Invasive plants negatively impact native, but not exotic, animals
Abstract
Despite our growing understanding of the impacts of invasive plants on ecosystem structure and function, important gaps remain, including whether native and exotic species respond differently to plant invasion. This would elucidate basic ecological interactions and inform management. We performed a meta-analytic review of the effects of invasive plants on native and exotic resident animals. We found that invasive plants reduced the abundance of native, but not exotic, animals. This varied by animal phyla, with invasive plants reducing the abundance of native annelids and chordates, but not mollusks or arthropods. We found dissimilar impacts among “wet” and “dry” ecosystems, but not among animal trophic levels. Additionally, the impact of invasive plants increased over time, but this did not vary with animal nativity. Our review found that no studies considered resident nativity differences, and most did not identify animals to species. We call for more rigorous studies of invaded community impacts across taxa, and most importantly, explicit consideration of resident biogeographic origin. We provide an important first insight into how native and exotic species respond differently to invasion, the consequences of which may facilitate cascading trophic disruptions further exacerbating global change consequences to ecosystem structure and function.
1 INTRODUCTION
The consequences of introduced species to ecosystem structure and function are wide-ranging (Levine et al., 2007; Strayer, Eviner, Jeschke, & Pace, 2006; Vilà et al., 2011) and continue to be an important research topic as the world becomes increasingly invaded (Guénard et al., 2018). The impact of invasive plants is most commonly expressed as impacts on native plants (e.g., Vila & Weiner, 2015) and ecological processes (e.g., nutrient cycles, Ehrenfeld, 2003). While important research continues into invasive plant–native plant and invasive plant–environment interactions, interest is on the rise for identifying impacts of invasive plants on other invasive plants (e.g., invasion meltdown, Simberloff & Von Holle, 1999), identifying the impacts of multiple invasive species (e.g., co-invasion, Tekiela & Barney, 2017), as well as impacts on resident animal populations and broader system-level changes (e.g., trophic cascades, Seibold, Cadotte, MacIvor, Thorn, & Müller, 2018). Multi-trophic interaction studies are more apparent and better documented in invasive animal (e.g., brown tree snake causing bird extinction, Wiles, Bart, Beck, & Aguon, 2003) and pathogen studies (David et al., 2017), but remain relatively rare in the invasive plant literature despite their potential cascading effects. This shift to studies that expand the invasive plant impact framework reflects the broader role introduced species have—one that can influence multiple direct and indirect interactions among a multitude of species and processes.
Effective conservation and management must be driven by a holistic understanding of the larger role invasive plants play in our ecosystems (Barney, 2016), including their effects on native plants, the environment, other invasive species, and food webs. Recent studies have begun evaluating the effects of invasive plants on resident animal populations, including changes to animal abundance, individual performance, and behavior, through both direct and indirect mechanisms. For example, some invasive shrubs are implicated in increased bird mortality (Borgmann & Rodewald, 2004; Schlossberg & King, 2010; Schmidt & Whelan, 1999), reduced nest success due to increased predation (Dodonov, Paneczko, & Telles, 2017), as well as changes to general habitat preferences (King, Chandler, Schlossberg, & Chandler, 2009). One recent high-profile example is the endangered willow flycatcher (Empidonax traillii extimus) that will now nest in the invasive tamarisk (Tamarix spp.) in the absence of native trees in the Western United States (Sogge, Sferra, & Paxton, 2008), leading to a temporary termination of a successful biocontrol program (Dudley & Bean, 2012).
In some cases, invasive plants have been found to mediate predator–prey interactions through habitat creation (Devore & Maerz, 2014) and refuge from predation (Dutra, Barnett, Reinhardt, Marquis, & Orrock, 2011). These indirect interactions can also be mediated via changes in leaf litter quantity or quality, as with the invasive European bird cherry (Prunus padus) that significantly reduces invertebrate prey subsidies to coho salmon (Roon, Wipfli, Wurtz, & Blanchard, 2016). In arthropods, much work has focused on predatory spiders as they can affect trophic cascades to prey and plants. For example, orb-weaving spiders have been shown to increase considerably in number following the introduction of new structure for web building from the invasive spotted knapweed (Centaurea stoebe; Dudek, Michlewicz, Dudek, & Tryjanowski, 2016; Pearson, 2009; Smith & Schmitz, 2015; Smith-Ramesh, 2017).
As individual studies of invasive plant impacts on animals accumulated, meta-analyses and reviews followed looking for larger trends. An early meta-analysis looked at the inverse, i.e., the role native and exotic herbivores have on invasive plant abundance (Parker, Burkepile, & Hayt, 2006), finding that native herbivores suppress and exotic herbivores facilitate invasive plants. In a systematic review of North American bird species impacted by invasive plants, Nelson et al. (2017) found neutral effects on abundance and mortality, but negative impacts on richness. An analogous review in arthropods (Spafford, Lortie, & Butterfield, 2013) found a decrease in species richness on invasive plant hosts compared to native hosts, but was not able to compare trophic levels due to data limitations. Schirmel, Bundschuh, Entling, Kowarik, and Buchholz (2016) showed via meta-analysis of 198 studies, that invasive plants significantly reduced animal abundance, diversity, and fitness; though these effects varied significantly among ecosystem, animal trophic level, and animal taxonomic group. Invasive plant effects were strongest in riparian systems, while birds and insects were the most strongly impacted animal groups. In a broad comparison of invasive plant effects on trophic levels, McCary, Mores, Farfan, and Wise (2016) found invasive plants to have differential impacts on animal populations in “brown” (i.e., detritivore) and “green” (i.e., herbivore and predator) trophic groups, though this depended strongly on the receiving ecosystem type. Namely, they found that grassland systems host no significant impacts on either food web at any trophic level, while wetlands are host to major impacts on green food webs, and forest invaders host various significant impacts on both green and brown food webs. These meta-analyses paint a compelling picture that invasive plants can have broad-scale negative effects on resident animals across broad taxonomic and trophic levels.
