Climate change does not affect the seafood quality of a commonly targeted fish
Abstract
Climate change can affect marine and estuarine fish via alterations to their distributions, abundances, sizes, physiology and ecological interactions, threatening the provision of ecosystem goods and services. While we have an emerging understanding of such ecological impacts to fish, we know little about the potential influence of climate change on the provision of nutritional seafood to sustain human populations. In particular, the quantity, quality and/or taste of seafood may be altered by future environmental changes with implications for the economic viability of fisheries. In an orthogonal mesocosm experiment, we tested the influence of near-future ocean warming and acidification on the growth, health and seafood quality of a recreationally and commercially important fish, yellowfin bream (Acanthopagrus australis). The growth of yellowfin bream significantly increased under near-future temperature conditions (but not acidification), with little change in health (blood glucose and haematocrit) or tissue biochemistry and nutritional properties (fatty acids, lipids, macro- and micronutrients, moisture, ash and total N). Yellowfin bream appear to be highly resilient to predicted near-future ocean climate change, which might be facilitated by their wide spatio-temporal distribution across habitats and broad diet. Moreover, an increase in growth, but little change in tissue quality, suggests that near-future ocean conditions will benefit fisheries and fishers that target yellowfin bream. The data reiterate the inherent resilience of yellowfin bream as an evolutionary consequence of their euryhaline status in often environmentally challenging habitats and imply their sustainable and viable fisheries into the future. We contend that widely distributed species that span large geographic areas and habitats can be “climate winners” by being resilient to the negative direct impacts of near-future oceanic and estuarine climate change.
1 INTRODUCTION
Climate change will have various direct and indirect impacts on marine and estuarine species and ecosystems. Climate stressors, including warming and acidification, can directly impact species physiologies (Davis et al., 2017; Deutsch, Ferrel, Seibel, Pörtner, & Huey, 2015; Portner & Knust, 2007), phenology (Asch, 2015; Edwards & Richardson, 2004) and distributions, (Cetina-Heredia, Roughan, Sebille, Feng, & Coleman, 2015; Coleman et al., 2017; Perry, Low, Ellis, & Reynolds, 2005; Sunday et al., 2015) that can cascade through indirect effects to alter entire ecosystems (Nagelkerken & Connell, 2015; Provost et al., 2017; Vergés et al., 2016; Wernberget al., 2016, 2018). While we have an emerging understanding of these ecological impacts, we know less about the effects of climate change on the provision of ecosystem goods and services to human populations.
Oceans and estuaries provide many beneficial goods and services, worth trillions of dollars (Costanza et al., 2014), with the production of seafood among the most important for sustaining growing human populations. Seafood production and consumption is not only socially and culturally important, but sustains the economies of many global communities and industries. With a predicted increase in the reliance of seafood as a source of protein under climate change (Delgado, Wada, Rosegrant, Meijer, & Ahmed, 2003; Gerland et al., 2014), understanding potential climate-induced alterations to seafood quality and quantity will be vital for ensuring long-term resource viability (Cooley, Lucey, Kite-Powell, & Doney, 2012; Lloret et al., 2015).
There is general consensus that future ocean conditions will negatively impact fish production (quantity) via different pathways, including decreases in species body size mediated by physiological constraints (Sheridan & Bickford, 2011), ability to locate prey and altered defence mechanisms (Cripps, Munday, & Mccormick, 2011; Pauly & Cheung, 2018; Pistevos, Nagelkerken, Rossi, Olmos, & Connell, 2015). However, relatively fewer studies have examined the potential for impacts of climate change on fish tissue quality and nutritional benefits. Changes in tissue quality could manifest as altered tissue biochemistry, including the composition of fatty acids (particularly polyunsaturated fatty acids; PUFAs), micronutrients and protein. Although there are no studies on fish, near-future warming and acidification decreased the nutritional quality of marine mollusc flesh through reduced proteins and lipids (Tate, Benkendorff, Ab Lah, & Kelaher, 2017), and altered fatty acid profiles (Anacleto et al., 2014; Valles-Regino et al., 2015) and concentrations of micronutrients, including copper and arsenic (Tate et al., 2017). Such changes not only affect the nutritional benefits of seafood, but could also manifest as altered palatability (i.e., taste and/or texture) or visual appeal. For example, for oysters (Crassostrea gigas), warming and acidification under future climate scenarios tended to increase appeal in some sensory properties (meat appearance and degustation), but failed to significantly impact palatability or appearance (Lemasson et al., 2017). Any substantial change in the nutritional quality and appeal of seafood may have implications for providing health benefits and sustenance to humans into the future.
