Delayed herbivory by migratory geese increases summer-long CO2 uptake in coastal western Alaska
Abstract
The advancement of spring and the differential ability of organisms to respond to changes in plant phenology may lead to “phenological mismatches” as a result of climate change. One potential for considerable mismatch is between migratory birds and food availability in northern breeding ranges, and these mismatches may have consequences for ecosystem function. We conducted a three-year experiment to examine the consequences for CO2 exchange of advanced spring green-up and altered timing of grazing by migratory Pacific black brant in a coastal wetland in western Alaska. Experimental treatments represent the variation in green-up and timing of peak grazing intensity that currently exists in the system. Delayed grazing resulted in greater net ecosystem exchange (NEE) and gross primary productivity (GPP), while early grazing reduced CO2 uptake with the potential of causing net ecosystem carbon (C) loss in late spring and early summer. Conversely, advancing the growing season only influenced ecosystem respiration (ER), resulting in a small increase in ER with no concomitant impact on GPP or NEE. The experimental treatment that represents the most likely future, with green-up advancing more rapidly than arrival of migratory geese, results in NEE changing by 1.2 µmol m−2 s−1 toward a greater CO2 sink in spring and summer. Increased sink strength, however, may be mitigated by early arrival of migratory geese, which would reduce CO2 uptake. Importantly, while the direct effect of climate warming on phenology of green-up has a minimal influence on NEE, the indirect effect of climate warming manifest through changes in the timing of peak grazing can have a significant impact on C balance in northern coastal wetlands. Furthermore, processes influencing the timing of goose migration in the winter range can significantly influence ecosystem function in summer habitats.
1 INTRODUCTION
Climate change is advancing spring and consequently altering the timing of species interactions (Cleland, Chuine, Menzel, Mooney, & Schwartz, 2007; Parmesan & Yohe, 2003; Post, Forchhammer, & Bret-Harte, 2009). For example, earlier plant emergence has advanced migration and breeding of some bird species during spring (Forchhammer, Post, & Stenseth, 1998; Inouye, 2000; Swanson & Palmer, 2009). However, not all biological processes are temperature-driven or respond the same way to temperature-induced changes (Clausen & Clausen, 2013; Ward et al., 2016), leading to situations where producers and consumers are no longer in sync (Inouye, 2000; Durant et al., 2005; Nakazawa & Doi, 2012). These phenological mismatches may be even greater at high latitudes where many herbivores migrate in the spring from temperate regions and do not experience local cues that may minimize the difference in timing of biological events between trophic levels. Mismatches may furthermore be greater at high latitudes because Arctic regions are warming faster than the temperate zone (Elmendorf, Henry, & Hollister, 2012). Phenological mismatches have changed many populations (Doi, Gordo, & Katano, 2008; Doiron, Gauthier, & Levesque, 2015; Post & Forchhammer, 2008; Ross, Alisauskas, Douglas, & Kellett, 2017; Visser, Noordwijk, Tinbergen, & Lessells, 1998) but could also alter ecosystem function, as the timing of interactions between primary producers and consumers could affect ecosystem processes, such as greenhouse gas fluxes (Kelsey et al., 2018).
With climate warming, we expect advanced growing seasons and changes in C sources and sinks (Forkel, Carvalhais, & Rödenbeck, 2016; Gonsamo, Chen, & Ooi, 2018). Earlier green-up in spring can influence CO2 exchange through processes that control photosynthesis (i.e., C uptake) or respiration (i.e., C loss) at the ecosystem scale (Randerson, Thompson, Conway, Fung, & Field, 1997). Advancing the growing season may increase CO2 uptake if earlier emergence results in greater vegetation biomass aboveground or a longer growing season (Cahoon, Sullivan, & Post, 2016; Gonsamo et al., 2018); but greater biomass will also enhance ecosystem respiration (Oberbauer, Tweedie, & Welker, 2007) partially offsetting any C gains at the ecosystem level. Furthermore, because early green-up is associated with warm temperatures, decomposition and microbial respiration in cold soils will respond to warming with greater CO2 release (Blok et al., 2018; Lupascu et al., 2014) although the response by photosynthesis appears greater up to this point (Forkel et al., 2016). High latitudes have been “greening” for over three decades (Bhatt et al., 2010; Ju & Masek, 2016), which suggests enhanced gross CO2 uptake by plants, but studies also demonstrate increased CO2 release by warmer soils and the importance of influences beyond direct responses to warming (Shaver et al., 2000; Lupascu et al., 2014).
Migratory herbivores also play a critical role in regulating CO2 exchange (Kelsey et al., 2016; Post & Pedersen, 2008; Sjögersten, Wal, & Woodin, 2008, 2012 ). First, herbivores remove aboveground tissue, temporarily reducing the green biomass responsible for CO2 uptake (Cahoon, Sullivan, Post, & Welker, 2012; Sjögersten et al., 2008, 2012 ). Grazed tissue is often replaced by young leaves with higher nitrogen (N) and higher rates of photosynthesis (Caldwell, Richards, Johnson, Nowak, & Dzurec, 1981; Sjögersten, Wal, Woodin, & Loonen, 2011), potentially offsetting any short-term reduction in CO2 uptake. Furthermore, grazing can alter root/shoot ratio (Ruess, Uliassi, Mulder, & Person, 1997), which may eventually affect the quantity and quality of belowground carbon inputs and alter ecosystem respiration (Woodin, Wal, Sommerkorn, & Gornall, 2009). Second, herbivores can hasten decomposition and nutrient cycling through fecal deposition and trampling potentially alleviating N limitations to microbial respiration or plant photosynthesis (Ruess, Hik, & Jefferies, 1989; Ruess et al., 1997; Zacheis et al., 2002). Finally, herbivores can affect soil respiration through changes to the physical environment by reducing shading, which warms and dries soils (Köster, Köster, Berninger, Heinonsalo, & Pumpanen, 2018; Welker, Fahnestock, Bilbrough, & Piper, 2004); warmer soils often promote greater N availability (Leffler, Klein, Oberbauer, & Welker, 2016).