Despite the accumulating evidence that invasive plants impact animal abundance, health, and behavior, it is not clear if invasive plants have differential impacts on native and exotic animals. This is an important distinction for several reasons. First, conservation efforts are focused on preserving native diversity which is under threat from a variety of global change factors, including invasive species (Downey & Richardson, 2016). Second, it has long been hypothesized that invasive species may facilitate each other in a cascading “meltdown” (Simberloff & Von Holle, 1999), though this is rarely studied across trophic levels (Smith-Ramesh, Moore, & Schmitz, 2017). The Parker et al. (2006) meta-analysis did look at native versus exotic animals, but in the inverse—the effects of animals on invasive plants—and only focused on herbivores. It is reasonable to expect that native and exotic animals of all trophic levels may differentially respond to plant invasions. Like native plants, native animals have long coevolutionary relationships with the resident plants and animals that may be altered or disrupted upon arrival of an abundant exotic plant (Carvalheiro, Buckley, & Memmott, 2010). Exotic animals lack this coevolutionary history, which has unknown consequences to their success following invasive plant arrival (Prior, Robinson, Meadley Dunphy, & Frederickson, 2014). As the world's ecosystems become increasingly invaded (Seebens et al., 2018), it is vital to broaden the perspective of potential outcomes to the resident biota as this bears directly on conservation, management, and policy.
Our objective was to determine whether invasive plants differentially impact native and exotic animals. Specifically, we set out to find whether or not native and exotic animals show negative, positive, or no impact following association with invasive plant species. Additionally, because the impact of invasive plants is often context-dependent (Schirmel et al., 2016; Vilà et al., 2011), we sought to identify the ecological factors that influence the impact of invasive plants on native and exotic resident animals, including time since invasion (Strayer et al., 2006). Just as the majority of invasive plant–plant interactions have focused on consequences to native plants (Downey & Richardson, 2016), we predicted that invasive plant–animal studies would be biased toward impacts on native animals, due to conservation concern. Thus, following the methods of other studies that found strong research biases across multiple dimensions of invasive plant studies (Hulme et al., 2013), we also performed a qualitative assessment of these publications. We also considered other sources of research bias, including study spatial and temporal scales, as well as resident ecosystem and response animal taxonomic group and trophic level.
2 MATERIALS AND METHODS
2.1 Literature search
We conducted a search for publications that investigated the effect of invasive plants on animals using the online database ISI Web of Science on December 6, 2017. We used the Boolean search term ‘(alien OR exotic OR invas* OR nonnative OR non-native OR nonindigenous OR non-indigenous) AND (plant) AND (diversity OR community OR richness OR biodiversity OR abundance OR complexity OR fitness) AND (trophic OR food web OR specie* interaction* OR food cycle OR food-cycle OR cascade)’ which was derived from collating keywords from 48 publications that closely matched our topic. We further supplemented our database with publications that were included in an earlier, related meta-analysis (Schirmel et al., 2016). This search resulted in 574 publications that we then reviewed in detail.
Studies were excluded from our database if they met the following criteria: (a) were not relevant or not peer reviewed; (b) did not investigate a single invasive vascular plant; (c) had no non-invaded control site (control site includes uninvaded sites or lowest invader abundance when on a gradient); (d) assessed biocontrol species (biocontrol agents are not considered resident animals in the context of our objectives); (e) were conducted in an ecosystem other than terrestrial, emergent wetland, or marsh; (f) were not observational (i.e., manipulative/experimental studies were excluded); and (g) were reviews, meta-analyses, or modeling papers. The remaining studies were then excluded if their (h) response was not an animal, or their (i) response animal(s) were not identified to species, as nativity can only be determined when identified to species. This resulted in 77 studies, which we used to evaluate study design biases (hereafter referred to as “bias studies”). Of these 77 “bias studies,” only studies that were not excluded by the following criteria were used in the meta-analysis: studies were excluded if they (j) did not include mean ± error and sample size and (k) if the study recorded only native or only exotic response animals (not both). This resulted in 12 remaining studies (hereafter “meta studies”) appropriate for our meta-analysis (see Figure S1 and Table S1). A citation list for the 77 studies included is located in the Appendix.
