Presence of an invasive species reverses latitudinal clines of multiple traits in a native species
Abstract
Understanding the processes driving formation and maintenance of latitudinal clines has become increasingly important in light of accelerating global change. Many studies have focused on the role of abiotic factors, especially temperature, in generating clines, but biotic factors, including the introduction of non-native species, may also drive clinal variation. We assessed the impact of invasion by predatory fire ants on latitudinal clines in multiple fitness-relevant traits—morphology, physiological stress responsiveness, and antipredator behavior—in a native fence lizard. In areas invaded by fire ants, a latitudinal cline in morphology is opposite both the cline found in museum specimens from historical populations across the species’ full latitudinal range and that found in current populations uninvaded by fire ants. Similarly, clines in stress-relevant hormone response to a stressor and in antipredator behavior differ significantly between the portions of the fence lizard range invaded and uninvaded by fire ants. Changes in these traits within fire ant-invaded areas are adaptive and together support increased and more effective antipredator behavior that allows escape from attacks by this invasive predator. However, these changes may mismatch lizards to the environments under which they historically evolved. This research shows that novel biotic pressures can alter latitudinal clines in multiple traits within a single species on ecological timescales. As global change intensifies, a greater understanding of novel abiotic and biotic pressures and how affected organisms adapt to them across space and time will be central to predicting and managing our changing environment.
1 INTRODUCTION
Latitudinal gradients and clines are key features of biogeography found across taxa and ecosystems (De Frenne et al., 2013; Fuhrman et al., 2008; Hawkins, Porter, & Felizola Diniz-Filho, 2003; Roy, Jablonski, Valentine, & Rosenberg, 1998). These patterns describe how factors such as diversity, abundance, and species’ traits vary across space and in association with biotic and abiotic environmental factors (Willig, Kaufman, & Stevens, 2003). The study of latitudinal clines has provided insight into how key organismal traits, such as body size and fecundity, impact fitness (Cardillo, 2002; Jonsson & L'abée-Lund, 1993; Sibly, 1991). Latitudinal clines are incorporated into theory and development of models explaining life-history variation and the role of plasticity and adaptation in shaping traits (Niewiarowski, 1994; Reznick & Ghalambor, 2001). Some latitudinal clines are well known in certain taxa, such as those described by Bergmann's, Allen's, and Gloger's Rules, and are considered to be driven by abiotic factors such as temperature and season length (Meiri & Dayan, 2003; Millien et al., 2006). Much research has focused on the role that abiotic factors, especially thermal regimes (Chen, Hill, Ohlemüller, Roy, & Thomas, 2011; De Frenne et al., 2013; Huey, Gilchrist, & Hendry, 2005), play in structuring latitudinal clines. However, biotic factors, such as mutualisms, symbioses, parasite–host interactions, or predator–prey relationships, may also be important drivers of clines and have received increasing study (Merino et al., 2008; Moles, Bonser, Poore, Wallis, & Foley, 2011; Schemske, Mittelbach, Cornell, Sobel, & Roy, 2009). Crucially, both abiotic and biotic factors theorized to drive formation of clines are shifting as a result of global environmental change.
Research into the biogeographical impacts of abiotic and biotic factors has taken on a new importance in the current era of rapid anthropogenic change (Araújo & Luoto, 2007). Changes including warming, increasing climatic variability, and invasion of non-native species (Butchart et al., 2010) produce novel selective regimes (Parmesan, 2006; Sax et al., 2007). Some species may not be able to successfully adapt to these changes, especially those from polar (Kerby & Post, 2013; Mills et al., 2013) or alpine regions (Dullinger et al., 2012). However, many species do adapt to novel selective regimes (Strauss, Lau, & Carroll, 2006), and biogeographical patterns, including latitudinal clines, can be altered in the process. Clines in genetic variation, body size, and phenology have shifted to track changes in abiotic conditions (Gardner, Heinsohn, & Joseph, 2009; Millien et al., 2006; Yang & Rudolf, 2010). Similarly, species introduced to novel environments recreate clines found in their native range and develop new clines, within as few as 20 years (Huey et al., 2005; Huey, Gilchrist, Carlson, Berrigan, & Serra, 2000; Johnston & Selander, 1964; Lee, 2002; Liu, Li, & McGarrity, 2010). Studying effects of rapid change on clines can help us understand environmental factors underlying the formation of clines and provide insight into how novel ecological pressures alter species’ traits (De Frenne et al., 2013; Teplitsky & Millien, 2014).