How phenological mismatch influences the direction and magnitude of CO2 exchange will depend on the competing or complementary influences of season advancement and herbivory (Kelsey et al., 2018). Phenological mismatch may generate greater CO2 uptake if season advancement and delayed grazing both result in enough new photosynthetic tissue to offset any increase in autotrophic and heterotrophic respiration (Cahoon et al., 2016; Forkel et al., 2016). Conversely, any CO2 uptake advantage incurred by an early or longer growing season may be partially offset by delaying grazing if herbivore fecal deposition is critical in supplying N to green plants or delayed grazing results in considerable self-shading by taller vegetation (Ruess et al., 1997). Finally, mismatch can result in CO2 losses if grazing occurs early relative to green-up leading to reductions in leaf area before leaves can replenish stored C used to produce them in the first place (Archer & Tieszen, 1983; Beaulieu, Gauthier, & Rochefort, 1996).
The Yukon–Kuskokwim Delta (Y-K Delta) of western Alaska is experiencing rapid climate change and has great potential for phenological mismatch between green-up of coastal wetland vegetation and the arrival of migratory geese. Our study addresses the question: Will a phenological mismatch between timing of green-up and timing of goose arrival result in higher or lower rates of ecosystem respiration (ER), net ecosystem exchange (NEE), and gross primary productivity (GPP, the sum of NEE and ER) during the growing season? We anticipate that advancing the growing season will result in greater net CO2 uptake (i.e., the response of GPP will exceed that of ER) and that delaying or removing grazing from the system will have the same effect due to increased photosynthetic biomass. Consequently, if geese arrive into an ecosystem where green-up has advanced and they graze “phenologically late,” we expect greater CO2 uptake during the growing season.
2 MATERIALS AND METHODS
2.1 Study system
All research was conducted in the coastal wetlands on the Tutakoke River (61.25 N, −165.62 W) in the Yukon–Kuskokwim Delta (Y-K Delta) in western Alaska (Supporting Information Figure S1). The Y-K Delta encompasses 75,000 km2 of subarctic wetland and tundra between the Yukon and Kuskokwim Rivers, and along the coast of the Bering Sea. Temperatures vary from means of ca. –14˚C during winter to ca. 10˚C during summer (Jorgenson & Ely, 2001), and much of the area is covered in ice and snow from late autumn to mid-spring although no permafrost exists near the coast. These coastal systems are comprised of recent emergent and submerged surficial deposits creating tidal flats and deltaic plains. Our experiment was conducted in a brackish wet sedge meadow on the active floodplain. The meadow is 10–20 cm higher than adjacent tidal channels, the soil is silty loam underlain with deposits of silts and sands and has neutral soil pH (Jorgenson, 2000). Soil moisture content typically exceeds 50% during the growing season (A. J. Leffler, unpublished data), and soils are approximately 3.4% C and 0.2% N by weight between 0 and 15 cm soil depth (R. T. Choi, unpublished data).
The Y-K Delta is an important breeding area for migratory geese including Pacific black brant (Branta bernicla nigricans), emperor geese (Chen canagica), cackling geese (B. hutchinsii minima), and greater white-fronted geese (Anser albifrons). Carex ramenskii, a salt-tolerant sedge, is the dominant species within 3 km of the coast (Jorgenson, 2000; Kincheloe & Stehn, 1991). Carex ramenskii has a shorter, more nutritious growth form (often referred as C. subspathacea) that forms grazing lawns, which are more productive than taller vegetation (Ruess et al., 1997). Pacific black brant are highly selective foragers and greatly prefer grazing lawns associated with brackish ponds (Person et al., 2003; Ruess et al., 1997). Pacific black brant and the other abundant grazer in the system (cackling geese) consume aboveground vegetation in the grazing lawns and do not grub (Person et al., 2003).
The Tutakoke River is in the core nesting area for Pacific black brant with ca. 500 nests km2 (Fischer, Williams, & Stehn, 2017). Here, the timing of the most intense summer grazing is closely linked to hatch date (Supporting Information Figure S2). Adult geese use grazing lawns as soon as the lawns start growing but females do not graze substantially during the ca. 25-day incubation period (Eichholz & Sedinger, 1998). Rather, the intensity of grazing in the breeding area increases substantially following hatch as goslings begin to consume vegetation and females recover from nutrient deficits incurred during incubation of eggs (Sedinger & Raveling, 1990). Intense grazing occurs until geese move inland toward brood-rearing areas ca. 40 days later (Mickelson, 1975). Mean hatch dates (1983–2016) have varied between 11 June and 30 June (Fischer et al., 2017) with median hatch among years of 21 June; hatch has been observed as early as 3 June and as late as 9 July (Fischer, Stehn, & Walters, 2008).