2.2 Data collection
For each study, we recorded growth habit for the invasive plant species. We also used online databases (e.g., www.cabi.org, www.eol.org, www.catalogueoflife.org, www.iucnredlist.org) to estimate the time since first introduction of the invasive plant to the study location and recorded the spatial extent used for that determination. Each study was classified into ecosystem type as a “wet” ecosystem (e.g., wetland, marsh, riparian, etc.) or a “dry” ecosystem (e.g., forest, grassland, scrubland, etc.) and by geographic region. For every response animal, we recorded information on nativity (i.e., exotic or native to the study site), phylum, and trophic group. Nativity was assessed using similar online databases as above. In cases when we were unable to determine animal nativity, we removed that animal from the meta-analysis. For trophic groups, we categorized response animals as detritivores, primary consumers, secondary consumers, and omnivores based on online databases and species classifications. Further, we classified each response variable into a response type (abundance, density, fitness, survival, performance, growth, behavior); however, only studies that reported response animal abundance or density qualified for the meta-analysis. From the studies that reported abundance or density, we extracted sample size, mean, and standard error or standard deviation from text, tables, or from figures using ImageJ (Abràmofff, Magalhães, & Ram, 2005). When necessary, raw data were obtained from either the authors or the Supporting Information, from which we were able to calculate mean abundances and standard deviations for each response animal. For studies that included more than one site, we considered each site independently. If a study spanned multiple years, we included only observations from the final year of the study. Finally, when a study analyzed the effects of an invasive plant across an invasion gradient (e.g., high to low abundance of the invasive plant), we considered only the two extreme contrasts.
2.3 Meta-analysis
2.3.1 Effect size calculation
We calculated the standardized mean difference of each response animal abundance in invaded and non-invaded sites using Hedges' d. Hedges' d is a unitless, unbiased estimator of the standardized mean difference when sample sizes are small (Hedges & Olkin, 1985). In the context of our meta-analysis, negative values of Hedges' d indicate invasive plants had a negative effect on response animal abundance and positive values of Hedges' d indicate invasive plants had a positive effect on response animal abundance (see “Effect Size Calculation” in the Supporting Information for more detailed information on effect size calculation).
2.3.2 Statistical analysis
All studies included in the meta-analysis reported multiple observations (i.e., each study had >1 effect size), which violates the assumption of meta-analysis that effect sizes are independent of one another (as discussed by Gurevitch & Hedges, 1999; Nakagawa, Noble, Senior, & Lagisz, 2017 among others). To account for sampling dependence within the study, we chose to perform multilevel, mixed-effects meta-analyses where a nested effect of observation within study was included in each model. This allowed us to take into account the hierarchical dependence of multiple observations extracted from individual studies.
To assess the overall mean effect and heterogeneity, we performed an initial multilevel model with a random, nested effect of observation within the study. We then investigated the sources of heterogeneity by assessing ecologically relevant moderator variables (similar to predictor/independent variables in traditional linear models). To determine if invasive plants have an effect on native and exotic response animals, we fit each model with the moderator nativity, and, because there are multiple factors that might influence the impact of invasive plants on native and exotic animals, we explored the following moderators in combination with response animal nativity: invasive plant growth habit, ecosystem type, and trophic group and phylum of response animals. We ran a separate meta-analysis for each moderator with nativity. We could not analyze more than one moderator at a time with nativity, because our sample size was small and we did not have a sufficient number of replicates across moderator levels when more than one moderator was added to the model with nativity. Additionally, we investigated the overall effect of invasive plants on response animals in “wet” versus “dry” ecosystems by performing a single meta-analysis using all qualified studies that recorded either abundance or density (n = 41) and including the moderator ecosystem type. We only analyzed a level (subgroup) of a moderator if the level had at least three studies.
To determine if the impact of invasive plants on animals changes over time, we performed two separate meta-analytic models including the moderator time since introduction and the random effect of observation nested within study. In the first model, we sought to assess the impact of invasive plants on the abundance of animals in general. As such, we included all studies that met our inclusion criteria and reported abundance or density (n = 40). For the second model, we used only the 12 papers that recorded both exotic and native animals, because we wanted to determine if the impact of invasive plants over time is different for native and exotic response animals. In this model, we included the interaction term of time since introduction and animal nativity.
The overall heterogeneity among effect sizes was assessed using Cochran's Q and the heterogeneity statistic, I2. Cochran's Q tests the null hypothesis of homogeneity among effect sizes (Cochran, 1954). p Values are obtained when the Q-statistic is compared to the chi-squared distribution where a significant p value is evidence of heterogeneity among effect sizes. I2 values range between 0% and 100% and are interpreted as the percent of variability across effect sizes due to heterogeneity rather than sampling error (Higgins & Thompson, 2002). To evaluate the influence of the moderators on the impact of invasive plants on native and exotic animals, we used the Q test. In a meta-analytic model that includes moderators, the total heterogeneity (QT) is partitioned into residual heterogeneity (QE) and model heterogeneity (QM), where QM tests the null hypothesis that effect sizes are equal. All analyses were conducted in R (3.5.0; R Core Team, 2018) using the metafor package (Viechtbauer, 2015).
2.3.3 Publication bias analysis
We evaluated publication bias via funnel plot visualization, where we plotted the meta-analytic residuals against the inverse of the sampling errors (i.e., precision). An asymmetrical funnel plot is an indication of publication bias. In addition to graphical assessment, we used a modified Egger's regression as proposed by Nakagawa and Santos (2012) on the meta-analytic residuals to investigate publication bias statistically. A significant intercept indicates that there is evidence of publication bias. We chose to use the meta-analytic residuals for two reasons: (a) the raw effect sizes in our dataset violate the assumption of Egger's regression that effect sizes are independent of one another; and (b) funnel plot asymmetry may reflect actual heterogeneity that we account for in our models via the addition of moderator variables. Additionally, we assessed the possibility of a time-lag bias by observing the relationship between publication year and effect size. A time-lag bias occurs if studies with significant effect sizes are published before studies that find nonsignificant effects.
