Restricted gene flow and local adaptation highlight the vulnerability of high-latitude reefs to rapid environmental change
Abstract
Global climate change poses a serious threat to the future health of coral reef ecosystems. This calls for management strategies that are focused on maximizing the evolutionary potential of coral reefs. Fundamental to this is an accurate understanding of the spatial genetic structure in dominant reef-building coral species. In this study, we apply a genotyping-by-sequencing approach to investigate genome-wide patterns of genetic diversity, gene flow, and local adaptation in a reef-building coral, Pocillopora damicornis, across 10 degrees of latitude and a transition from temperate to tropical waters. We identified strong patterns of differentiation and reduced genetic diversity in high-latitude populations. In addition, genome-wide scans for selection identified a number of outlier loci putatively under directional selection with homology to proteins previously known to be involved in heat tolerance in corals and associated with processes such as photoprotection, protein degradation, and immunity. This study provides genomic evidence for both restricted gene flow and local adaptation in a widely distributed coral species, and highlights the potential vulnerability of leading-edge populations to rapid environmental change as they are locally adapted, reproductively isolated, and have reduced levels of genetic diversity.
Introduction
Today, it is widely accepted that managing coral reefs for resilience needs to be performed at the ecosystem level in order to mitigate the effects of stressors associated with climate change (Nyström et al., 2000; Hughes et al., 2005, 2010). While this high-level approach is important for management purposes, the future of coral reef ecosystems is ultimately hinged upon population-level responses of scleractinian corals, which are the foundation species of coral reefs. As a result, an understanding of resilience in reef-building coral populations is central to understanding the response of coral reef ecosystems to rising green house gases.
One fundamental component of resilience to climate change is the capacity of corals to recover from disturbance events through the dispersal of larvae and the maintenance of connectivity (Bernhardt & Leslie, 2013). In general, marine populations occur along a spectrum of connectivity, from demographically open populations that regularly exchange larvae and rely on recruitment from outside sources to demographically closed populations that are isolated from one another and are more or less self-sustained by locally sourced recruits (Waples & Gaggiotti, 2006). When connectivity among reef systems is high, areas that elude damage can act as a lifeline to impacted areas by providing larval recruits to facilitate a recovery. Isolated reefs, however, are entirely reliant on local sources of larvae to maintain healthy populations, and if these sources are compromised, recruitment levels may drop below and ecological threshold of recovery and populations will fail to be maintained (Hoegh-guldberg et al., 2007; Smith et al., 2007; Gilmour et al., 2013).
Another fundamental component of resilience is the capacity to adapt to rising temperatures and increasing disturbance events (Bernhardt & Leslie, 2013). This hangs on the ability to form novel phenotypes that match the environment, which is largely dependent on standing genetic variation (Barrett & Schluter, 2008; Tigano & Friesen, 2016). Higher levels of genetic diversity provide a greater probability of achieving allelic combinations that confer beneficial phenotypes. Mutations offer an additional pathway to achieving beneficial phenotypes, but this process is much slower than from standing genetic variation, where the alleles are already present in the population and at higher frequencies. In addition, many corals are locally adapted to different thermal environments and exhibit various levels of bleaching resistance (Barshis et al., 2013; Bay & Palumbi, 2014; Palumbi et al., 2014; Schoepf et al., 2015; Howells et al., 2016), so the exchange of beneficial genetic variants among populations via larval dispersal will also play a key role in the adaptive capacity of populations in the future (Dixon et al., 2015; Kleypas et al., 2016).
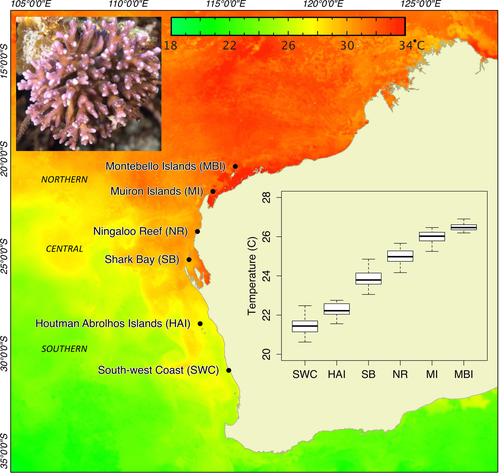
Studies that characterize spatial patterns of gene flow, genetic diversity, and local adaptation can be valuable tools for management aiming to delineate evolutionarily significant units, prioritize areas for spatial conservation, and predict population-level responses to a changing climate (Palsbøll et al., 2007; Funk et al., 2012; Tulloch et al., 2015). Here, we apply a genome-wide analysis of genetic variation in a reef-building coral species across a temperate–tropical transition zone in Western Australia, spanning 10 degrees of latitude and a mean temperature range of five degrees Celsius. We utilize a genotyping-by-sequencing (GBS) approach to explore both neutral population structure and patterns of local adaptation in the scleractinian coral Pocillopora damicornis, one of the few reef-building species that thrives in both the extreme environments of the northwest (20°S) and the temperate waters of the southwest (32°S). The outcomes of this research provide important information for understanding the vulnerability of coral populations to a changing climate and for developing species threat maps to prioritize areas for spatial conservation management.