Much research on how global change alters latitudinal clines has focused on how changes in abiotic conditions drive shifts in genotypes and phenotypes (Edeline, Lacroix, Delire, Poulet, & Legendre, 2013; Gardner et al., 2009; Millien et al., 2006; Rombouts et al., 2009). Some types of global environmental change, including biological invasions, provide valuable insights into the role of biotic factors in driving the formation and alteration of latitudinal gradients and clines (Bruno, Fridley, Bromberg, & Bertness, 2005). Invasive species occur over large geographical extents and can apply strong selective pressures to native species (Salo, Korpimäki, Banks, Nordström, & Dickman, 2007; Sax & Gaines, 2008). This provides an opportunity to assess how rapid changes in biotic factors affect existing clines. Additionally, while most studies of changes in biogeographical clines address one focal trait, under some conditions, including biological invasions, multiple traits within a species can shift simultaneously (Langkilde, 2009; Shine, Brown, & Phillips, 2011). Here, we examine the role of novel predation pressure, a biotic factor, in altering latitudinal clines in multiple traits in a single species using a system of invasive predatory ants and native lizards.
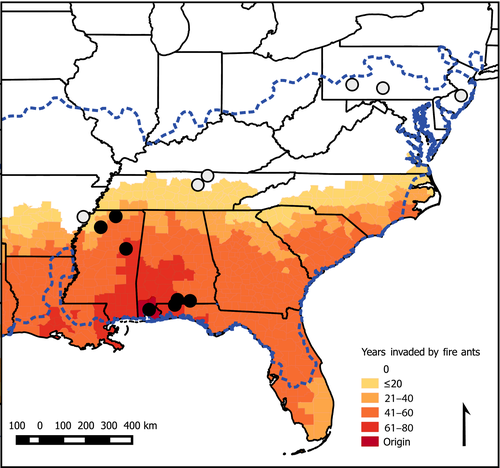
Red imported fire ants (Solenopsis invicta) were introduced through the port of Mobile, AL, USA, in the 1930s and have spread throughout the southeastern Unites States (Figure 1). They consume and can exert novel selective pressures upon native species including eastern fence lizards (Sceloporus undulatus) (Allen, Epperson, & Garmestani, 2004; Langkilde, 2009). Lizards, and especially fence lizards, have been valuable organisms for studying ecogeographical rules (Angilletta, Niewiarowski, Dunham, Leaché, & Porter, 2004; Du, Warner, Langkilde, Robbins, & Shine, 2010; Niewiarowski, 1994). Fence lizard populations are known to differ in a suite of traits, including antipredator behavior, stress (glucocorticoid) responsiveness, and morphology, according to whether or not they have been invaded by fire ants (Graham, Freidenfelds, Mccormick, & Langkilde, 2012; Langkilde, 2009). Lower latitude populations of fence lizards, which have been invaded by fire ants for longer periods of time (Figure 1), are more behaviorally responsive to fire ants and have correspondingly longer hindlimbs and higher stress responsiveness, adaptations which support increased survival of fire ant attacks (Graham et al., 2012; Langkilde, 2009), as compared to higher latitude, fire ant-naïve populations. These previous studies assume a causal effect of fire ant presence but have not addressed whether climatic factors or pre-existing latitudinal clines in antipredator behavior, stress responsiveness, or hindlimb length might explain the observed patterns in these traits as well as, or better than, the presence of fire ants. For these three traits, we examined whether observed patterns represent pre-existing clines or instead are due to changes in pre-existing clines that correspond to novel pressure by invasive fire ants.
2 MATERIALS AND METHODS
2.1 Field collection
Eastern fence lizards were captured via noose and by hand at 13 sites (seven invaded by fire ants and six uninvaded) across the latitudinal range of this species from 2006 to 2014 (Figure 1). Measurements of morphology, physiological responsiveness to a stressor, and antipredator behavior were taken on subsets of these (Supporting Information Table S1).