Additionally, the timing of green-up varies among years by over 30 days in the Y-K Delta (Supporting Information Figure S2). Using the day of year when the 50% maximum NDVI is achieved as a vegetation phenology metric, green-up has varied between 23 May and 25 June during 1982–2016 (D. Douglas, unpublished data; NDVI methods follow Brook, Leafloor, Abraham, & Douglas, 2015). Consequently, phenological mismatch can occur in years with early green-up and late goose hatch and will become more likely in the future with earlier spring green-up.
Finally, the Tutakoke population and Pacific black brant in the Y-K Delta may be in decline leading to less grazing in the future. Brant populations peaked in the 1990s, and data suggest that recruitment was inadequate to maintain a stable or growing population (Sedinger, Nicolai, Lensink, Wentworth, & Conant, 2007), with first-year and adult survival declining between 1990 and 2015 (Leach et al., 2017). Consequently, the extent of grazing lawn may decline in the future as the sedge reverts to the taller growth form (Person et al., 2003).
2.2 Experimental design
In May 2014, we initiated a factorial experiment to examine the interaction between timing of green-up and timing of grazing on ecosystem processes in the Y-K Delta (Table 1, Supporting Information Figure S2). Our study consisted of five experimental blocks within grazing lawns associated with brackish pond margins. Each block contained eight 1.7-m × 0.85-m experimental plots with season advancement treatments (two levels: ambient and advanced) crossed with a timing-of-grazing treatment (four levels: early, typical, late, and none) yielding 40 plots total across the five blocks. These plots were fenced from early May until mid-August to prevent any grazing outside the specified times in the experiment. We included one additional unfenced “background” plot per block that experienced natural grazing throughout the experiment and no season advancement. The distance between blocks ranged from ca. 70 to ca. 700 m. The experiment continued for three years (2014–2016) using the same plots and treatment combinations. Local weather conditions were monitored during the experiment using a weather station (Campbell Scientific, Logan, UT) that recorded air temperature, humidity, incident sunlight (photosynthetically active radiation [400–700 nm], PAR), wind speed and direction, and precipitation.
| Treatment | Phenology | Mismatch | Result | ||
|---|---|---|---|---|---|
| Season | Grazing | Season | Grazing | ||
| Advanced | Early | −3 | −3 | 0 | Geese match early green-up with early grazing |
| Ambient | Early | 0 | –3 | –3 | Geese arrive early but current green-up time |
| Advanced | Typical | −3 | 0 | 3 | Early green-up with no change in grazing |
| Ambient | Typical | 0 | 0 | 0 | Typical conditions of the system |
| Advanced | Late | −3 | 3 | 6 | Early green-up with geese arriving late |
| Ambient | Late | 0 | 3 | 3 | Normal green-up with late goose grazing |
| Advanced | None | −3 | N/A | N/A | Early green-up and no grazing |
| Ambient | None | 0 | N/A | N/A | Normal green-up and no grazing |
Note
- Season and grazing indicate if they started early, at the typical time, or late with the value indicating time in weeks. Resulting mismatch is the sum of phenological change in season and grazing. Season advancement in our treatment is estimated at three weeks from shoot growth measurements. N/A indicates treatments where mismatch is not possible since grazing was removed from the system.
We advanced the growing season using two conical passive-warming open-top chambers (OTC; Marion, Henry, & Freckman, 1997) adjacent to each other to ensure adequate space for experimental measurements. Chambers were 85-cm diameter at the base tapering to 50 cm at top, and 30-cm tall. Chambers were installed on ca. 1 May each year. Previous use of similar chambers in Arctic systems warmed mean air and soil temperature by ca. 1–2˚C (Leffler et al., 2016). Open-top chamber warming effects decline as the growing season progresses and solar radiation declines; but OTCs accelerate early season plant growth (Sullivan & Welker, 2005; Post & Pedersen, 2008). We removed OTCs from all plots on 1 July (they were also removed temporarily during experimental grazing) once they had advanced the growing season (see below). We monitored air and soil temperature (10 cm height and depth) using microloggers (models DS1921G/Z; Maxim Integrated, San Jose, CA) inside and outside the OTCs. We monitored season advancement by measuring the height of new green vegetation every 2–3 weeks in 2014 and weekly in 2015 and 2016. Height was measured on ten shoots in a fixed 10 cm × 10 cm quadrat in each plot.
We manipulated timing of grazing by constructing goose exclosures (ca. 7.6 m2) around paired ambient and advanced growing season plots that had the same grazing treatment and only allowed grazing within the exclosure during experimentally dictated times between early May and mid-August; grazing in the background plots remained unconstrained. Prior to the experiment, ca. 20 female Pacific black brant were captured locally each year and held for the summer in a fenced area after clipping flight feathers; geese were provided supplemental feed ad libitum. Geese were located and captured from nests, which constrained the earliest date grazing treatments could begin. Each experimental treatment consisted of two geese inside the experimental exclosure for four 24-hr grazing bouts separated by 12 days. Prior to each grazing bout geese were held without food to allow any recently consumed material to pass the digestive system (Prop & Vulink, 1992); following each bout, geese were held for an additional two hours and the feces produced were collected and returned to the experimental exclosure. The period of the experiment was from 1 May until 15 August or 3.5 months; thus, we created the same grazing intensity in each goose grazing treatment of 7.2 goose-hours m−2 month−1. This treatment was similar to a previous controlled-grazing study in the same population of geese (Herzog & Sedinger, 2004). This grazing intensity resulted in similar biomass within the experiment as that observed in grazing lawns with unconfined grazing (Herzog & Sedinger, 2004; Person, Babcock, & Ruess, 1998). The early, typical, and late grazing treatments started 30 May, 20 June, and 9 July, respectively (Supporting Information Figure S2), and lasted 37 days. These dates were selected to vary the timing of grazing by geese and goslings to approximate a 30-day variation in the range of earliest to latest hatch dates (3 June to 9 July) of Pacific black brant observed during the past three decades in the Tutakoke River population (Fischer et al., 2008, 2017) and to account for logistical challenges of using actual goose grazing in our experiments rather than simulating grazing with clipping. Early and typical as well as typical and late overlapped in time by ca. 2 weeks (Supporting Information Figure S2); overlap of all three treatments would have required considerably more captive geese.