Materials and methods
Sample collection and screening
Adult coral colonies of P. damicornis were collected from six reef systems along the coastline of Western Australia: Montebello Islands (MBI), Muiron Islands (MI), southern Ningaloo Reef (NR), Shark Bay (SB), the Houtman Abrolhos Islands (HAI), and the South-west Coast (SWC) (Fig. 1). Samples were collected within a two-kilometer area at depths between two and 10 meters, and tissue was preserved in 100% Analytical Reagent grade ethanol. High levels of morphological overlap within the genus Pocillopora can confound accurate species identification (Schmidt-Roach et al., 2013; Marti-Puig et al., 2014; Thomas et al., 2014), so all samples were screened using a fluorescence-based real-time quantitative PCR (qPCR) approach. This approach targets a single nucleotide polymorphism in a hypervariable mitochondrial open reading frame (Flot and Tillier 2007) that can accurately distinguish P. damicornis (haplotype α; Schmidt-Roach et al., 2014) from other morphologically similar species within the genus Pocillopora in Western Australia (Thomas et al., 2016). Genomic DNA was extracted using the DNeasy Tissue Kit (Qiagen) and samples confirmed as P. damicornis using the qPCR approach were subsequently screened across a panel of microsatellite markers (Starger et al., 2008) to rid the sample set of any replicate multilocus genotypes prior to high-throughput sequencing. Microsatellite markers (Pd3-004, Pd2-006, Pd3-008, Pd3-009) were multiplexed and amplified in 10 μL reactions using the Qiagen Multiplex PCR Kit and following thermal cycling conditions in Starger et al. (2008). Size fragment analysis was performed with fluorescent-labeled primers in gene-marker v.1.90 (Softgenetics, State College, PA, USA) using automated scoring of alleles with manually prepared bins. All scores were checked manually to minimize genotyping errors.
SNP genotyping
Genome-wide single nucleotide polymorphism (SNP) data were generated at Diversity Arrays Technology (DArT; http://www.diversityarrays.com/) using a GBS approach (DArTseq). DArTseq represents a combination of DArT complexity reduction methods and Illumina technologies and is similar to restriction site-associated DNA sequencing (RADseq; Davey et al., 2010). DArTseq-reduced representation library development protocol is optimized for different organisms and applications by selecting the most appropriate complexity reduction method (size of the representation and the fraction of a genome selected for the assays). Four methods of complexity reduction were tested, and the PstI-HpaII method was selected. Genomic DNA was processed in digestion/ligation reactions principally as per Kilian et al. (2012), but replacing a single PstI-compatible adaptor with two different adaptors corresponding to two different restriction enzyme overhangs. The PstI-compatible adapter includes the Illumina flow cell attachment sequence, sequencing primer sequence, and barcode region. The reverse adapter contains the flow cell attachment region and HpaII-compatible overhang sequence. Sequencing was carried out on a single lane of an Illumina Hiseq2500 and processed using proprietary DArT analytical pipelines (DArTsoft14). We retained DArT loci that were genotyped in more than 75% of samples, with a minor allele frequency (MAF) >0.05 and mean sequence coverage >5×. The filtered dataset was blasted against available Symbiodinium clade B (Shoguchi et al., 2013) and clade C (C.X. Chan, http://refuge2020.com/) genomes, as well as four Symbiodinium transcriptomes (199 999 sequences; clades A, B, C3, and D) online at http://www.bio.utexas.edu/research/matz_lab/matzlab/Data.html to ensure that all loci belonged to the coral host and not their endosymbiotic algae.