For the first time, we examine the interactive effects of near-future climate change (warming and acidification) on the growth, health and nutritional properties of a recreationally and commercially important fish; yellowfin bream (Acanthopagrus australis). This species occurs throughout south-eastern Australia (southern Queensland to southern New South Wales/Victoria; Iwatsuki 2013, Stewart, Hegarty, Young, Fowler, & Craig, 2015) whereas a panmictic stock (Roberts & Ayre, 2010) they undertake migrations from estuarine to oceanic areas for spawning (Curley, Jordan, Figueira, & Valenzuela, 2013; Pollock, 1984). Such movements are facilitated by wide salinity (<10–35 PSU) and temperature tolerances (19–38°C) (Froese & Pauly, 2017; Gray, 2015; Uhlmann, Broadhurst, & Millar, 2015). Along with numerous closely related congenerics, which are distributed more broadly throughout the Indo-Pacific (Iwatsuki 2013), yellowfin bream have a relatively higher market value than many coharvested species (Curley et al., 2013; Froese & Pauly, 2017). Given their wide distribution and putative tolerance to a range of abiotic conditions, we suggest yellowfin bream and species of Acanthopagrus, in general, may be relatively resilient to near-future warming and acidification.
2 MATERIALS AND METHODS
To test whether the growth, health and tissue quality of yellowfin bream may be impacted by climate change, an experiment utilizing twenty independent 230-L round outdoor mesocosms (80 cm diameter × 45 cm high) was configured at the National Marine Science Centre (NMSC) aquaria, Coffs Harbour, Australia (30°16′3.70″S, 153°8′15.31″E). The mesocosms were arranged to have an orthogonal combination of ambient (“current”) and future pCO2 (~430 and ~960 ppm, respectively) and temperatures (~22 and 25°C, respectively; n = 5 mesocosms per treatment; Supporting Information Table S1). These treatments corresponded to 2,100 predictions for climate change scenario RCP 8.5, a high emissions trajectory prediction (IGBP et al., 2013).
Sea water from the adjacent ocean was pumped into the NMSC flow-through aquarium system and filtered at 50 μm prior to entering each mesocosm at 2.5 L/min. Water temperatures in the mesocosms were controlled using heater–chiller units (Aquahort Ltd., Omana Beach, New Zealand). The pCO2 concentrations were manipulated by bubbling CO2-enriched air via a gas mixer (PEGAS 4000MF) through the CO2-enriched header tanks, while ambient air was diffused through stones in the current treatment header tanks. Water temperature, conductivity (units) and pH were measured daily (Hach HQ40d multi probe; Supporting Information Table S1). Total alkalinity for each treatment was measured weekly using a potentiometric titration (888 Titrando, Metrohom, USA) of 40 µm filtered, Hg fixed water samples. These water quality measurements were then used to calculate the partial pressure of CO2 (pCO2) using the CO2SYS program (Pierrot et al., 2009).
Before starting the experiment, ~230 yellowfin bream (~20–26 cm total length; TL) were collected from the middle of its range in eastern Australia at Maclean, New South Wales (NSW, −29.464°E, 153.172°S) during short (~15 min) deployments of paired penaeid trawls fitted with codends made from 27 mm knotless netting (Broadhurst & Millar, 2009). Catches were emptied on to a wet sorting tray, and yellowfin bream were collected, transported and placed into multiple 3,000-L aerated and flow-through holding tanks at the NMSC for an acclimation period (>6 months). During this period, fish were fed a diet of school prawns (Metapenaeus macleayi) and 4 mm commercial pellets at a rate ~1% biomass/day. The ambient water temperature (17.6–20.7°C; mean ± SD of 19.1 ± 0.9°C), salinity (33.5–37.0; 34.9 ± 0.1 ppt) and DO (6.7–8.1; 7.5 ± 0.4 mg/L) in the holding tanks remained within the tolerance ranges for yellowfin bream. In total, 13% of fish died and all within the first 2 weeks of confinement, with no subsequent mortality.