2.4 Study design bias analysis
Similar to other studies of possible research bias (e.g., Hulme et al., 2013; Warren, King, Tarsa, Haas, & Henderson, 2017), we sought to provide a detailed census of the studies evaluating the effects of invasive plants on animals. We did this using descriptive statistics of the 77 bias studies, which include the 12 meta studies. Due to the small sample size, we were unable to construct contingency tables or perform other analyses to be included for most of this section; instead, we present a summary of the identified research and two chi-squared tests investigating our hypothesis that researchers are more likely to study native animals and specific taxa.
To understand the general research status, we classified the response animal nativity, ecosystem type, location, invasive plant family, and publication year in each study. We reviewed the distribution of the number of unique response animal classes and species in each meta study. To determine whether researchers focused on native animals or specific taxa, we reviewed each of the 77 papers and determined if (a) the study was designed to focus on a specific response animal; and (b) the authors specifically mentioned the nativity of the response animal within the paper text. The former was completed by reading the entire paper and determining if the methods were designed to study a specific animal species (e.g., Malo et al., 2012 who focused on a single native rodent), or rather designed to capture a variety of animal species in a specific group of interest (e.g., Petillon et al., 2006 who studied any arachnid present, regardless of nativity or species). The second was completed by searching for the terms “native” OR “exotic” OR “invasive” (which also returned “non-native” and “nonnative”) and seeing if the use of either term was ever applied to a response animal. We then used a chi-squared test with Yates' continuity correction to compare this information to whether each study recorded only native or only exotic animals or whether a study recorded both exotic and native animals.
3 RESULTS
3.1 Meta-analysis
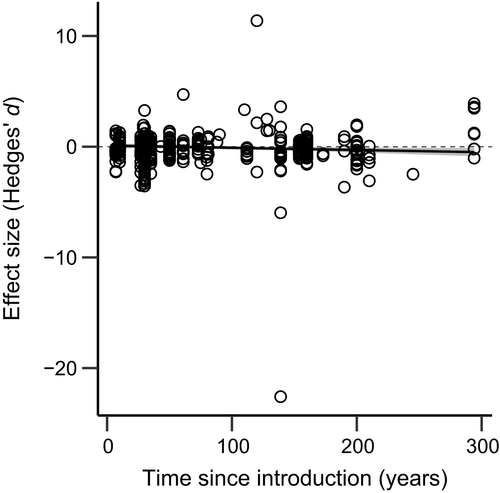
We found overall heterogeneity among effect sizes to be high (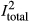 = 73.1%, Q = 573.7, df = 185, p < .001; Table 1). The high heterogeneity was not surprising considering that the 12 studies included in the meta-analysis spanned a diverse number of plant species, animals, ecosystems, and geographic locations which we attempted to account for by including moderator variables in the analyses. Overall, the among-study variance accounted for 16.8% of the heterogeneity and the within-study variance accounted for 56.2% of the heterogeneity.
= 73.1%, Q = 573.7, df = 185, p < .001; Table 1). The high heterogeneity was not surprising considering that the 12 studies included in the meta-analysis spanned a diverse number of plant species, animals, ecosystems, and geographic locations which we attempted to account for by including moderator variables in the analyses. Overall, the among-study variance accounted for 16.8% of the heterogeneity and the within-study variance accounted for 56.2% of the heterogeneity.
| Q | df | p | |
|---|---|---|---|
| Overall model | |||
| Overall | 573.74 | 185 | <.001 |
| Phylum × Nativity | |||
| Nativity | 9.14 | 1 | .003** |
| Phylum | 3.13 | 3 | .371 |
| Nativity × Phylum | 12.31 | 3 | .006** |
| Ecosystem type × Nativity | |||
| Nativity | 0.003 | 1 | .958 |
| Ecosystem type | 0.50 | 1 | .48 |
| Nativity × Ecosystem type | 1.92 | 1 | .166 |
| Ecosystem type | |||
| Ecosystem type | 2.79 | 1 | .095* |
| Trophic group × Nativity | |||
| Nativity | 0.002 | 1 | .964 |
| Trophic group | 0.42 | 3 | .935 |
| Nativity × Trophic group | 1.33 | 3 | .722 |
| Invader growth habit × Nativity | |||
| Nativity | 0.0001 | 1 | .992 |
| Growth habit | 0.26 | 2 | .880 |
| Nativity × Growth habit | 1.79 | 2 | .408 |
| Time since introduction × Nativity | |||
| Nativity | 0.66 | 1 | .417 |
| Time since introduction | 0.36 | 1 | .548 |
| Nativity × Time since introduction | 0.03 | 1 | .863 |
| Time since introduction | |||
| Time since introduction | 5.22 | 1 | .022** |
Note
- Significant influence of moderator variables is indicated at p < .05 (**) and p < .1 (*).