2.3 CO2 exchange measurements
We measured exchange of CO2 between atmosphere and biosphere using a short-term closed circulation system to quantify NEE and ER during all three years the experiment was conducted. Each plot was measured ca. twice weekly with measurements taking place ±3 hr of solar noon beginning in late May and ending in the middle of August. The system consisted of a clear acrylic chamber with a temperature and humidity sensor (model CS215; Campbell Scientific, Logan, UT) and a fan for air circulation; the chamber was connected through an air pump (ca. L min−1 flow rate) to an infrared gas analyzer (model 820; Licor Inc., Lincoln, NB) using ca. 150 cm of Bev-A-Line tubing. A datalogger (model CR800; Campbell Scientific, Logan, UT) integrated chamber sensor and CO2 measurements at 1 Hz. We installed PVC collars ca. 5 cm into soil in each plot to seal the chamber to the soil. Collars were installed annually at least 48 hr prior to any measurements and were not moved during the year; the same collar positions were used each year of the study. The vegetation in the collars was not disturbed, and we saw no evidence of drying in the collars through the experiment. Each measurement of a plot consisted of a NEE sample for two minutes, a 30- to 45-s period to allow the chamber to return to ambient conditions, followed by an ER measurement (two minutes) with the acrylic chamber covered in an opaque shroud to stop photosynthesis. Flux of CO2 for NEE and ER was determined with a nonlinear curve fit of chamber CO2 over time between 30 and 120 s following chamber closure with the slope of the curve at the time of chamber closure as the instantaneous flux; GPP was calculated from NEE and ER (Rogers et al., 2011; Leffler et al., 2016). During measurements, PAR was 1,013 ± 437 (SD) µmol m−2 s−1 and air temperature was 13.2 ± 3.9˚C. Negative and positive values indicate CO2 uptake and CO2 release, respectively, at the ecosystem scale.
2.4 Data analysis
All analysis employed a linear mixed model framework with model selection performed using AIC. Models were fit using the nlme package within the R statistical computing environment (R Development Core Team, 2014), and error is expressed as 95% confidence intervals from bootstrapped model coefficients.
First, we quantified the effect of OTCs on season advancement. The response variable was mean vegetation height and included categorical predictors of year and treatment (ambient or advanced), a continuous predictor of day of year, all interactions, and a random plot within block effect. Data included in this analysis were collected before 1 July (the date of OTC removal) and from plots that did not experience grazing before OTC removal (i.e., late and no grazing plots). The resulting model coefficients were used in a system of equations to calculate the date on which the advanced season plots achieved the same height as the ambient season plots on 1 July.
Next, we fit a basic model to predict CO2 flux in continuously grazed “background” plots that received no experimental manipulation. The model was fit to NEE, ER, or GPP as response variables with continuous covariates of quadratic day of year to account for predictable changes in CO2 exchange during the growing season (i.e., peak season maximum NEE) and continuous environmental covariates known to influence CO2 flux. For models of ER, we include air temperature as the environmental covariate because respiration typically increases as air temperature increases. Air temperature was log-transformed prior to inclusion in models because the relationship between temperature and ER is typically modeled as an exponential function (Shaver et al., 2013). For models with NEE or GPP, we include PAR as the covariate because NEE and GPP are primarily light-driven processes based on leaf-level photosynthesis. While the relationship between NEE or GPP and PAR typically is modeled with a rectangular hyperbola (Shaver et al., 2013), our measurements were made at higher light levels near midday where a simple linear fit was appropriate. All models included random effects of plot within block within year. We do not make inference on year-to-year differences in CO2 exchange (i.e., we do not include a fixed “year” effect); rather, we consider differences among years part of the random effects and draw inference among experimental treatments. For all model output, ER is reported at 15˚C and NEE and GPP are reported at PAR of 1,000 µmol m−2 s−1.
Finally, we tested for statistically significant effects of season advancement and altered timing of grazing on CO2 flux in the experimentally manipulated plots. We used the above-described model as the base model and built additional candidate models with additive and interactive fixed effects of our experimental treatments. We tested ten candidate models for ER (Supporting Information Table S1), and NEE and GPP (Supporting Information Table S2) and selected the model with the lowest AIC as the model with the most support; these models are presented graphically. Since there was considerable variation in PAR and temperature among measurements, we present all model output for ER standardized at 15˚C and all model output for NEE and GPP standardized at PAR = 1,000 µmol m−2 s−1 to allow for unbiased comparisons under conditions where neither temperature nor light were limiting CO2 exchange.