Genetic diversity and gene flow
To remove loci potentially under directional or balancing selection, we used the fdist2 method in lositan (Antao et al., 2008), which estimates expected heterozygosity and unbiased FST values for each locus to generate a global neutral distribution for FST under Wright's Island model (Wright, 1931). This approach represents a conservative method for identifying neutral loci, and minimizes the retention of loci under weak selection that could bias downstream analyses. Analyses were based on 500 000 simulations assuming an infinite alleles mutation model and using neutral mean FST, 0.95 confidence intervals, and a false discovery rate of 0.1. Loci with probabilities between 0.05 and 0.95 were retained as putatively neutral loci and screened for conformity to Hardy–Weinberg equilibrium (HWE) using genodive 2.0b.27. We corrected for multiple comparisons using the Benjamini–Hochberg method (Benjamini & Hochberg, 1995).
The following analyses were calculated using the neutral dataset. Genetic diversity (expected heterozygosity) was calculated using the r package ‘poppr’ (Kamvar et al., 2014). Genetic distance was calculated using Nei's distance (Nei, 1972), and a distance-based phylogenetic reconstruction (UPGMA) was also performed using ‘poppr’. Bootstrap support for branch nodes was based on 1000 replicates. Discriminant analysis of principle components (DAPC) was performed using ‘adegenet’. No prior information about sampling location was used, and the optimal number of principle components and discriminant functions to retain was determined by maximizing the alpha score, which represents the difference between the observed and random discrimination (Jombart et al., 2010). Finally, admixture analyses were performed using the Bayesian Markov chain Monte Carlo clustering approach in structure 2.3.4 (Pritchard et al., 2000). We set K to range from 1 to 10 (10 replicates per K) with no prior location information, correlated allele frequencies, a burn-in of 10 000 followed by 100 000 MCMC replicates. The optimal number of populations (K) in the dataset was determined following the ΔK method (Evanno et al., 2005) as implemented in structure harvester (Earl & vonHoldt, 2012).
Local adaptation
To identify outlier loci putatively under selection, we used two different population differentiation approaches. Firstly, we screened all loci using the fdist2 method under a hierarchal island model in arlequin (Excoffier & Lischer, 2010). Hierarchical genetic structure across samples can result in numerous false positives in outlier genome scans, and arlequin addresses this issue by accounting for population structure and allowing for lower migration rates among groups than among populations within groups (Excoffier & Lischer, 2010). The hierarchical model was implemented using groupings based on the neutral distance-based phylogenetic reconstruction, and analyses were based on 100 000 simulations with 1000 demes and 100 groups to simulate. Loci that fell outside the 0.99% quantile were considered candidates for directional selection. Secondly, we utilized a Bayesian model-based approach in bayescan 2.0 (Foll & Gaggiotti, 2008), which has been shown to have lower type 1 error rates than fdist2 approaches (Narum and Hess 2011). Analyses were conducted using default settings. Loci under selection were defined as those with q-values <0.05. One limitation of these genome-wide scans for selection is that they are not designed to test hypotheses about the environmental factors driving selective pressures but rather represent a ‘blind’ scan for loci under selection (Villemereuil and Gaggiotti 2015). So, to increase support for local adaptation to environmental variation along our latitudinal cline, we utilized an ecological association (EA) approach in bayescenv, which allows inferences about the specific environmental variable driving selection at particular loci (Villemereuil and Gaggiotti 2015). We tested for genotype by environment correlations with allele frequency and four temperature variables: mean (Tmean), maximum (Tmax), minimum (Tmin), and variance (Tvar). Temperature data were acquired from NOAA Coral Reef Watch 5-km Regional Virtual Stations (http://coralreefwatch.noaa.gov), and values were averaged across 12 years (2004–2014) and standardized to represent distances as per developers' instructions (Villemereuil and Gaggiotti 2015). To determine whether any of the outlier loci identified by the three different approaches were in genic regions that coded for proteins, we blasted reference nucleotide sequences (69 bp) to a publically available P. damicornis transcriptome (Mayfield et al., 2014).
Results
Sample collection and screening
Our initial sample set of 397 colonies was reduced to 294 (74%) that could be reliably amplified and confidently called as P. damicornis using the fluorescence-based qPCR assay (Table S1). Screening across a panel of five microsatellites revealed that clonality among these samples was high and further reduced our sample set to 124 unique multilocus genotypes for which we could reliably amplify and confidently score, of which 70 produced sufficient quality libraries for high-throughput sequencing. Due to the remote locations of sample collection, there were some instances where preserved samples were stored at ambient temperatures (often exceeding 30°C) for several weeks. As a result, this likely resulted in DNA degradation and could explain the high failure rate for reduced representation library development.