2.2 Behavioral testing
In 2006–2014, captured lizards (n = 487) were placed in cloth bags and transported to areas with active fire ant mounds for behavioral testing. We gently disturbed fire ant mounds and exposed lizards to sublethal fire ant attack for up to 1 min. We recorded whether they either fled from the attack or adopted crypsis by remaining motionless [for details of the methodological approach see Langkilde (2009)].
2.3 Blood and CORT sampling
During the breeding season in May–July, 2011 and 2013, we assessed physiological responsiveness to a stressor in female fence lizards. We measured this as changes in corticosterone (CORT), the primary stress-relevant hormone in reptiles (Romero, 2004), following a standardized stressor. We measured baseline levels of CORT in blood samples obtained from fence lizards (n = 95) via the retro-orbital sinus within 2 min of first disturbing each animal. Lizards were then subjected to a standardized stressor (confinement in a cloth bag) for 30 min, and an additional blood sample was taken (post-stressor sample). CORT responsiveness was calculated as the difference in CORT concentrations between the post-stressor and baseline samples. Blood samples were stored on ice until centrifugation, when plasma was pipetted off and stored at −20°C until hormone quantification.
CORT was quantified using commercially available enzyme immunoassay (EIA) kits (Corticosterone High Sensitivity EIA Kits, Immunodiagnostic Systems Ltd., Gaithersburg, MD) previously validated for use in this species (Trompeter & Langkilde, 2011). Plasma samples were diluted 90% with assay buffer so that levels of CORT fell within the detectable range of the assay's standard curve. Each sample was run in duplicate following the kit instructions. Coefficients of variation were calculated using control samples provided in the kits. Mean intraassay coefficient of variation was 2.30% (range: 1.71%–3.36%) and the mean interassay coefficient of variation (plates = 7) was 5.36%.
2.4 Morphological traits
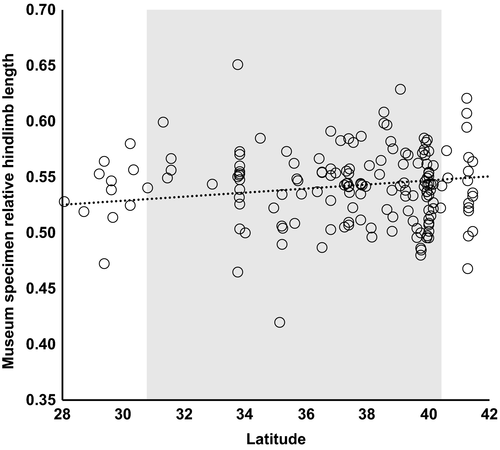
In 2006–2014, we measured hindlimb length and snout-vent length (SVL) of field-caught fence lizards [n = 1,450; see supplementary materials of Langkilde (2009) for details]. Relative hindlimb length was calculated by dividing lizard hindlimb length by SVL. To account for observer differences in measurement error, all measurements of relative hindlimb length were standardized to a reference population (Lee, Supporting Information Table S1) from which all observers measured lizards. Hindlimb length was also calculated as residuals from a regression of ln-transformed hindlimb length on ln-transformed SVL, and results were qualitatively similar. Hindlimb length and SVL were measured in fence lizard museum specimens (n = 165, from 84 unique sites) collected from across the latitudinal range of this species at sites that were not invaded by fire ants at the time of collection (Quarantined Areas, 2016; Supporting Information Figure S1). All preserved lizards were measured by one observer (T.L.). Values of hindlimb length cannot be compared between live and preserved specimens due to different measurement approaches required for preserved specimens but are consistent within these groups.
2.5 Climatic analyses
As abiotic conditions are often theorized to drive formation and maintenance of latitudinal clines, we assessed whether relevant climatic variables chosen a priori could be driving observed patterns in lizard traits. For each study site, we extracted values of selected variables from the BioClim database at a 2.5′ resolution (Hijmans, Cameron, Parra, Jones, & Jarvis, 2005), allowing us to integrate conditions over all capture localities at each site and the surrounding area. Because climatic variables are often strongly correlated, we removed variables from our a priori set that had high correlations with other climatic variables (≥0.9) to construct a more parsimonious set of variables. Our final set of variables included annual mean temperature (bio1), mean diurnal range (bio2), precipitation in the warmest quarter (bio18), and elevation (elev).