The experiment commenced on 27 September 2017, well after the known spawning period (Curley et al., 2013) to avoid confounding results with reproductive factors or sexes of fish, although most were juveniles based on established sizes at maturity (20–24 cm fork length; FL). On the first day of the experiment, yellowfin bream in two of the 3,000-L holding tanks were anaesthetized using 30 mg/L Aqui-S in sea water. Twenty individuals were randomly selected across both holding tanks, measured to the nearest 0.1 mm for TL, FL and standard length (SL) and weighed (nearest 0.1 g) by being secured in a foam mount on a digital benchtop scale before being transferred to the mesocosms (one fish per mesocosm). Only one fish was placed in each mesocosm to avoid competitive interactions.
Each fish was acclimatized in its new environment (i.e., a mesocosm) for 1 week, prior to beginning gradual commencement of the seawater manipulations to treatment levels over a 2-day period. Fish were fed school prawns to satiation throughout the experiment. After 40 days, each fish was again anaesthetized with 30 mg/L Aqui-S solution, removed from their tanks and secured in a foam block before an ~1 ml blood sample (0.4–2.5 ml) was collected using a heparinized syringe and 23 gauge needles following Broadhurst et al. (2005). Immediately following blood collection, two microhematocrit tubes were filled for each individual and centrifuged for 120 s at 15,800 rpm/13,700 × g to calculate the haematocrit (Hct) packed cell volume (PCV) and a glucometer (TruTrack, Nipro, Australia) was used to determine blood glucose levels.
After being blood sampled, fish were euthanized (150 mg/L Aqui-S), weighed and measured for TL, FL and SL as above and dissected for tissue and organ sampling as below. Sex was noted where possible (although many fish were juveniles and could not be reliably sexed). Among those individuals that could be reliably sexed, ratios were roughly 1:1. Fish were then filleted and subsamples of the flesh were snap frozen in liquid nitrogen and transferred to −80°C prior to lipid analysis and the remaining flesh was stored at −20°C until further processing for proximate and elemental analyses.
Samples of the flesh (1 g) were weighed on an analytical balance (±0.0001 g) then dried in the oven at 60°C until they obtained a stable weight, then reweighed and the difference used to calculate moisture content. The dry samples were then transferred to a muffle furnace (Barnstead thermolyne 30400 furnace) at 400°C for 4 hr to obtain the ash content. The Kjeldahl nitrogen method was used to calculate total nitrogen, according to standard protocols (AOAC, 1995), with a factor of 6.25 to convert to crude proteins.
Lipids were extracted from the flesh using 1:2 chloroform: methanol according to standard procedures (Folch, Lees, & Sloane Stanley, 1957) in analytical grade solvents (Sigma-Aldrich). Separation of the polar layer was induced using 0.9% NaCl solution in a separating funnel. The lipophilic (chloroform) layer was then dried on a Buchi rotary evaporator (470 mbar, 40°C). The extract was transferred to a preweighed vial in a small volume of hexane and dried under a stream of high purity N2 gas, then reweighed to obtain the weight of lipid extract. The total lipid concentration for each extracted sample was determined as per cent lipid in the fresh weight (% g/g).
Subsamples of the total lipid extract (200 µl suspended at 25 mg/ml in isopropanol) were analysed by liquid chromatography–mass spectrometry (LC-MS) using a Phenomenex Synergi C18 (5 µm, 250 mm × 4.6 mm) LC column in an Agilent Technologies 1260 Infinity series HPLC with attached mass spectrometry unit (Agilent Technologies 6120 Quadrupole LC/MS). The mobile phase consisted of a solvent gradient of methanol and isopropanol (IPA) with 0.05% trifluoroacetic acid (TFA) added to both solvents at a flow rate of 300 µl/min. The gradient started with 10% IPA, increasing to 25% over 25 min, then up to 95% at 40 min, then held for 10 min before dropping back to 10% and held for 5 min. UV detection was set 210, 230, 280 and 360 nm, while total ion current was set to positive ion mode in the MS. Major peaks were compared to lipid standards with characteristic fragment ions from the total ion current (TIC) used to assist identifying free fatty acids and phospholipids, while TIC was characteristically suppressed for di- and triglycerides.