3 RESULTS
3.1 Environmental conditions and experimental treatments
Conditions at the study site during the growing season were generally cool and wet (Supporting Information Figure S3). Mean air temperature during the growing season was between 10.1 and 12.4˚C for the period 1 June to 15 August in 2014–2016. Mean daily temperatures were between 6.9 and 12.5˚C in June, between 11.1 and 13.4˚C in July, and between 12.0 and 14.4˚C in August. Total precipitation varied between 87 and 109 mm among the years of study. Soil temperatures at 10 cm depth during the growing season varied between ca. 5 and 13˚C with a mean of ca. 10˚C.
The OTCs raised temperature between 1 June and 1 July (Supporting Information Figure S4). The impact of the OTCs on mean air temperature was between 1.0 (2014) and 1.7˚C (2016), while the impact on soil temperature was smaller, between 0.6˚C (2016) and 1.0˚C (2014). Maximum differences achieved near solar noon were ca. 2.8˚C warmer in the advanced season plots; at night, OTCs were up to 0.4˚C cooler than ambient. For the remainder of the season following removal of the OTCs on 1 July, differences in temperature were <0.3˚C between season advancement and ambient treatments.
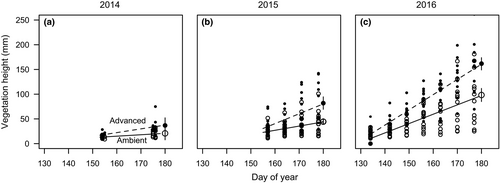
The effect of the OTCs in advancing the growing season was apparent each year of the study (Figure 1). In plots that were either not grazed or grazed only after the OTCs were removed, the modeled height of vegetation in the grazing lawns was 37, 78, and 163 mm in the season advancement treatment and 18, 42, and 99 mm in the ambient treatment in late June of 2014, 2015, and 2016, respectively. Rate of growth differed between ambient and season advancement treatments. The highest rate of growth (3.1 mm d-1) occurred in the season advancement treatment in 2016, while the ambient treatment had a rate of growth of 1.9 mm d-1 the same year. Modeled rates of growth indicate the season was advanced by 22, 18, and 21 days at the end of June in 2014, 2015, and 2016, respectively.
3.2 Background CO2 exchange within grazing lawns
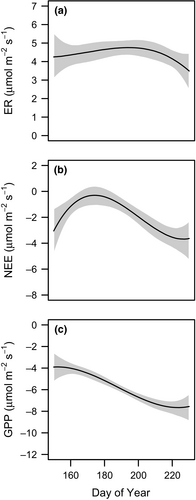
CO2 exchange within background plots largely followed predictable patterns during the growing season (Figures 2, 3, and Supporting Information Figure S5). Ecosystem respiration in grazing lawns, standardized to 15˚C, varied between 3.5 and 4.8 µmol m−2 s−1 with a seasonal average of 4.4 µmol m−2 s−1. Net ecosystem exchange in grazing lawns, standardized to 1,000 µmol m−2 s−1 PAR, varied between –0.3 and –3.7 µmol m−2 s−1 with a seasonal average of –1.8 µmol m−2 s−1. There was a distinct seasonal pattern in NEE with values close to zero in late June and early July, followed by greater CO2 uptake from mid-July to early August, and finally diminishing uptake of CO2 by mid-August. Gross primary productivity in grazing lawns, standardized to 1,000 µmol m−2 s−1 PAR, varied between –3.9 and –7.7 µmol m−2 s−1 with a seasonal average of –5.9 µmol m−2 s−1. The seasonal pattern indicated increasing CO2 uptake from early June until early August, followed by diminishing uptake by mid-August.
3.3 Differences in CO2 exchange among experimental treatments
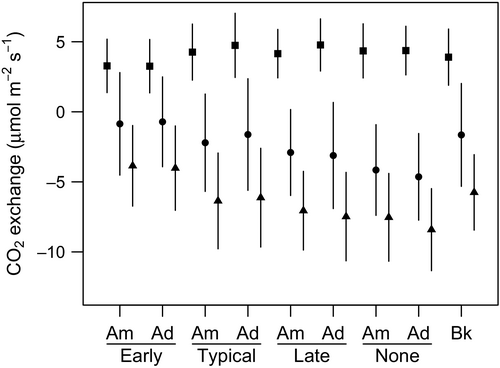
Experimental treatments had a significant influence on ER, NEE, and GPP (Figures 3 and 3). Model selection results indicate that altered timing of grazing was included in the top model for each of the components of CO2 exchange (Table 2, Supporting Information Tables S3–S5); other supported models within ∆AIC < 2 of the top model incorporated season advancement. In each case, timing of grazing interacted with other model predictors while season advancement was additive. For ER, the top model included an interactive grazing effect and an additive season advancement effect with a model weight of 77% (Table 2). For NEE and GPP, the top model contained only the interactive grazing effect weighted at 73% and 62%, respectively; but models with an additive effect of season advancement were weighted at 27% and 38%, respectively (Table 2, Figure 4).