2.6 Statistical analyses
We used a generalized linear mixed model with a logit link and a binomial distribution to assess the effect of latitude on behavior with fixed effects for latitude, invasion status, sex, and the interaction between sex and invasion status; random effects for site nested within invasion status and year; and SVL as a covariate. CORT responsiveness was calculated as the difference between post-stressor and baseline CORT levels, and CORT concentrations were inverse hyperbolic sine transformed to meet assumptions of normality. We constructed a linear mixed model to assess the effect of latitude on CORT responsiveness with fixed effects for latitude, invasion status, and year; a random effect for site nested within invasion status; and SVL as a covariate. We used a linear mixed model to assess the effect of latitude on relative hindlimb length of museum specimens with fixed effects for sex, latitude, and the interaction between sex and SVL; a random effect for site; and SVL as a covariate. We used a linear mixed model to assess the effect of latitude on morphology with fixed effects for latitude, invasion status, sex, and the interaction between sex and SVL; a random effect for site nested within invasion status; and SVL as a covariate. Parsimonious final models were constructed using likelihood ratio tests to eliminate unnecessary interactions and fixed effects. In each model, we separately estimated the slope and intercept of the trait's relationship with latitude at sites invaded and uninvaded by fire ants and compared the slopes using one-tailed t tests. If the slopes were significantly different, we inferred that the presence of fire ants affected the relationship of the target trait with latitude. To test whether abiotic factors better explain observed patterns in lizard morphology, stress responsiveness, and behavior, we competed models with climatic predictors against the final model for each trait which included fire ant invasion and latitude, as well as the null model for each trait which contained intercepts and the same random effect structure as each full model. Four models with one climatic predictor each (elev, bio1, bio2, or bio18) were produced for each trait by removing fire ant invasion and latitude as factors in each final model and substituting a single climatic predictor while retaining other covariates and a random effect for site. Climatic predictors were introduced singly to avoid issues with multicollinearity between abiotic factors. In a similar fashion, to test whether the pattern of relative hindlimb length in museum specimens collected prior to fire ant invasion was due to abiotic factors, we competed models with the same single climatic predictors against a model including latitude and a null model with the same random effect structure. Akaike's information criterion corrected for small sample sizes (AICc) was calculated for each model. For each trait, the model with the lowest AICc was judged to perform best. Models were checked for violations of assumptions of normality by visually inspecting standard plots of residuals. Analyses were conducted using SPSS v. 23 (IBM Corp.) and R v. 3.3.1 (R Core Team, 2016).
3 RESULTS
In areas uninvaded by fire ants, fence lizard populations show a decreasing incidence of crypsis behavior with increasing latitude (Figure 2a; β = −0.312, F1,8 = 20.080, p < 0.001). However, in areas invaded by fire ants, this latitudinal cline is reversed (difference in slopes: t = 5.824, p < 0.001), and lizards are most likely to break crypsis and flee in response to fire ants in lower latitude populations with longer history of fire ant invasion (Figure 2a; β = 0.393, F1,8 = 13.78, p < 0.001, n = 487).
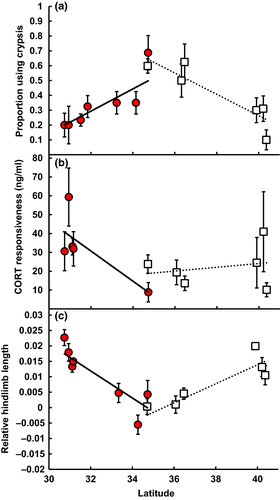
In areas uninvaded by fire ants, stress responsiveness of female fence lizards shows a positive, but nonsignificant trend with latitude (β = 0.121, F1,8.23 = 2.528, p = 0.149). However, this relationship is significantly different in populations from fire ant-invaded areas (Figure 2b; difference in slopes: t = 2.423, p = 0.017), where populations at the lowest latitudes have the greatest increases in CORT following exposure to a standardized stressor (β = −0.269, F1,12.78 = 3.640, p = 0.079, n = 95).