SNP genotyping
The DArT proprietary pipeline produced 26 271 high-quality SNPs, of which 5484 (21%) met our more stringent selection criteria. All datasets used in subsequent analyses can be found on the Open Science Network (doi: 10.17605/OSF.IO/YNKH8). A Blastn analysis against reference Symbiodinium genomes and transcriptomes indicated that loci that passed through the DArT pipeline did not belong to the symbiont. This result can be attributed to dartsoft14, DArT PL's proprietary software's capacity to filter symbiont and bacterial sequences in SNP marker selection. This is achieved through training the program to distinguish allelic sequence variants from orthologous sequences based on the Mendelian behavior of loci in thousands of control crosses across a large diversity of organisms (A. Killian, personal communication). Approximately 83% of filtered loci (n = 4562 loci) passed our filtering steps for neutrality. Among the 4562 neutral loci, <1.0% was out of HWE in more than half of the populations. As a result, the 4562 neutral loci comprising 4056 contigs, with a mean FST of 0.115, were retained as the neutral dataset (doi: 10.17605/OSF.IO/YNKH8).
Genetic diversity and gene flow
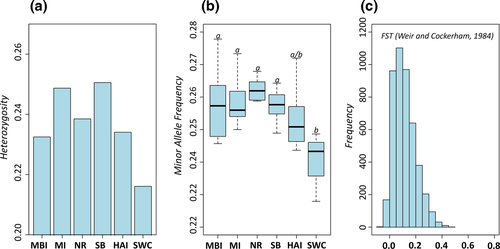
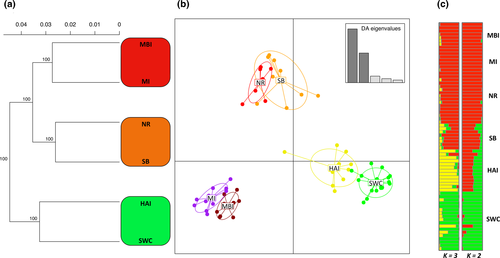
Plots of minor allele frequency and heterozygosity using the neutral dataset followed a curvilinear relationship with latitude and were highest toward the center of the species distribution and lowest in the most southern region sampled (Fig. 2a, b). There were no statistically significant differences in minor allele frequency among northern and central range populations. In contrast, SWC was significantly different from northern and central populations, but not HAI (Table S2). The distance-based phylogenetic reconstruction revealed a clustering pattern consistent with geographic region of sample collection and showed the northern, central, and southern range populations to cluster together with strong bootstrap support (Fig. 3a). DAPC was consistent with the phylogenetic reconstruction and showed a tight clustering pattern of populations within regions (Fig. 3b). Admixture analysis in structure revealed K = 2 as the most likely number of populations (Fig. S1) and showed HAI to be admixed between SWC and reefs to the north (Fig. 3c). At K = 3, HAI formed its own distinct cluster.
Local adaptation
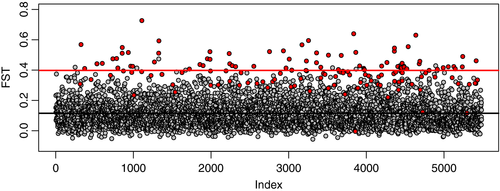
Genome-wide scans for selection using the entire filtered dataset (n = 5484 loci) under an fdist2 hierarchal island model in arlequin returned 153 loci. A Bayesian approach in bayescan returned 48 loci, 44 of which were also identified by arlequin (Fig. S2). An ecological association approach implemented in bayscenv identified a total of four loci to be under selection: one locus that showed strong correlations with Tvar, Tmean, and Tmax, and an additional three that showed strong correlations with Tmax. No loci showed strong correlations with Tmin. Two of the four loci identified by bayscenv were also identified by both arlequin and bayscan (Fig. S2). Together, these three outlier approaches identified 159 loci belonging to 139 unique sequences with a mean FST of 0.398 (Fig. 4; doi: 10.17605/OSF.IO/YNKH8). See Table S3 for a complete list of all loci identified by the three different approaches. We were able to recover protein annotations for 26 (19%) of these sequences using a publically available P. damicornis transcriptome (Mayfield et al., 2014). These loci had homology to green fluorescent proteins, as well as proteins of the ubiquitin, NACHT, and immunoglobulin families, among others (Table S4).