| Model | logLik | AIC | ∆LogLik | ∆AIC | df | weight |
|---|---|---|---|---|---|---|
| ER | ||||||
| AIRT*DOY*DOY2*GRZ + SEA | −3661.4 | 7396.8 | 45.1 | 0 | 37 | 0.768 |
| AIRT*DOY*DOY2*GRZ | −3663.9 | 7399.8 | 42.6 | 3.1 | 36 | 0.165 |
| AIRT*DOY*DOY2*SEA + GRZ | −3678.2 | 7402.5 | 28.3 | 5.7 | 23 | 0.044 |
| AIRT*DOY*DOY2+GRZ + SEA | −3686.5 | 7405.0 | 20.0 | 8.2 | 16 | 0.013 |
| NEE | ||||||
| PAR*DOY*DOY2*GRZ | −5375.5 | 10823.1 | 163.6 | 0 | 36 | 0.73 |
| PAR*DOY*DOY2*GRZ + SEA | −5375.5 | 10825.1 | 163.6 | 2.0 | 37 | 0.27 |
| PAR*DOY*DOY2*SEA*GRZ | −5361.9 | 10859.8 | 177.3 | 36.8 | 68 | <0.001 |
| PAR*DOY*DOY2 + GRZ | −5507.9 | 11045.8 | 31.2 | 222.8 | 15 | <0.001 |
| GPP | ||||||
| PAR*DOY*DOY2*GRZ | −4049.3 | 8170.6 | 190.1 | 0 | 36 | 0.62 |
| PAR*DOY*DOY2*GRZ + SEA | −4048.8 | 8171.6 | 190.6 | 1.0 | 37 | 0.38 |
| PAR*DOY*DOY2*SEA*GRZ | −4035.7 | 8207.4 | 203.8 | 36.8 | 68 | <0.001 |
| PAR*DOY*DOY2 + GRZ | −4205.3 | 8440.6 | 34.1 | 270.1 | 15 | <0.001 |
Note
- ER, ecosystem respiration; NEE, net ecosystem exchange; GPP, gross primary productivity; AIRT, air temperature; PAR, photosynthetically active radiation; DOY, day of year; GRZ, grazing treatment; SEA, season advancement treatment.
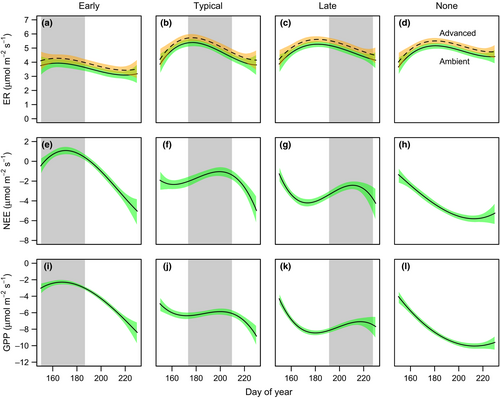
The rate of ER was affected significantly by timing of grazing and season advancement (Table 2). Season advancement increased respiration, and this effect was most evident in the typical and late grazing treatments (Figures 3 and 4). Overall, the season advancement influence on ER was 0.34 µmol m−2 s−1 when standardized to 15˚C, but was as great as 0.78 µmol m−2 s−1 in the late grazing treatment. The primary difference in ER between grazing treatments was lower ER in the early grazing treatment compared to the others. Early grazing treatments had a mean respiration rate of 3.69 µmol m−2 s−1 compared to means between 4.9 and 5.0 µmol m−2 s−1 for the other treatments. Consequently, early grazing can suppress ER by up to 26%.
Net ecosystem exchange was affected significantly by timing of grazing (Table 2, Figure 3). Altering the timing of grazing changed NEE primarily by moving the time of maximum CO2 uptake during the growing season (Figure 4). While the season advancement affect only altered NEE by <0.1 µmol m−2 s−1 at a PAR of 1,000 µmol m−2 s−1, altered timing of grazing produced a range of NEE from –0.9 µmol m−2 s−1 in the early grazing treatment to –4.5 µmol m−2 s−1 in the treatment with no grazing. Typical and late grazing treatments were intermediate at –2.0 and –3.2 µmol m−2 s−1, respectively. Consequently, among the treatments with grazing, altered timing can decrease NEE by over 50%, or increase it by over 60% compared to the treatment designed to mimic current conditions (ambient season and typical grazing treatment).
We observed similar results for GPP as for NEE with timing of grazing primarily modifying CO2 uptake (Table 2, Figure 3). Similar to NEE, altered grazing changed the time of maximum CO2 uptake during the spring and summer (Figure 4). Altered timing of grazing produced a range of GPP from –4.2 in the early grazing treatment to –8.4 when grazing was removed. Typical and late grazing treatments were intermediate at –6.3 and –7.4 µmol m−2 s−1, respectively. Among treatments with grazing, altering the timing of grazing can decrease GPP by 35% or increase GPP by over 15% compared to the typical grazing treatment.
4 DISCUSSION
We demonstrate three critical impacts of climate change on northern ecosystems. First, altering the timing of grazing by Pacific black brant geese relative to the timing of green-up in a subarctic coastal wetland significantly impacted CO2 exchange between the biosphere and the atmosphere. Second, of the two experimental factors (shifting the timing of grazing or advancing the growing season), timing of grazing had the largest effect on CO2 exchange suggesting processes that alter the timing of peak grazing in the future can have a large impact on C balance in northern coastal wetlands. Finally, because the timing of grazing is linked to timing of migration by Pacific black brant, processes that influence migration at different latitudes from the breeding range can have a substantial impact on ecosystem function in the far north. Overall, the direct effect of climate change on phenology of green-up is small compared to the indirect effect expressed through changes in the timing of peak grazing.