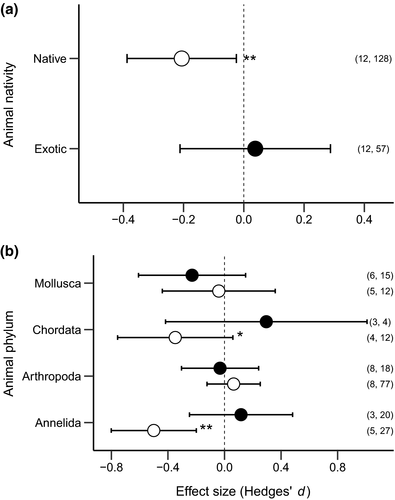
There was a significant negative effect of invasive plants on native animals but not on exotic animals (Figure 1a), but the negative effect was contingent on animal phylum (Qinteraction = 12.31, p = .006; Figure 1b). Abundance of native annelids (Q = 10.62, p = .001) and, to a lesser extent, native chordates (Q = 2.804, p = .094) was negatively impacted by invasive plants, while native mollusks and arthropods were not impacted by invasive plants (Figure 1b). We observed no effect of invasive plants on exotic animals at any taxonomic level (Figure 1b). Further, invader growth habit, ecosystem type, and response animal trophic group in combination with animal nativity were not significant (Table 1). However, we found that invasive plants had a significant negative impact on animals in “wet” ecosystem types (Q = 5.14, p = .023; Figure 2) but not in “dry” ecosystem types (Q = 0.04, p = .833; Figure 2).
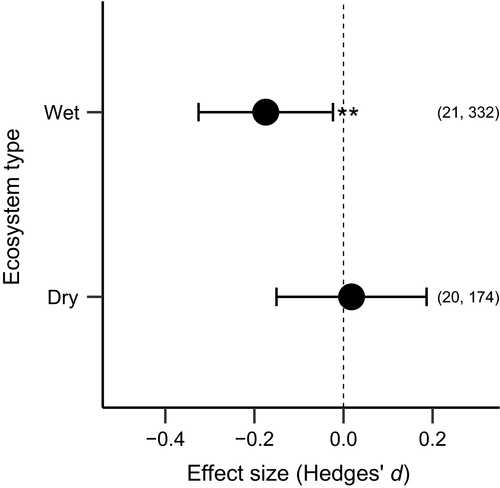
Animal abundance decreased significantly with longer invasive plant residence times (Q = 5.22, p = .022; Figure 3). However, invasive plant impacts over time did not vary between native and exotic animals (Q = 0.03, p = .823; Table 1).
3.2 Publication bias
There was significant funnel plot asymmetry in the meta-analytic residuals of the overall model (bo = −0.760, p = .002; Figure S2a). To determine if this asymmetry was the result of publication bias or represented true heterogeneity in the effect sizes, we also evaluated the meta-analytic residuals from the model assessing the interaction of nativity and phylum. After accounting for nativity and phylum, the modified Egger's regression no longer indicated significant asymmetry of the meta-analytic residuals (bo = −0.345, p = .144; Figure S2b), suggesting that heterogeneity, and not publication bias, is driving the asymmetry. Additionally, we found no evidence of time-lag bias (bo = −0.23, p = .280; Figure S3).
3.3 Study design bias
Of the 77 publications that evaluated the effect of invasive plants on animals, 56 assessed only native animal species, one assessed only exotic animal species, and 20 assessed both (ignoring response animals with unknown nativity).
These 77 studies were published between 1981 and 2017, with a peak between the years of 2005 and 2010 (Figure 4a). Most (75.3%) studies were conducted in North America or Europe, and were most common in forests (28.6%), marsh (20.8%), grassland (15.6%), and riparian (14.3%) ecosystems (Figure 4b,c). Dramatically, the invasive plant species in the Poaceae family were the focus of 33.8% of the total studies (26 of the 77, Figure 4d), with Phragmites australis the focus of seven of those 26 studies.
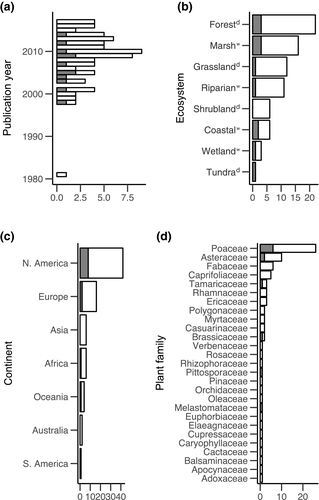
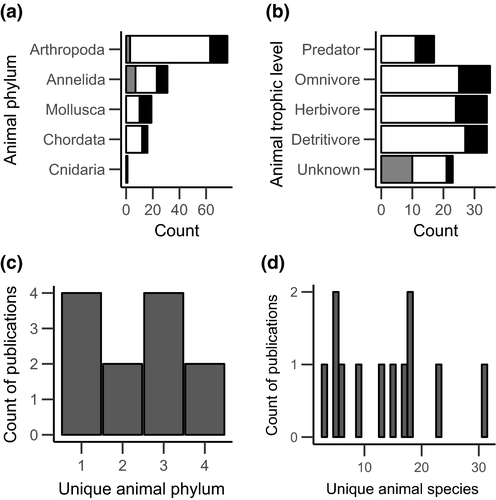
The meta studies looked at a total of 11 invasive plant species in six families, with 50% (six studies) being Poaceae (Figure 4d). There were 143 unique response animal species (206 total observations), comprising 98 natives, 35 exotics, and 10 with unknown nativity. Distribution within animal phyla and trophic level (when known) was uneven (Figure 5a,b). Out of the meta studies, four looked at only response animals within a single phylum, while eight looked at response animals from two to four phyla (Figure 5c). The distribution of the number of response animal species in each paper ranged from 3 to 31 (Figure 5d).
We found no evidence of a statistical bias between a manuscript's response animal nativity and mention of nativity or species-centric design (χ2 = 0, df = 1, p = 1.000 and χ2 = 2.23, df = 1, p = .135, respectively; Figure S5).