Discussion
Our results indicate that high-latitude reefs in Western Australia are characterized by restricted gene flow and reduced genetic diversity. In addition, genome-wide scans for selection identified a number of outlier loci previously known to be associated with thermal tolerance in corals. These results provide genomic evidence for both neutral and adaptive differentiation in high-latitude coral populations, indicating that these high-latitude populations are reproductively isolated, locally adapted, and characterized by reduced genetic diversity. As a result, they may be more vulnerable than tropical populations to rapid environmental change due to a limited capacity to adapt.
Populations of P. damicornis in Western Australia display high levels of genetic differentiation, which suggests restricted gene flow and a limited exchange of larvae among geographically distant regions. Within regions, however, populations appear to maintain moderate levels of gene flow and connectivity. This was particularly the case in the northern region, where we detected low levels of genetic differentiation between coral populations at the Montebello and Muiron Islands, which are separated by approximately 180 km. Central range populations also showed a similar pattern, and there was little difference between southern Ningaloo Reef and Shark Bay samples. In contrast, we detected strong signs of divergence within the southern range populations. Interestingly, the Houtman Abrolhos Islands appear to be a genetic hybrid zone that connects central and southern range populations. Genetic tension zones often occur in areas of transition between two biomes (Adrion et al., 2015), and the Houtman Abrolhos Islands sit in a region of convergence of tropical and temperate waters, located on the edge of the continental shelf in the path of the warm poleward-flowing Leeuwin Current. The islands are the southernmost coral reef in the Indian Ocean and harbor a diverse assemblage of both tropical and temperate species (Veron & Marsh, 1988; Hutchins & Pearce, 1994; Watson & Harvey, 2009). Little is known about coral larval recruitment in the islands, but coral communities have been previously thought to be reliant on the Leeuwin Current and larvae from the north to maintain healthy populations (Simpson, 1985; Babcock et al., 1994). Similarity in community composition between the Houtman Abrolhos Islands and central range populations indicates an evolutionary connection, but recent analyses using Lagrangian particle tracking models suggest that the islands are largely self-seeding and are not connected on ecological time scales (Markey et al., 2016). Furthermore, high levels of genetic differentiation have been identified in populations of P. damicornis between Ningaloo Reef and the Houtman Abrolhos Islands based on allozyme analyses (Whitaker, 2006). P. damicornis is a brooding coral species, with the majority of larvae recruiting locally (Stoddart, 1984); however, the species is capable of rare long-distance dispersal (Ayre & Hughes, 2000; Thomas et al., 2014), which could counteract the effects of genetic drift but is unlikely to provide enough recruits to aid in the recovery of coral communities following a disturbance.
In addition to restricted gene flow, high-latitude coral populations in Western Australia are characterized by reduced levels of genetic diversity. These results are consistent with past studies that identified reduced levels of genetic diversity in high-latitude populations in Australia (Ayre & Hughes, 2004; Miller & Ayre, 2008; Noreen et al., 2009). Patterns of genetic diversity are often linked to environmental gradients, with the highest levels of genetic diversity occurring where conditions are optimal and declining in either direction, as conditions become less favorable (central-marginal model; Brussard, 1984). Standing stock genetic variation is a key driver of the adaptive capacity of populations, and the low levels of genetic diversity in high-latitude populations identified here suggests that these populations have a reduced capacity to adapt. Corals of the South-west Coast occur in a temperate environment where they spend approximately 20% of their time below 20°C. An alternative scenario might be that only a subset of alleles is needed to survive temperate climates and that there is the potential for these populations to expand southward. The 2010/2011 marine heatwave resulted in the tropicalisation of temperate marine ecosystems along the central coast of Western Australia (Wernberg et al., 2012; Bennett et al., 2015). A number of sites experienced severe declines in canopy-forming kelp species and an increase in both tropical fish biomass and coral recruits (Wernberg et al., 2016). This climate-driven ecosystem reconfiguration altered key ecosystem processes, providing resilience to the phase shift and prevented kelp species from recovering (Bennett et al., 2015). Although this event did not extend the ranges of tropical species (Veron & Marsh, 1988), it represents a glimpse into the future tropicalization of temperate reefs farther to the south. As the climate continues to warm and isotherms shift poleward, the question that remains for leading-edge coral populations is whether the southward expansion of temperate alleles can outrun the rate at which temperatures increase.