Fence lizard specimens collected prior to fire ant invasion across the full latitudinal range of this species show a positive relationship between relative hindlimb length and latitude (Figure 3; β = 0.0018, F1,80.6 = 5.681, p < 0.020, n = 165), with potential drivers of this pattern including temperature and precipitation (Supporting Information Table S2). This trend is not different from the relationship between hindlimb length and latitude in present-day fence lizard populations uninvaded by fire ants (Figure 2c; β = 0.0031, F1,7.4 = 9.692, p < 0.016, n = 706; difference in slopes of historical vs. present-day: t = −1.101, p > 0.271). The direction of this cline is reversed, however, in populations invaded by fire ants (difference in slopes of uninvaded vs. invaded: t = 4.444, p < 0.001), which have proportionally longer limbs at lower latitudes (Figure 2c; β = −0.0044, F1,8.5 = 9.949, p < 0.013, n = 744).
Models constructed using climate data had higher AICc values than models including fire ant presence for all three traits (Table 1).
| Trait | Model | K | Log-likelihood | AICc | ΔAICc |
|---|---|---|---|---|---|
| Morphology | Fire ant invasion | 9 | 3,878.137 | −7,738.149 | — |
| Elevation | 7 | 3,870.995 | −7,727.913 | 10.236 | |
| bio1 | 7 | 3,870.704 | −7,727.329 | 10.820 | |
| bio2 | 7 | 3,872.783 | −7,731.488 | 6.661 | |
| bio18 | 7 | 3,872.419 | −7,730.760 | 7.389 | |
| Null | 3 | 3,587.215 | −7,168.413 | 569.74 | |
| Stress responsiveness | Fire ant invasion | 7 | −145.114 | 305.515 | — |
| Elevation | 5 | −148.784 | 308.242 | 2.727 | |
| bio1 | 5 | −148.781 | 308.235 | 2.720 | |
| bio2 | 5 | −148.740 | 308.155 | 2.640 | |
| bio18 | 5 | −147.517 | 305.708 | 0.193 | |
| Null | 3 | −158.395 | 323.053 | 17.538 | |
| Antipredator behavior | Fire ant invasion | 8 | −292.170 | 600.641 | — |
| Elevation | 6 | −305.863 | 621.851 | 21.210 | |
| bio1 | 6 | −305.920 | 621.965 | 21.324 | |
| bio2 | 6 | −305.578 | 621.281 | 20.640 | |
| bio18 | 6 | −303.411 | 616.947 | 16.306 | |
| Null | 3 | −308.472 | 620.969 | 20.328 |
Note
- Change in AICc (ΔAICc) for each model calculated as the difference in AICc for each model from the top performing model for each trait.
4 DISCUSSION
For all three traits we examined in fence lizards—antipredator behavior, physiological stress responsiveness, and relative hindlimb length—we found evidence that clines are significantly different where lizards coexist with fire ants than in areas which remain unoccupied by fire ants. The putative trait changes we observed in areas invaded by fire ants are all in directions that confer adaptive advantages to lizards responding to fire ant attacks.
Fence lizards often employ crypsis (i.e., remaining still and not fleeing) as an antipredator tactic. In areas uninvaded by fire ants, we found that use of crypsis by fence lizards decreases with latitude (Figure 2a). This pattern coincides with observations and predictions in other organisms that antipredator behavior should decrease with latitude, likely as a consequence of reduced predator diversity and abundance at higher latitudes (Díaz et al., 2013). However, in areas invaded by fire ants, using crypsis as an antipredatory behavior is ineffective, as lizards which do not flee from fire ants are envenomated by additional ants and may quickly be overwhelmed (Freidenfelds, Robbins, & Langkilde, 2012). In this situation, increases in fleeing behavior (i.e., decreases in crypsis) are adaptive and allow escape from and survival of fire ant attacks (Langkilde, 2009). In areas invaded by fire ants, the relationship between latitude and use of crypsis shifted to become positive, with the lowest levels of crypsis occurring in the southernmost populations. This pattern matches predictions that adaptive increases in lizard anti-ant behavior should be greater in populations exposed to fire ants for longer periods of time (Langkilde, 2009) and corresponds to other observations of predator-driven natural selection on behavior in lizards (Lapiedra, Schoener, Leal, Losos, & Kolbe, 2018).
