling, McDonough MacKenzie, Fridley, Kalisz, & Primack, 2019).
If global change drivers involve increases or decreases in resource availability other than light (e.g. N deposition increasing soil N availability: Falkengren-Grerup, Brunet, & Diekmann, 1998; past arable land use increasing P availability: Blondeel et al., 2019; or climate change decreasing growing season precipitation: IPCC, 2013), the overstorey might act as a buffer attenuating direct responses of the understorey. Persistence of light limitation is often considered as the main mechanism that lowers the understorey's response to global change (see for example De Frenne et al., 2015). Understorey responses to an increase of resource availability might even become negative as increased resource availability also enhances overstorey growth leading to a stronger understorey–overstorey competition for light. The understorey's nutrient-cycling capacity, however, might respond differently. As nutrients tend to accumulate in plant biomass as a response to elevated nutrient availability in the soil (Aerts & Chapin, 1999), the understorey's nutrient-cycling capacity might potentially increase following an increase of nutrient availability. P accumulation in understorey plants due to this so-called luxury consumption has, for example, been reported for multiple species (e.g. Baeten et al., 2011; Tessier & Raynal, 2003).
The overstorey might also play a buffering role when global change involves changes in growing conditions, such as temperature and air humidity. Multiple studies have reported upon the overstorey's capacity to decouple above from below canopy atmospheric conditions (e.g. Davis et al., 2019; Von Arx, Graf Pannatier, Thimonier, & Rebetez, 2013), giving rise to lower climate change-induced temperature or VPD increases at the forest floor than those measured in open field conditions (De Frenne et al., 2019; Von Arx et al., 2013). Due to this buffering, which will be stronger under closed canopy conditions, global changes experienced by the understorey can be less severe than those experienced by the overstorey, potentially leading to smaller functional responses in the understorey. This buffering effect of the overstorey, however, does not necessarily hold for all global change drivers and associated changes in growing conditions. The overstorey can, for example, actively contribute to soil acidification (De Schrijver et al., 2012), leading to a potential acceleration of changes in soil acidity under a closed canopy, with adverse effects on understorey growth (Falkengren-Grerup, Brunet, & Quist, 1995; Haynes & Swift, 1986).
Consequently, it is clear that to investigate changes in understorey functioning, one also needs to take into account responses of the overstorey to global change. This is especially true when changes in the relative importance of the understorey for temperate forest functioning are being investigated. Changes in the understorey's relative importance, as defined in Table 1, will depend on the overstorey's functional response in two ways. The overstorey's functional response will alter not only the ratio's denominator, but also its counter via the mechanisms discussed above. For the functions considered in this review, we expect that direct functional responses to global change in the overstorey and the understorey tend to go in the same direction but that, due to competition with the overstorey, an increase/decrease in overstorey functioning often results in a lower increase/decrease of understorey functioning. Whether this will result in a decrease or increase of the relative importance of the understorey under global change will depend on the direction and magnitude of overstorey and understorey responses to global change. Assuming that overstorey density and composition can be used to predict the overstorey's contribution to forest functioning and after aggregating composition and biomass effects on overstorey and understorey functioning, the pathways in Figure 2 can be simplified to those in Figure 3, with pathway A representing the functional response of the overstorey to global change, B the functional response of the understorey to global change and C the functional response of the understorey to changes in overstorey functioning.