4 DISCUSSION
Our meta-analysis, which is the first to examine nativity-based animal responses, supports the hypothesis that native and exotic animals are differentially impacted by invasive plants. We found that native animals were negatively impacted by invasive plants while exotic animals were not. Importantly, within these impacts, animal taxonomic groups showed different responses, with native annelids and chordates showing the strongest effect of invasive plants. Interestingly, no studies were designed to test for differential impacts based on nativity and most studies did not identify animals to species. Additionally, the studies comprising our analyses reflect similar biases in invasive plant studies (Hulme et al., 2013) that are limited to few geographies, ecosystems, and invasive species.
4.1 Native versus exotic animals
Overall, we found that invasive plants had no effect on exotic animal abundance (i.e., no apparent facilitation or inhibition), but reduced the abundance of native animals. Despite our growing understanding of the role invasive plants play in the landscape, we still lack important information on whether native and exotic resident species respond differentially to plant invasion. Elucidating possible nativity-based differences in resident species', especially animal, responses would inform the tension of whether invasive species are the drivers or passengers of ecosystem change (Turkington & MacDougall, 2005), and whether invasion meltdown extends between multiple trophic groups. This would also inform the cascading consequences of managing invasive plants. For example, Rodewald, Shustack, and Hitchcock (2009) showed that native cardinals nesting in invasive Lonicera maackii shrubs fledged 20% fewer offspring, while others have found Cedar waxwings (Bombycilla cedrorum) feeding on Lonicera morrowii fruit develop novel plumage coloration that may affect breeding behavior (Witmer, 1996). In contrast, native species may have increased performance or fitness in response to plant invasion. For example, Schlossberg and King (2010) found increased nest success for native gray catbirds in invasive versus native substrates. Thus, there are many compelling motivations for parsing invasive plant ecological impacts among native and exotic animals.
The interactions between invasive plants and resident animals play important roles in both biotic resistance (the ability of a system to oppose invasion) and meltdown (the facilitation of exotic species by other exotic species). We found that invasive plants negatively impacted native annelids and chordates, though they did not impact native mollusks and arthropods. Annelids, some of which are at risk, are important organisms for many ecosystem processes. For example, the giant Palouse earthworm (Driloleirus americanus), which is believed to be on the brink of extinction by conservation agencies, is severely impacted by habitat destruction and competition with non-native earthworms (Xu, Johnson-Maynard, & Prather, 2013). However, exotic earthworms are responsible for altering important forest ecosystem processes (e.g., Li, Fisk, Fahey, & Bohlen, 2002). Here we found that invasive plants reduced native annelid abundance and neither suppressed nor facilitated exotic annelids; thus, having a compound negative impact on native systems. We also found that invasive plants reduced native chordate abundance. Chordates are a large, diverse group of organisms, including vertebrates, that play a multitude of important ecosystem functions across a range of trophic levels. Reducing chordate abundance could have far-ranging and cascading impact on recipient ecosystems. Famous examples include the cascading shifts in vegetation and enormous expansion of herbivore populations following the elimination of wolves in Yellowstone National Park, USA (Beschta & Ripple, 2015). While we found no differential effects across trophic levels, reductions in native annelid and chordate abundance could precipitate profound changes in ecosystem structure and function, as well as cascading effects to other trophic levels.
The concept of “invasion meltdown,” or the exotic–exotic facilitation of runaway invasion, has intrigued ecologists since it was proposed (Simberloff & Von Holle, 1999), though empirical tests remain limited (Simberloff, 2006). Regarding plant–animal interactions in invasion meltdown, Simberloff and Von Holle (1999) discussed two primary relationships: exotic animals as pollinators and dispersers, and exotic animals as ecological disturbers. These can both be broadly categorized as exotic animals facilitating invasive plant establishment (i.e., increases in invasive plant richness via exotic animals), with their inverse falling under the same meltdown umbrella (i.e., invasive plants facilitating invasive animal establishment). Though their predictions speak less to our objective of the effects of invasive plants on native/exotic animal abundance, exotic plant facilitation of exotic animal population expansion seems a logical extension of the meltdown concept. Our findings provide moderate support for invasion meltdown, or at a minimum do not contradict its central thesis, or our abundance-related amendment. In our study, we could not test whether the invasive plants facilitated the establishment of the exotic animals, though we did find that exotic animal abundance was neither facilitated nor reduced by invasive plants. Importantly, very few studies we examined (21 of 77 studies, 27%) recorded exotic animals at all. Our results did find that native animal abundance decreased following plant invasion, the consequence of which may in some cases facilitate exotic animal invasion by decreasing competition or opening new niches. As others have stated (Simberloff, 2006), meltdown is likely idiosyncratic and will be best tested with purposefully designed studies.
Interestingly, we found no evidence that invasive plant impacts on exotic or native animals varied based on trophic level. In contrast, McCary et al. (2016) found that invasive plants differentially impacted “green” (e.g., herbivores) and “brown” (e.g., detritivores) food webs. They found that invasive plants decreased primary consumer abundance and increased secondary consumers. However, like nearly all previous studies of invasive plant impacts, they did not parse resident animal biogeography—rather they found broader trends. The cascading effects of invasive plants could drive important changes across trophic levels (Smith-Ramesh, 2017), the understanding of which would be meaningfully informed by parsing these effects among native and exotic residents. For example, management practices may be directed toward the exotic drivers of cascading effects, rather than the passengers of such change (HilleRisLambers, Yelenik, Colman, & Levine, 2010).