Increases in circulating levels of glucocorticoid stress hormones, a form of physiological stress responsiveness, facilitate organismal responses to a variety of challenges (Romero, 2004) and may covary with environmental stressors across latitudes (Breuner et al., 2003). Generally, stress responsiveness in clutching females is predicted to decrease with latitude during the breeding season to maximize fitness (Wingfield, 1988). However, previous work in Sceloporus has shown no evidence for a latitudinal cline in stress responsiveness of breeding females (Hews & Abell Baniki, 2013). In areas uninvaded by fire ants, we found that stress responsiveness in breeding female fence lizards is weakly and nonsignificantly associated with latitude (Figure 2b), in agreement with previous research. In contrast, in populations of lizards co-occurring with fire ants, the relationship between latitude and responsiveness is significantly different. Lizards from populations at the lowest latitudes have the highest levels of stress responsiveness (Figure 2b), suggesting an incipient shift in stress physiology (McCormick, Robbins, Cavigelli, & Langkilde, 2017). This physiological response supports more effective escape from fire ants (Trompeter & Langkilde, 2011) and primes lizards to flee from future attacks (Langkilde, Thawley, & Robbins, 2017), thus supporting observed adaptive shifts in antipredator behavior.
In areas uninvaded by fire ants, we found a significantly positive cline between relative hindlimb length and latitude (Figure 2c). This relationship was the same as that observed in museum specimens collected from areas free of fire ants at the time of collection (Figure 3), suggesting the cline predates fire ant invasion. However, this cline is reversed in populations co-occurring with fire ants, with southern populations having longer hindlimbs. Increased hindlimb length in eastern fence lizards is adaptive in fire ant-invaded populations as this trait supports higher sprint speeds (Miles, 1994) during flee behaviors and improves removal of attacking fire ants by twitch behaviors (Langkilde, 2009). Additionally, this pattern of increasing hindlimb length at lower latitudes, where fence lizard populations have coexisted longer with fire ants, matches the prediction that this adaptive trait should increase with the evolutionary time that populations have been exposed to fire ants (Langkilde, 2009).
It is possible that environmental factors other than fire ant presence or contemporary processes co-occurring with fire ant invasion have driven the observed clines in these traits. However, models using our selected set of abiotic variables (temperature, precipitation, and elevation) performed worse than those including fire ant presence in predicting observed patterns in traits (Table 1). For CORT responsiveness, however, precipitation in the warmest quarter of the year performed similarly to the model including fire ant invasion (ΔAICc = 0.193), suggesting that this climatic factor could influence stress responsiveness. However, as there is no a priori prediction for this relationship in this system or related literature, and CORT responsiveness can play a role in facilitating adaptive behavior (Langkilde et al., 2017), we do not consider it strong evidence against the effect of fire ants on stress responsiveness. For hindlimb length, analyses using specimens preserved in museums show that abiotic factors, including precipitation in the warmest quarter of the year, may have contributed to the historic cline in this trait (Supporting Information Table S2). The current pattern in hindlimb length diverges strongly from this historical cline, suggesting that the pattern we observed is driven by recent, nonclimatic change.
An additional consideration is that the invasion of non-native species may be related to other landscape-level factors, such as land use or habitat type, or co-occurs with other contemporary processes. For example, fire ants typically occupy open, disturbed habitats, so changes in land use could contribute to the pattern we observe here. However, fence lizards also favor open, disturbed habitat, and all of our study populations were found in similar disturbed edges of mixed forests, suggesting that land use differences are not driving observed clines. There may be other contemporary processes that we have not considered that could explain our results. These findings support further research into the importance of biotic environmental changes, such as the expansion of invasive species, vs. other contemporary processes in the formation of clines.