Altering the timing of grazing by Pacific black brant in the Y-K Delta can alter the seasonal patterns of CO2 exchange in the system and has more consequential influences on CO2 uptake rather than CO2 release. Early season grazing by Pacific black brant suppressed ER but delayed grazing did not have the opposite effect (Figure 4). Early grazing in the system resulted in geese removing the youngest aboveground tissue and likely resulted in suppression of root growth (Archer & Tieszen, 1983) and consequently respiration from belowground tissues. Grazing intensity by geese can have varying effects on ER depending on the ecosystem; in mesic tundra grazing reduced ER by ca. 50% compared to ungrazed controls, but moderate grazing nearly doubled ER in a wet tundra system (Sjögersten et al., 2012).
Timing of grazing had a larger effect on NEE and GPP than ER with the potential for early grazing to cause net C loss from the system in the late spring and early summer; delaying grazing enhanced NEE and GPP (Figure 4). Early grazing greatly suppressed GPP, causing NEE to exceed 0 for much of the late spring and early summer. In fact, early grazing eliminated net CO2 uptake between the onset of grazing and early July, a considerable length of time given the already short growing season in high latitude ecosystems. Conversely, delayed grazing allowed rapid increases in GPP in the spring, resulting in high rates of CO2 uptake in the early to mid-summer. Subsequent declines in GPP due to late grazing were smaller and corresponded with post-peak CO2 uptake such that declines in GPP associated with grazing were concurrent with the onset of senescence. We are unaware of other studies that altered the timing of grazing in Arctic or subarctic coastal systems and examined CO2 exchange as a response variable. However, previous studies report decreased cumulative height and biomass with early compared to late season grazing or no grazing (Archer & Tieszen, 1983) and no influence of timing of grazing between early July and mid-August on plant growth (Beaulieu et al., 1996). Because NEE and GPP are closely tied to leaf area (Shaver et al., 2013), our results are broadly consistent with these studies suggesting early grazing diminishes overall production in the system. One possible mechanism may be that belowground carbohydrate stores are minimal in grazing lawns in late spring (Archer & Tieszen, 1983; Beaulieu et al., 1996) and removal of these reserves before they can contribute to substantial new leaf production is detrimental to future C uptake. High-intensity grazing by geese also suppressed NEE and GPP compared to lower intensity or grazing removal from a wet tundra system (Sjögersten et al., 2008).
Carbon uptake substantially increased in response to removal of grazing from this coastal wetland. The largest effect of grazers on C uptake is removal of photosynthetic tissue although the impact of grazing on plant functional group composition can also be substantial (Cahoon et al., 2012; Metcalfe & Olofsson, 2015; Post & Pedersen, 2008; Ylänne, Stark, & Tolvanen, 2015). We did not quantify changes in species composition, but we expect them to be minimal because we were largely working in a monoculture grazing lawn. We did observe that removal of grazing from the system resulted in taller vegetation during the growing season. The extent of grazing lawns in the coastal wetlands of western Alaska is linked to goose abundance and foraging intensity (Person et al., 2003), and long-term data suggest possible declines in goose numbers in the Y-K Delta (Leach et al., 2017; Sedinger et al., 2007). Such declines would likely result in greater C uptake in the system as grazing lawn reverts to the taller growth form (Person et al., 2003; Ruess et al., 1997).
Studies excluding mammalian and avian herbivores from Arctic systems suggest varying effects on C uptake depending on the ecosystem and species involved. NEE remained at ca. +1 and –1 µmol m−2 s−1 in wet and mesic tundra systems, respectively, when grazing by geese was removed compared to treatments receiving light grazing, but virtually all net C uptake ceased in high-intensity grazing plots in wet tundra (Sjögersten et al., 2008). Conversely, removal of grazing or grubbing by geese nearly doubled GPP to over 3.5 µmol m−2 s−1 (Sjögersten et al., 2011; Van der Wal, Sjögersten, & Woodin, 2007). Exclusion of caribou and muskoxen from shrub and graminoid tundra, however, only altered GPP by ca. 15% (Cahoon et al., 2012). Our results are consistent in magnitude to these other Arctic grazed systems.
The most consequential influence of season advancement was through enhanced early season growth and increased ER. Vegetation in the advanced season plots in late June was over 50% taller than in the ambient season plots, and rate of growth was 60% greater in advanced compared to ambient season treatments during spring 2016. Despite clear enhancement of growth, the change in ER was <10% of the season-long mean and only exceeded 10% in the treatments where grazing was also delayed. Furthermore, the growth enhancement did not result in greater CO2 uptake. Most studies involving passive OTC simulate season-long warming rather than advanced growing season and demonstrate small changes in ER, GPP, and NEE (Boelman et al., 2003; Cahoon et al., 2012; La Puma, Philippi, & Oberbauer, 2007; Leffler et al., 2016; Sharp, Sullivan, Steltzer, Csank, & Welker, 2013; Welker, Brown, & Fahnestock, 1999; Welker, Fahnestock, Henry, & O'dea, K. W., & Chimner, R. A., 2004) and increased plant biomass in Arctic systems (Doiron, Gauthier, & Lévesque, 2014; Hollister, Webber, & Bay, 2005; Wookey et al., 1995). At the same site, a concurrent study suggested season-long warming can increase abundance of Salix ovalifolia in the system (Carlson, Beard, & Adler, 2018). The nonsignificant season advancement effect on GPP/NEE (Figure 4) suggests that any increase in stem elongation in the season advancement plots (Figure 1) was offset by a decline in photosynthetic rate per unit area or a reduction in leaf area, a result possibly suggesting N limitation in the system which is common at high latitudes (Mack, Schuur, & a. G., Bret-Harte, M. S., Shaver, G. R., & Chapin III, F. S., 2004; Sistla et al., 2012; Leffler & Welker, 2013) and in northern coastal wet meadows (Ruess et al., 1997). Alternatively, our measurements were instantaneous plot-level CO2 exchange values and cannot account for possible greater season-long NEE associated with earlier leaf expansion although length of growing season is not necessarily correlated with cumulative C uptake (Starr, Oberbauer, & Pop, 2000; White & Nemani, 2003).