A 200 µl subsample of the lipid extract was derivatized using BF3 in methanol for fatty acid methyl esters (FAMEs) analysis according to Valles-Regino et al. (2015). Fatty acid composition was analysed using gas chromatography (GC; Agilent 6890 N) on a BPX 70 capillary column (70% cyanopropyl polysilphenylene-siloxane, 50 m × 0.22 mm × 0.25 μm), coupled with a flame ionization detector (FID). The GC oven temperature was programmed at 100°C, held for 5 min and then increased at 5°C/min until the final temperature 240°C was obtained, with high purity helium as the carrier gas at a linear flux of 1 ml/min. Identification of FAMEs was based on retention time, peak and elution order compared to FAMEs standard test mix (SUPELCO 37-Component FAME Mix) and marine test mix PUFA No.1 (Marine Source, Analytical Standards, Sigma-Aldrich). To identify unmatched peaks including docosapentaenoic acid, supplementary analysis using gas chromatography–mass spectrometry (GCMS) was undertaken using an Agilent 6890 GC coupled to Agilent 5973 mass spectrometer (Valles-Regino et al., 2015) and matched to the NIST14 and Wiley275 mass spectral libraries.
The total elemental analysis of flesh subsamples (1 g) was undertaken by the Environmental Analysis Laboratory (EAL), Southern Cross University (National Association of Testing Authorities, Australia (NATA); Accreditation Number 14960). Samples were digested in a mixture of HNO3 (25%) and HCl (75%) (1:3, v/v) using a hot-block acid digestion procedure. Elemental concentrations were analysed on an inductively coupled plasma (ICP) spectrometer (NexION 300 D series) with ESI SC-FAST Auto Sampler (Perkin Elmer, Waltham, MA, USA) and were determined on a wet-weight basis.
2.1 Statistical analyses
Multivariate and univariate analyses were done using PERMANOVA in the PRIMER 6.0 & PERMANOVA + add-on package (Anderson, Gorley, & Clarke, 2008). For each individual variable, data were analysed using Euclidian distances with 9,999 permutations and pairwise tests used to ascertain the nature of any significant differences. Multivariate analyses were also done for lipid and fatty acid composition (standardized) and macro- and microelements (normalized) using Euclidean distance. For all analyses, there were two orthogonal, fixed factors; temperature (ambient 22°C and future 25°C) and acidification (ambient and future) with n = 5 replicate tanks.
3 RESULTS
All fish survived the duration of the experiment, ate well and were in visible good health. There were significant increases in TL, FL and SL (PERMANOVA p ≤ 0.05) and a marginally nonsignificant (p = 0.07) but biologically large (50%) increase in weight under elevated temperature treatments (Table 1, Figure 1). After 40 days, fish were, on average, about 10 g heavier and 4 mm longer in tanks at 25°C than those in tanks at 22°C (Figure 1). There was no effect of acidification on changes in fish morphometrics, or an interaction with temperature (PERMANOVA, p > 0.05, Table 1).
| Temperature | pCO2 | Temperature × pCO2 | ||||