Our results show that adaptive changes to novel pressures can occur simultaneously across multiple pathways and encompass multiple traits (morphology, physiology, and behavior). The adaptive values of trait shifts are linked, as changes in stress responsiveness and hindlimb length support increased flight behaviors (Langkilde, 2009; Langkilde et al., 2017; Trompeter & Langkilde, 2011), allowing an integrated organismal response to a single novel biotic threat. Evidence suggests that heritable variation may underlie some trait shifts in fence lizards, as limb length in Sceloporus is heritable (Langkilde, 2009; Tsuji, Huey, Berkum, Garland, & Shaw, 1989) and changes in response to selection in lizards (Calsbeek & Irschick, 2007), while transgenerational factors affect both antipredator behavior (Robbins & Langkilde, 2012) and stress responsiveness (McCormick et al., 2017). However, the relative contributions of genetic variation, epigenetic effects, and phenotypic plasticity to the formation of these clines are not yet clear and bear further investigation.
These results reveal that pre-existing latitudinal clines and the continuing presence of selective regimes that drove their formation may not preclude adaptation to environmental change even if novel pressures run counter to pre-existing ones. In fact, latitudinal clines in a variety of traits are labile (Brommer, Hanski, Kekkonen, & Väisänen, 2015; Gardner et al., 2009; Huey et al., 2005; Umina, Weeks, Kearney, Mckechnie, & Hoffmann, 2005). Variability, whether heritable or plastic, is a precondition for adaptive responses (Ayrinhac et al., 2004; Hoffmann, Shirriffs, & Scott, 2005). The existence of a cline may indicate that enough variation exists within a species for a given trait to allow adaptation to a range of environmental conditions. Conversely, lack of variation in key traits, especially across gradients of important environmental factors, could indicate species that may be unlikely or unable to adapt to environmental change (Bell, 2013; Carlson, Cunningham, & PaH, 2014; Hoffmann & Sgrò, 2011).
Both biotic (e.g., invasive species, habitat alteration) and abiotic (e.g., climate change) factors are changing increasingly rapidly as a result of human activities (Butchart et al., 2010). Whether and how species can adapt quickly enough to novel pressures imposed by anthropogenic activities are currently two of the most pressing ecological questions (Carlson et al., 2014; Huey, Buckley, & Du, 2018). Our research demonstrates that reversal of clines in the presence of novel selective pressures can occur in multiple traits over a broad portion of a species’ range within a short time frame [<80 years, ≈40 generations (Langkilde, 2009)], a period similar to those observed for clinal shifts in response to climate change or species’ introductions to novel areas (Hoffmann & Sgrò, 2011; Huey et al., 2005; Johnston & Selander, 1964). Although these clinal shifts are adaptive, they deflect traits from their original evolutionary trajectories (Figure 2) and thus may mismatch animals to the conditions under which they historically evolved (e.g., Thawley & Langkilde, 2017). As fire ants invade more northern lizard populations, we expect trait values in these populations to respond by shifting from historical norms, providing a further powerful test of the ability of invasive species to reshape clines in this system. This work shows that biotic factors, such as the presence of a novel invader, may serve as selective pressures over large geographical extents, in this case, their presence not just shifting but actually reversing clines. Given that biotic factors can quickly alter latitudinal clines, future studies should address if biotic factors may mediate or be the primary cause of changes in patterns currently attributed to abiotic drivers such as climate change. As species introductions accelerate and novel ecological communities become more common (Moyle & Light, 1996; Strauss et al., 2006), biotic interactions may have an increasingly important role to play in structuring latitudinal gradients and clines.
ACKNOWLEDGEMENTS
We thank the following people and institutions: Solon Dixon Forestry Education Center (logistical support), H. John-Alder (access to field sites), D. Cavener (access to plate reader), Langkilde Lab members and B. Chitterlings (assistance in the field, contribution of datasets, and comments on manuscript), and the American Museum of Natural History, Museum of Comparative Zoology, and the Yale Peabody Museum of Natural History and staff (access to specimens). Funding was provided by a Gaylord Donnelley Environmental Fellowship, the Eppley Foundation for Research, National Geographic, the American Museum of Natural History, and the National Science Foundation (IOS-1051367, DEB-0949483) to T.L., and a Pennsylvania State University Summer Discovery Grant to M.G-B. This research was conducted under permits from the respective states and authorities. All procedures were approved by the Institutional Animal Care and Use Committees of Yale University, the Pennsylvania State University and Auburn University.