Lastly, we found that invasive plant impacts increase with residence time (Figure 4), though this did not differ between native and exotic animals. There is increasing attention to accounting for important factors that may modulate invasive plant impacts, with abundance and time since introduction being most common. For example, Tekiela and Barney (2017) found that the impacts of two invasive grasses varied strongly by their relative abundance. Likewise, Dostal et al. (2013) found the impact of giant hogweed (Heracleum mantegazzianum) on native plants declined within 30 years of initial invasion. Unlike invader abundance, the time since invasion of many plant invaders is not known at the site level. This can be estimated for woody plants via annual growth rings, but for herbaceous species time since introduction requires local knowledge. Thus, for our analysis we used the most accurate information available; extracted from the paper when stated, or gleaned from records at the smallest spatial scale available (Figure S4). Our results suggest animal abundance decreases with plant invader residence time, which further supports rapid intervention to mitigate impacts.
4.2 A crisis in taxonomic expertise and collaboration
We hypothesized that native animals would be more commonly studied than exotic animals for their conservation importance. However, we found that no studies were designed to test for differential native and exotic responses, and less than half of the studies (40.3%) stated an intention to evaluate response animal nativity in any capacity (native, exotic, or both). Despite this lack of apparent a priori nativity consideration, the majority of studies identifying animals to species sampled only native animals (72.7%). This suggests a few intriguing possibilities: (a) the sampling designs somehow biased their collection toward native animals; or (b) exotic animals are far less common in plant-invaded landscapes than native animals. While the former seems improbable, the latter is a fascinating proposition, and both make interesting future research topics.
Importantly, a large proportion of the studies we reviewed, including those from previous meta-analyses (e.g., Schirmel et al., 2016), grouped animals into larger taxonomic or functional groups (74 of 151, 49.0%, Figure S2). The ability to identify organisms to species is important for describing the world's flora and fauna, and critical for developing an accurate understanding of invasive species impacts. Invaders are often cited as critically threatening native plants (e.g., Dueñas et al., 2018) and animals (e.g., Clavero & García-Berthou, 2005), yet these impacts cannot be fully understood unless we can identify the species being impacted. Theory suggests that the resident community may respond differentially depending on the biogeographic origin of its constituents (Grosholz, 2005), which we discuss in detail below. To test for differential effects between native and exotic response organisms requires that the study either (a) be designed to test this hypothesis or (b) the response organism be identified to species to determine nativity ex post facto. Our analyses of the existing literature identified deficiencies in both.
First, research on invasive plant impacts is likely dominated by plant ecologists who primarily focus on plant–plant interactions and plant–environment interactions. Our analysis suggests that plant–animal studies as currently carried out present limitations to interpreting the results due to a lack of animal identification. Grouping animals into broad taxonomic or functional groups may simply be a limitation of the group performing the work, which would be rectified via collaboration with experts. Thus, there may be an opportunity for increased collaboration among plant and animal researchers, which echo broader calls for inter- and transdisciplinary research (Balmford & Cowling, 2006; Jordan et al., 2016; Vaz et al., 2017).
Secondly, the lack of animal identification may be a result of a general lack of expertise, which may be particularly acute in understudied regions of the world and for rare species. There have been widespread calls to action for increased taxonomic training in response to the dwindling number of trained taxonomists across a range of disciplines (Drew, 2011; Packer et al., 2018). This crisis is particularly acute as global change continues to drive another great extinction, which may wipe out species before they can be identified and described. In fact, within invasive plant science, Pysek et al. (2013) identify a lack of taxonomic ability, citing the critical need to be able to identify exotic plant species quickly and correctly to mitigate the invasion before it gets out of hand. Thus, we strongly emphasize the critical need for identifying response taxa to species, whether plant or animal, and echo the calls of others for increased training and resources for species identification. A holistic understanding of invasive plant impacts (and, thus, conservation, management, and policy) is contingent on explicit consideration of resident biogeographic origin.
4.3 Wet versus dry differences
Previous studies have found differences in invasive plant impacts across ecosystems (e.g., McCary et al., 2016; Schirmel et al., 2016); unfortunately, we lacked adequate replication to test for variation among ecosystems. However, we did have studies spanning seven ecosystem types across the globe, which we grouped into “wet” and “dry.” We found that resident animal abundance was reduced by invasive plants in “wet” ecosystems, with no change in abundance in “dry” ecosystems; though there was no evidence that this impact was different for native and exotic animals. This suggests that the impact of invasive plants on resident animals is larger in wet ecosystems (e.g., marsh, riparian, wetland). This is consistent with Schirmel et al. (2016), who found a similar pattern, that coastal, riparian, and lake ecosystems had large decreases in animal abundance, with no clear pattern in “dry” ecosystems, though they did not explicitly test for a “wet/dry” ecosystem effect. They posit three possible reasons for higher impacts in riparian systems that seem well suited to all “wet” ecosystems: (a) frequent and sometimes large disturbance; (b) these systems are “downhill” and are the recipients of upland systems (e.g., nutrients, runoff, propagules); and (c) are common dispersal corridors for propagules. These conditions may increase invasive plant richness and abundance in wetter environments (Catford & Jansson, 2014), thereby exaggerating their ecological impacts, including those on resident animals. Studying the drivers and consequences of invasive plant-native/exotic resident animal interactions across different ecosystems would greatly enhance our understanding of these complex dynamics.