|---|---|---|---|---|---|---|
| Pseudo F | p value | Pseudo F | p value | Pseudo F | p value | |
| Morphometrics | ||||||
| Weight | 4.03 | 0.07 | 0.66 | 0.42 | 0.19 | 0.19 |
| TL | 9.31 | 0.01 | 0.55 | 0.45 | 0.33 | 0.57 |
| FL | 4.11 | 0.05 | 0.09 | 0.56 | 0.09 | 0.78 |
| SL | 6.13 | 0.02 | 0.00 | 0.94 | 0.00 | 0.93 |
| Blood analysis | ||||||
| Haematocrit PVC | 0.14 | 0.73 | 0.14 | 0.74 | 0.46 | 0.52 |
| Glucose | 0.54 | 0.49 | 0.01 | 0.92 | 0.67 | 0.45 |
| Proximate composition | ||||||
| Moisture | 1.48 | 0.26 | 0.20 | 0.71 | 0.07 | 0.84 |
| Ash | 0.06 | 0.82 | 0.38 | 0.55 | 3.28 | 0.09 |
| Total protein | 3.73 | 0.08 | 0.62 | 0.44 | 0.10 | 0.76 |
| Total lipids | 0.01 | 0.91 | 0.00 | 0.96 | 1.38 | 0.25 |
| Lipid compositiona | 0.93 | 0.47 | 1.02 | 0.40 | 0.93 | 0.46 |
| Free fatty acids | 1.90 | 0.21 | 0.01 | 0.91 | 0.01 | 0.92 |
| Phospholipids | 1.33 | 0.31 | 0.49 | 0.53 | 1.04 | 0.38 |
| Triglycerides | 2.48 | 0.15 | 0.41 | 0.56 | 0.70 | 0.44 |
| Fatty acids compositiona | 0.25 | 0.83 | 0.30 | 0.79 | 0.66 | 0.55 |
| Saturated fatty acids | 0.16 | 0.6867 | 0.29 | 0.59 | 0.59 | 0.47 |
| Monounsaturated fatty acids | 0.21 | 0.6620 | 0.10 | 0.76 | 1.21 | 0.30 |
| Polyunsaturated fatty acids | 0.01 | 0.9176 | 0.40 | 0.55 | 0.15 | 0.70 |
| n−3 | 0.01 | 0.9223 | 0.46 | 0.53 | 0.09 | 0.78 |
| n−6 | 0.30 | 0.5869 | 0.12 | 0.74 | 0.23 | 0.63 |
| n−3/n−6 ratio | 0.25 | 0.6494 | 0.49 | 0.52 | 0.02 | 0.89 |
| Macroelementsa | 0.17 | 0.87 | 0.23 | 0.82 | 0.04 | 0.99 |
| Microelementsa | 3.12 | 0.06 | 0.65 | 0.51 | 0.68 | 0.48 |
Note
- Significant values in bold.
- a Multivariate analysis.
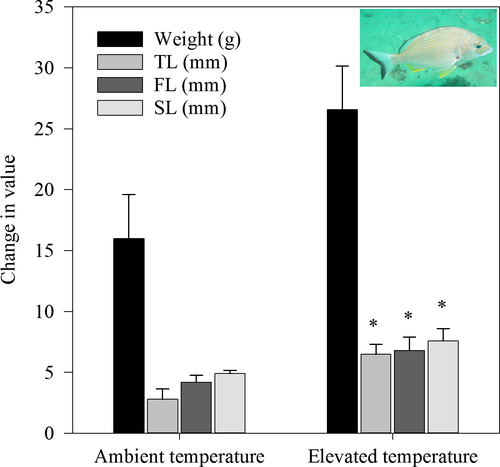
There was no influence of either temperature or acidification (or their interaction) on blood haemolysis (haematocrit PCV) or glucose levels (PERMANOVA, p > 0.05; Table 1). The proximate composition of the flesh was also unaffected (Table 1) with an average of 75.0 ± 1.0% moisture content and 5.8 ± 0.7% ash per g wet weight (PERMANOVA, p > 0.05). The protein content varied from 18–22 g/100 g dry flesh with no significant difference under elevated temperature or pCO2 conditions (PERMANOVA, p > 0.05, Table 1).
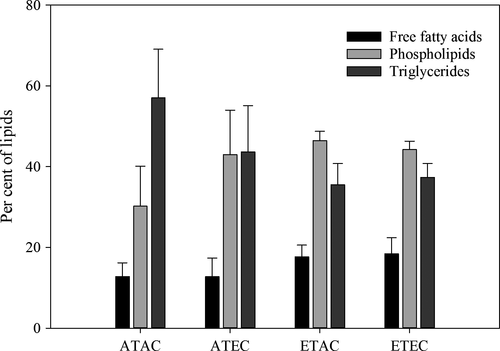
The total extracted lipids comprised on average 1.3 ± 0.4% per g wet weight, with no difference according to experimental conditions (PERMANOVA, p > 0.05, Table 1). There was a nonsignificant trend for a higher percentage of phospholipids and free fatty acids relative to triglycerides in the total lipid composition under elevated temperature treatments (Figure 2). The fatty acid profile was dominated by saturated and monounsaturated fats (>70%), with a high proportion of steric and oleic acids (Table 2). The dominant PUFA was linoleic (C18:2; ~10%), with low levels of all other omega 6 (n-6) and omega 3 (n-3) PUFAs, and an n-3:n-6 ratio <1 (Table 2). There was no change in the proportion of SFA, MUFA and PUFAs or fatty acid composition under elevated temperature or acidification (PERMANOVA, p > 0.05, Tables 1 and 2).