Our experimental manipulation produced CO2 exchange values similar to those observed in unmanipulated grazing lawns. We consider the ambient season and typical grazing treatment to most closely represent the current condition, consequently the difference between current condition and unmanipulated grazing lawn was 0.13 µmol m−2 s−1 for ER, 0.39 µmol m−2 s−1 for NEE and 0.45 µmol m−2 s−1 for GPP (Figures 4 and 5). These differences are small compared to the range of values observed following altered timing of grazing. Moreover, the ambient season and typical grazing treatment resulted in similar aboveground biomass as in the background plots (R. T. Choi, unpublished data).
The most probable future phenological mismatch is where geese arrive late in the Y-K Delta system relative to timing of green-up, resulting in enhanced C uptake. Future CO2 exchange in the Y-K Delta will be influenced by timing of goose arrival since season advancement had little influence on NEE (Figure 4). Compared to the current condition (typical grazing), early arrival suppresses mean NEE by 1.1 µmol m−2 s−1 while delayed arrival enhances NEE by 1.2 µmol m−2 s−1. Consequently, delayed arrival relative to green-up, the most likely future state, will increase CO2 uptake. However, data from 2014–2016 at our study site, and recent findings from elsewhere in the Arctic, suggest that geese are already arriving and initiating nests earlier (Fischer et al., 2017; Lameris, Jeugd, & Eichhorn, 2018), potentially mitigating any enhancement of CO2 uptake resulting from the phenological mismatch in coastal wetlands. In fact, our results suggest that stronger goose responses to climate change (i.e., reduced phenological mismatch) reduces CO2 uptake relative to the typical grazing/ambient season treatment by forcing grazing earlier into the growing season. The most dramatic change in the system, however, would follow population declines or relocation during the growing season that eliminates grazing resulting in ∆NEE = –2.5 µmol m−2 s−1. We cannot ignore this possibility given recent reports of declining Pacific black brant survival in the Y-K Delta (Leach et al., 2017).
Our study was designed to examine the interacting influences of season advancement and timing of grazing by migratory geese on processes related to CO2 exchange. Our grazing treatment timing was based on the earliest and latest observed hatch dates over the past four decades and are consequently biologically representative of this system currently while also suggestive of future grazing when extreme events are likely to be more common in response to a warming climate. One aspect of mismatch we cannot address here is the natural covariance of event timing – early green-up years are associated with early hatch (Supporting Information Figure S9). We conducted our experiment with fixed dates rather than relative dates based on phenology of green-up to ensure repeatability among years.
Our experimental manipulation suggests that the influence of climate change on the timing of migratory goose grazing in the Y-K Delta of western Alaska may have a larger impact on ecosystem CO2 exchange than direct changes in response to a locally warming climate. Worldwide, grazers have a substantial influence on NPP and ecosystem-level CO2 exchange (Augustine & McNaughton, 2006; Knapp et al., 1999; Lara, Johnson, Andresen, Hollister, & Tweedie, 2017; Welker, Fahnestock, Bilbrough, et al., 2004), but here we suggest that influence is modified by timing of grazing. Moreover, the timing of peak grazing is linked to timing of arrival to the breeding range (Eichholz & Sedinger, 1998; Lindberg, Sedinger, & Flint, 1997), which is determined by climate and weather conditions in their southern winter range and in staging sites along the migration route (Bauer, Gienapp, & Madsen, 2008; Clausen & Clausen, 2013). Our results illustrate how response of climate-induced changes in ecosystem function in northern latitude coastal wetlands could be driven by processes hundreds to thousands of kilometers further south. These biological teleconnections represent some of the greatest unknowns in migration ecology and climate change impacts on high latitude ecosystems in the future.
ACKNOWLEDGEMENTS
We thank R. Hicks, M. Holdredge, H. Braithwaite, J. Ferguson, L. Carlson, K. Lynöe, and T. De Masters for assistance in the field. Logistic support was provided by CH2MHill PolarField Services. We also thank J. Sedinger and “Sedinger Camp” for sharing their expertise in conducting research in the Y-K Delta. D. Douglas generously provided NDVI data and modeled green-up dates for our study site. Two anonymous reviewers shared comments that greatly improved the manuscript. All applicable institutional and/or national guidelines for the care and use of animals were followed (IACUC permit USU2004). Collections were permitted by the Alaska Department of Fish & Game (16-023) and US Fish & Wildlife Service (Migratory Bird Scientific Collection permit: MB28352B-0). US Fish & Wildlife also permitted research in the Yukon Delta National Wildlife Refuge (Special Use Permit FF07RYKD00-14-06). This work was supported by the National Science Foundation (ARC1304523, ARC1304879, & DGE1633756). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.