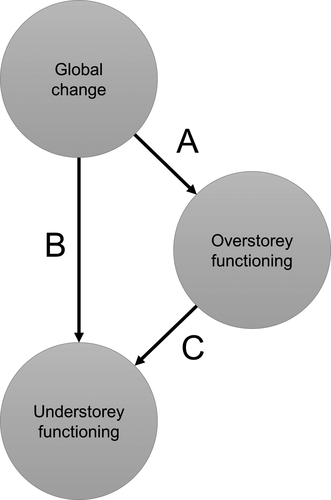
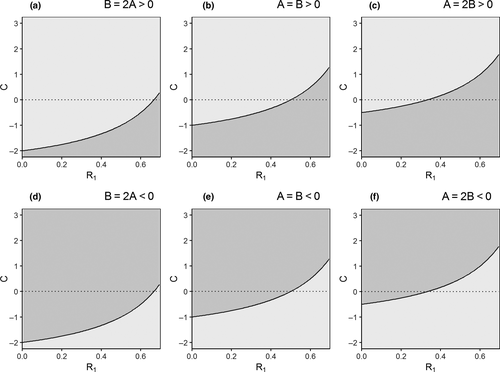
Assuming linear, non-interactive relationships as depicted in Figure 3, we can deduce expected changes in the understorey's functional importance (for calculations, see Table S5). For example, we more often expect an increase of the relative importance of the understorey when direct responses to global change are negative for both the overstorey and the understorey (A,B < 0; Figure 4d–f). Especially when the overstorey is more sensitive to global change than the understorey (A > B) or when competition with the overstorey is strong (C « 0). When the direct responses to global change are positive both for the understorey and the overstorey (A,B > 0), we expect opposite trends (Figure 4a–c). Considering responses to CO2 enrichment as an illustration, for example, overstorey productivity has been found to respond positively to elevated CO2 concentrations, while understorey responses were rather modest (Ellsworth, Thomas, Crous, & Palmroth, 2012; Kim, Oren, & Qian, 2016), suggesting that for this function and this global change driver, A likely exceeds B. Kim et al. (2016) additionally found that the induced increase of overstorey LAI reduced light availability for the understorey, resulting in a negative indirect effect on the understorey (C < 0). Under elevated atmospheric concentrations of CO2 enrichment, we hence expect a decline in the relative functional importance of the understorey (Figure 4c). For most global change drivers and functions, however, we do not have this information at hand. One of the reasons for this might be the bias we noticed between global change drivers focussed upon in overstorey research (mostly temperature, precipitation and atmospheric CO2 concentrations) and those studied in understorey research (past and current land use, acidifying deposition and temperature).
Above, we only discussed overstorey effects on understorey functioning, while feedbacks might occur as well. Through competition for belowground resources and as a filter for tree regeneration (see Section 3.4), the understorey has the potential to alter the structure, composition and productivity of the overstorey. The strength of this feedback, however, is highly variable. Negative effects of understorey cover on overstorey productivity due to competition for belowground resources have mainly been reported for young stands and on shallow soils with a low water-holding capacity (e.g. Giuggiola et al., 2018; Miller, Zutter, Zedaker, Edwards, & Newbold, 1995; Watt et al., 2003), while evidence for feedbacks occurring in mature stands is scarce. Differences in rooting depth of understorey and overstorey plant species and asymmetric competition for light in mature stands both suggest weak competitive effects of the understorey. Although our data do not allow testing directions of effects, we assume that the negative correlations between overstorey and understorey functioning, as revealed by several of the reviewed studies (e.g. Jarosz et al., 2008; Vincke et al., 2005), are mainly a result of the mechanisms visualized in Figures 2 and 3 and not attributable to a feedback effect. However, our data do suggest that the effect of the understorey on tree regeneration cannot be neglected (Section 3.4), but whether these effects will alter overstorey functioning in the long term remains understudied (but see Thrippleton, Bugmann, & Snell, 2017).
5 OUTLOOK
Our review illustrates that the understorey's contribution to temperate forest functioning is significant but varies depending on the ecosystem function and the environmental context considered. These results show that understorey communities constitute an important functional component of temperate forests and should not be ignored when developing management strategies to safeguard temperate forest functioning. While including the most important aspects of understorey functioning, many functions are still missing. Our review on the importance of the understorey to regulate pathogen and pollinator dynamics clearly illustrates that additional research is needed to quantify the importance of these functions and eventually predict their response to global change. As detailed in Section 4, we argue that a simultaneous investigation of both overstorey and understorey functional responses to global change will be crucial to be able to predict changes in understorey functioning and the relative importance of the understorey for temperate forest functioning under global change. Our review, that specifically targeted data originating from these kind of studies, additionally shows that these studies are still very scarce, only available for a limited set of ecosystem functions and limit themselves to quantification, not yet targeting the effects of global change. This data gap provides new perspectives for future research.
ACKNOWLEDGEMENTS
K.V., E.D.L., M.P.P., H.B. and S.L.M. received funding from the European Research Council (ERC Consolidator Grant: PASTFORWARD 614839). D.L. and W.P. were supported by a fellowship of the Research Foundation-Flanders (FWO). P.D.F. received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (ERC Starting Grant: FORMICA 757833). S.M. was supported by a fellowship of the China Scholarship Council (CSC).
AUTHORS' CONTRIBUTION
KV conceived the idea of this review. All authors contributed to the selection of relevant publications and data gathering. KV and DL led the writing of the manuscript with individual contributions of all authors in Section 3. All authors reviewed the draft and gave final approval for publication.
Open Research
DATA AVAILABILITY STATEMENT
All data related to this manuscript can be found in the Supporting Information and will be made available on www.pastforward.ugent.be.