| Fatty acid | Trivial name | Ambient temperature (22°C) | Elevated temperature (25°C) | ||
|---|---|---|---|---|---|
| Current pCO2 | Future pCO2 | Current pCO2 | Future pCO2 | ||
| C14:0 | Myristic | 3.07 ± 0.63 | 2.91 ± 0.61 | 2.86 ± 0.12 | 2.82 ± 0.31 |
| C16:0 | Palmitic | 25.02 ± 2.74 | 24.23 ± 2.83 | 24.67 ± 0.99 | 24.54 ± 1.14 |
| C18:0 | Stearic | 7.03 ± 0.51 | 6.74 ± 1.09 | 6.54 ± 0.28 | 7.01 ± 0.39 |
| Total SFA | 35.59 ± 3.81 | 33.88 ± 3.99 | 34.06 ± 1.25 | 34.36 ± 1.50 | |
| C16:1 | Palmitoleic | 7.20 ± 0.55 | 7.32 ± 1.1 | 7.34 ± 0.30 | 6.86 ± 0.36 |
| C18:1(n−9) | Oleic | 31.85 ± 3.61 | 32.96 ± 4.76 | 34.16 ± 1.64 | 32.40 ± 1.02 |
| Total MUFA | 39.04 ± 3.61 | 40.28 ± 5.76 | 41.50 ± 1.68 | 39.25 ± 1.19 | |
| C18:2(n−6) | Linoleic (LA) | 10.62 ± 1.14 | 10.97 ± 1.39 | 10.50 ± 0.83 | 10.28 ± 0.76 |
| C18:3(n−3) | a-Linoleic (ALA) | 1.23 ± 0.19 | 1.36 ± 0.36 | 1.13 ± 0.14 | 1.11 ± 0.17 |
| C20:2 (n−6) | Eicosadienoic | 2.53 ± 0.36 | 2.28 ± 0.52 | 2.34 ± 0.28 | 2.24 ± 0.45 |
| C20:4(n−6) | Arachidonic (AA) | 1.96 ± 0.89 | 1.77 ± 1.14 | 1.61 ± 0.42 | 2.36 ± 0.20 |
| C20:5(n−3) | Eicosapentaenoic (EPA) | 1.92 ± 0.69 | 1.97 ± 0.73 | 1.70 ± 0.25 | 2.19 ± 0.14 |
| C22:5(n−3) | Docosapentaenoic (DPA) | 1.78 ± 0.64 | 1.64 ± 0.68 | 1.82 ± 0.56 | 1.89 ± 0.29 |
| C22:6(n−3) | Docosahexaenoic (DHA) | 5.33 ± 2.4 | 5.85 ± 3.70 | 5.13 ± 0.99 | 6.21 ± 0.43 |
| Total PUFA | 25.37 ± 5.52 | 25.84 ± 6.25 | 24.43 ± 0.79 | 26.38 ± 1.55 | |
| n−3 | 10.26 ± 3.82 | 10.82 ± 5.08 | 9.98 ± 1.22 | 11.41 ± 0.85 | |
| n−6 | 15.12 ± 1.81 | 15.02 ± 1.85 | 14.45 ± 0.46 | 14.98 ± 1.06 | |
| n−3:n−6 | 0.66 ± 0.19 | 0.71 ± 0.29 | 0.69 ± 0.11 | 0.76 ± 0.07 | |
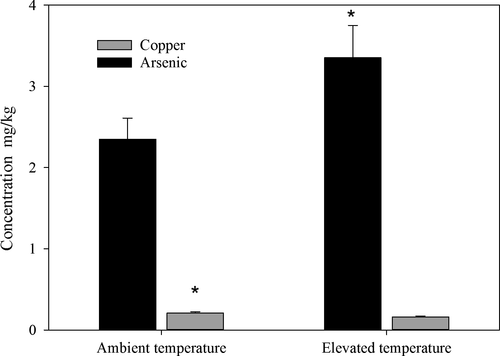
The dominant macroelements in the flesh were potassium (>4 mg/g), phosphorous, sulphur and sodium (on average >1 mg/g), followed by magnesium (>0.4 mg/g), calcium, silicon (on average >0.1 mg/g), iron and aluminium (0.002–0.4 mg/g; Supporting Information Table S2). There were no significant effects of elevated temperature or acidification on the overall macroelemental composition (PERMANOVA, p > 0.05 for all factors; Table 1) or any individual macroelement (Supporting Information Table S2). All microelements were recorded at below maximum recommended limits for seafood, except for vanadium (average 1.5 ± 0.03 mg/kg vs. upper limit of 0.01 mg kg−1 day−1 ATSDR, 2015). There was a slight tendency for temperature to change the composition of microelements overall (PERMANOVA, p = 0.06, Table 1), and this was likely affected by significant decreases in copper and increases in arsenic at elevated temperatures and nonsignificant trends for decreases in lead at elevated temperatures (Figure 3, Supporting Information Table S2). No other microelement or macroelement showed differences between treatments (PERMANOVA, p > 0.05, Supporting Information Table S2).