4.4 Biased and limited information
Invasive species science has been fraught with a variety of biases and seemingly axiomatic truisms that have in some instances triggered long debates among researchers (e.g., Brown & Sax, 2004; Davis et al., 2011; Gilroy, Avery, & Lockwood, 2017). It is important to take stock of these issues to ensure the foundations, assumptions, and limitations of the field are well understood. For example, in their assessment of the ecological impact of invasive plants, Hulme et al. (2013) found important biases toward studying a small number of invasive plants, in limited ecosystems, measuring too few and biased response variables, all of which overrepresented North America and Europe. From this, they concluded that we lack a broad and inclusive understanding of the ecological impacts of invasive plants. These biases and limited information also hamper effective management (Barney, 2016). Thus, we conducted a similar bias assessment of the studies of invasive plant impacts on native/exotic resident animals.
We found that invasive plants in the families Poaceae (26 of 77 study species) and Asteraceae (10 of 77) were overwhelmingly the most commonly studied, with P. australis (7 of 77) being the single most studied species. Similar to patterns found by Hulme et al. (2013), the vast majority of studies used in our analyses occurred in North America and Europe, with nearly all occurring since 2000. The latter suggests that the study of invasive plant impacts on animals is a rather new field of interest, perhaps linked to an expanding appreciation for the ecological impacts of invasive plants.
Importantly, our assessment of study designs found no bias in results if the authors stated an intention to study native or exotic animals. However, as described above, over half of the studies in our analysis sampled only native animals, while only 26.0% (20/77) sampled both native and exotic animals. Most of the studies we examined were designed to look at the effects of invasive plants on a specific group of animals (e.g., birds, spiders). Thus, while 50% of studies sampled no exotic animals, it is entirely possible there were exotic animals present outside of those being studied. Arthropods were sampled several fold more often than any other taxonomic group (Figure 5), though nativity was not assigned to the vast majority. Surprisingly, 75% of the “meta” studies evaluated response animals in ≥2 phyla, with most studies recording responses to >10 animal species, and some >30. Of course, this comes with large caveats in the small sample size (12 studies), and biases in studied ecosystems and invasive plants (Figure 5). It seems clear that coordinated surveys of invaded ecosystems are urgently needed (e.g., Barney et al., 2015; Kumschick et al., 2014)—ones that survey a broad range of taxa and ecosystem processes.
4.5 Conclusions and a call to action
Understanding the broad ecological impacts of invasive plants is critical to deploying mitigation strategies and informing conservation efforts effectively. Our work has identified differential impacts on native and exotic animals and defined important areas for future research efforts that have been largely overlooked to date. Despite calls for standardized impact definitions (e.g., Jeschke et al., 2014) and protocols (Barney et al., 2015; e.g., Kumschick et al., 2014), important gaps remain. For example, recording multiple metrics across a range of species and ecosystem processes (Barney, Tekiela, Dollete, & Tomasek, 2013), and placing invasive species within a food web context (Smith-Ramesh et al., 2017), which affords more accurate accounting of the lateral (i.e., within a trophic level) and cascading (i.e., “up” and “down” trophic levels) impacts. This expanded understanding is requisite for appropriate and pragmatic management. Our analysis revealed several gaps which we offer to future researchers for consideration.
First, taxonomic identification is as critical to understanding invasive species impacts as it is to studies of biodiversity. This broadened understanding will require appropriately designed studies with additional expertise in taxonomy. We found that most of the studies of the impacts of invasive plants on animals did not identify the animals to species. Thus, the “taxonomic expertise crisis” that is affecting the fields of entomology (Austen, Bindemann, Griffiths, & Roberts, 2016; Packer et al., 2018), botany (Prather, Alvarez-Fuentes, Mayfield, & Ferguson, 2004; Pyšek et al., 2013), benthology (Holzenthal, Robertson, Pauls, & Mendez, 2013), community ecology (Gotelli, 2004), and biology in general (Drew, 2011), are extended to invasion biology.
Secondly, clarifying the impacts of invasive species will require researchers to report the nativity of all organisms within the study. This is critical because determining this post facto is challenging at best, especially for rare, cryptic, and poorly studied species. The nativity status of surveyed species should be seen as vital to every study in invasion biology. Researchers should also identify the trophic position of the study and response organisms. As with nativity, this is often difficult to determine outside the study system. Estimates of the abundance and time since introduction of the exotic species within the study system would be greatly beneficial as there is increasing evidence that impacts vary with invader abundance (Sofaer, Jarnevich, & Pearse, 2018) and time (e.g., Dostál, Müllerová, Pyšek, Pergl, & Klinerová, 2013).
Our most important call is for more numerous and diverse empirical studies of the impacts of invasive plants on native and exotic taxa within the same sites across a range of linked trophic levels and ecosystems. Only with greater data availability and specificity will we be able to answer questions which are fundamental to invasion, restoration, and conservation ecology.
ACKNOWLEDGEMENTS
Special thanks to David Haak, Dan Atwater, and two anonymous reviewers for helpful comments on an earlier draft. We also acknowledge the Virginia Tech College of Agriculture and Life Sciences for partial support.
CONFLICT OF INTEREST
We declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
RF and GS performed the meta-analyses; RKB performed the publication analyses; ARH and GS performed the keyword search; ARH organized PRISMA details; all authors gathered raw data; JNB conceived the idea, oversaw all aspects, and finalized the revision, and all authors contributed to the final draft.
Open Research
DATA AVAILABILITY STATEMENT
All data are publicly available at https://data.lib.vt.edu/collections/rf55z785f.