Genome-wide scans for local adaptation identified a number of outlier loci under directional selection. Due to the limited genomic resources available for scleractinian corals, we were only able to annotate a subset of these outlier loci. Several of these loci had best blast hits to proteins previously identified to play a strong role in thermal tolerance and stress response in corals. For example, two outlier loci had best blast hits to proteins in the ubiquitin family. Ubiquitin is a cellular protein tag involved in protein turnover and the elimination of damaged cells. Ubiquitin is upregulated in corals under temperature stress (Desalvo et al., 2008; Voolstra et al., 2009; Granados-Cifuentes et al., 2013) and exhibits strong clinal patterns in corals along the Great Barrier Reef (Lundgren et al., 2013). In addition to ubiquitin, one sequence had a best blast hit to green fluorescent proteins, which play a photoprotective role in corals (Salih et al., 2000). Fluorescent proteins absorb higher energy light and are important in mitigating thermal stress to Symbiodinium by dissipating excess light energy and preventing chlorophyll excitation that leads to photoinhibition. They also have antioxidant properties that contribute to reducing the photoinhibitory effects of high levels of solar radiation (Palmer et al., 2009). Fluorescent proteins also rapidly decline in corals under environmental stress, making them a promising biomarker for coral thermal stress (Roth & Deheyn, 2013). Finally, a number of outlier loci blasted to genes involved in immunity and apoptosis such as proteins of the NACHT and immunoglobulin domains, which are key pattern receptors of the immune system and play an important role in the maintenance of symbiosis with Symbiodinium (Schwarz et al., 2008; Davy et al., 2012). Taken together, these data provide evidence that coral populations along the coastline of Western Australia are characterized by strong patterns of local adaptation in genic regions that are involved in thermal tolerance. It is likely that these genomic regions will face intensified selection as temperatures warm; however, how these patterns of local adaptation will influence the capacity of populations to adapt remains unknown. Furthermore, while this study has focused on coral as a solitary organism, thermal tolerance in corals is also dependent on their symbiotic relationship with co-exiting algae and microbial communities (Berkelmans & van Oppen, 2006; Howells et al., 2011; Kvitt et al., 2011; Tchernov et al., 2011). Therefore, when considering the potential adaptation of corals to thermal stress, future studies should explore patterns of adaptation within the entire coral biome, which will provide a more comprehensive understanding of thermal tolerance in coral populations across environmental gradients.
High-latitude reef environments are often thought of as a likely refuge for coral populations in the face of a changing climate (Glynn, 1996; Riegl, 2003), but the 2010/2011 marine heatwave showed that these reefs are not removed from the stressors associated with climate change. Furthermore, our results point to a reduced capacity of these systems to cope with warming conditions. As the local environment shifts and becomes more favorable for tropical populations, rare events of genetic rescue from the tropical north may represent a critical lifeline for these genetically depauperate populations. Unfortunately, a forecasted weakening of the Leeuwin Current in coming decades suggests that this mechanism of genetic rescue may be depressed if the poleward transport of larvae along the coastline is reduced (Feng et al., 2012). The current study sites exist within a network of marine reserves, but future scale-appropriate assessments of existing frameworks should monitor coral populations regularly for demographic and genetic changes.
Acknowledgements
This work was undertaken for the Marine Biodiversity Hub, a collaborative partnership supported through funding from the Australian Government's National Environmental SCIENCE Program (NESP). Montebello Island sample collections were funded by the Chevron-operated Wheatstone Project's State Environmental Offsets Program and administered by the Department of Parks and Wildlife. The Wheatstone Project is a joint venture between Australian subsidiaries of Chevron, Kuwait Foreign Petroleum Exploration Company (KUFPEC), Woodside Petroleum Limited, and Kyushu Electric Power Company, together with PE Wheatstone Pty Ltd (part owned by TEPCO). The manuscript was greatly improved by comments from M. vanOppen, M. Byrne, M. Bunce, and four anonymous reviewers. The authors would also like to extend thanks to J. Underwood for insightful conversation about the data.
Author contribution
LT, MS, and WJK conceived the study. LT, MS, WJK, and RE collected the samples. LT conducted laboratory work. LT, WJK, and MS analyzed data. LT, MS, WJK, and RE drafted the manuscript. All authors wrote the manuscript.