4 DISCUSSION
Although there is a consensus that future ocean conditions will likely evoke decreases in the production and sizes of marine fish, few studies have examined the influence of predicted increases in temperature and acidification on the quality and nutritional value of tissue. The data here show that elevated temperatures and pCO2-induced acidification predicted under conditions of near-future ocean warming increased the growth of yellowfin bream, and there was little change in their health or the quality and nutritional properties of muscle tissue. These results imply that this species and the fishery it underpins may be resilient to changes throughout oceans in the near future.
4.1 Effects of climate change on fish health and seafood quality
The finding that near-future ocean warming (an increase in 3°C from ambient as forecast for the end of this century) had little impact on the health and seafood quality of yellowfin bream is likely due to this species having a wide latitudinal range and inhabiting marine and estuarine habitats that naturally vary greatly in their abiotic characteristics (Curley et al., 2013; Froese & Pauly, 2017; Gray, 2015). Although the increase in temperature simulated here was above that usually experienced by yellowfin bream in the open ocean in the region where the experiment was completed (and where these fish were collected), it is well within the thermal range of the species (Curley et al., 2013) and within the range that can be experienced within individual estuaries. Moreover, acoustically tagged yellowfin bream from nearby areas have been known to migrate up to 250 km, including between estuarine and inshore open coastal habitats (Lowry, Becker, Folpp, Mcleod, & Taylor, 2017). Thus, a 3°C temperature increase is likely well within the range yellowfin bream can tolerate more broadly and suggests that near-future climate change may have negligible direct impacts on this widely distributed species, except perhaps near its low latitude limit. Indeed, modelling studies have shown that ecological generalists (e.g., species that display characteristics including omnivory and/or large latitudinal range size) are most likely to have already undergone climate-induced range extensions (Sunday et al., 2015), suggesting that such species are tolerant to the changing abiotic conditions expected in coming decades.
In contrast to our previous studies on a harvested gastropod, which incurred large reductions in protein and lipid content relative to moisture under near-future ocean conditions (Tate et al., 2017), we detected little change in the tissue chemistry of yellowfin bream. Unlike the gastropods, yellowfin bream do not need to catabolize their tissue to provide energy to cope with stress from future conditions of temperature and acidification. Nonetheless, we did find a nonsignificant trend for the percentage of triglycerides in yellowfin bream to be lower (relative to phospholipids and free fatty acids) under elevated temperature treatments, which is consistent with a weak stress response. Triglycerides are important energy reserves in fish (Bennett, Weber, & Janz, 2007) and are likely to be utilized by increased metabolism and higher growth rates observed at elevated temperature.
An increase in saturated relative to unsaturated fatty acids is a common consequence of organismal exposure to elevated temperatures, as a result of homeoviscous adaptation to maintain appropriate fluidity in cell membranes (Neidleman, 1987). However, unlike previous studies investigating ocean warming on molluscs (Anacleto et al., 2014; Martino & Cruz, 2004; Valles-Regino et al., 2015), yellowfin bream had no significant differences in their fatty acid composition. Previous studies on fish indicate that they also adapt to lower temperatures by increasing unsaturation in tissue lipids (Patton, 1975) and desaturase activity can be inhibited at higher temperatures (De Torrengo & Brenner, 1976; Qiang et al., 2017). However, the fish here had a relatively low proportion of polyunsaturated fatty acids (<30% vs. >40% in molluscs), and they contained very low levels of long-chained highly saturated omega 3 or omega 6 acids such as arachidonic acid (ARA C20:4), eicosapentaenoic acid (EPA C20:5) or docosapentaenoic acid (DPA C22:5), which are most susceptible to warming (Valles-Regino et al., 2015). Owing to relatively low omega 3 and 6 polyunsaturated fatty acids, and an n-3:n-6 ratio <1, yellowfin bream do not provide a premium source of fish oils that are good for human health (Simopoulos, 1991, 2008; Swanson, Block, & Mousa, 2012). Nevertheless, they do provide a good source of linolelaidic (C18:2) and monounsaturated fatty acids, which were not impacted under near-future ocean conditions.
There was also a slight tendency for temperature to change the composition of micronutrients overall, and this was driven by significant decreases in copper and increases in arsenic at elevated temperatures. The decrease in copper was in contrast to Tate et al. (2017), who found that elevated temperatures significantly increased copper content in the foot meat of a harvested predatory whelk (Dicathais orbita). We also found increases in arsenic and nonsignificant trends for a decrease in lead at elevated temperatures, but levels of these elements remained within safe levels (NHMRC, 2006). Again, this is in contrast to Tate et al. (2017) who found decreases in lead due to acidification (but not warming). Whelks are sedentary compared to yellowfin bream and have a narrower distributional range, which may explain why they generally responded more strongly to near-future ocean conditions. Nevertheless, these minor changes in mineral elements are unlikely to significantly impact the nutritional content of yellowfin bream tissue, but instead highlight that the influence of future oceans on seafood nutritional values will be species-specific and may be difficult to predict.
4.2 Effects of climate change on fish growth
The increase in growth of yellowfin bream under near-future ocean temperatures is in contrast to the literature that generally predicts fish production will decline due to a combination of decreases in body size (Sheridan & Bickford, 2011) associated with size-related physiological constraints, and changes in distributions and abundances (Cheung et al., 2012; Pauly & Cheung, 2018; Portner & Knust, 2007), as well as survival (Rosa et al., 2014). Because the yellowfin bream in our study were not near their critical upper temperature threshold in the elevated temperature treatment, it is likely that they experienced increased metabolism and certainly ate more (with fish fed to satiation) while remaining within size-related physiological constraints (Cheung et al., 2012; Pauly & Cheung, 2018; Portner & Knust, 2007). Previous studies have shown that yellowfin bream grow faster during summer and at their northern range (Curley et al., 2013). Moreover, our study resulted in relatively rapid increases in weight and size over a short period (40 days), which may not be sustained over longer time periods, as increasing body size and demand for oxygen goes beyond physiological limits of supply due to gill surface area (Pauly & Cheung, 2018). Nonetheless, there is uncertainty in how any inherent positive temperature effects on growth might translate in terms of climate change. Specifically, increased growth may not be realized if prey respond negatively to ocean changes or if yellowfin bream foraging efficiency is compromised (Gardiner, Atema, Hueter, & Motta, 2014; Munday et al., 2013). However, the omnivorous diet of yellowfin bream (Froese & Pauly, 2017) may facilitate adaptability to changing ecological landscapes.
It will be important to quantify if the increased growth of yellowfin bream found here was in the form of muscle, fat or other tissue because this has important implications for seafood quality and production. Similarly, given the sustainable harvesting of yellowfin bream is based on relationships between size, age and sex, understanding how the increases in size relates to maturation rates will be key for developing robust strategies for a sustainable fishery in a future of ocean change (Stewardson, Andrews, & Ashby, 2016). Nevertheless, intuitively, any realized increases in growth rates are likely to be positive in terms of life history (Curley et al., 2013) and may translate to greater absolute productivity in coming years.
5 CONCLUSION
Near-future changes to oceans predicted for the end of the century had few impacts on the health or nutritional quality of yellowfin bream, a recreationally and commercially important fish species from eastern Australia. Indeed, contrary to theory that predicts future oceans will decrease the size, production and nutritional quality of fish lipids, elevated temperatures increased the growth of yellowfin bream suggesting that short-term exposure to predicted near-future increases in ocean temperature is well within current physiological thresholds. We contend that fish species that have broad geographic ranges and habitats may sometimes be climate winners because they have plastic responses and can tolerate a range of abiotic conditions. The maintenance of seafood nutritional properties of such species will contribute to their fisheries viability in a future of increasing ocean climate change.
ACKNOWLEDGEMENTS
We thank Ashley Dowell, Analytical Research Laboratory, SCPS for facilitating the fatty acid and lipid analyses. Sean Blake is thanked for collecting the fish. Financial support was received through a SCU MERC Grant to KB and BPK and through the Australian Research Council via DP150104263 to BPK.




