Additive impacts of experimental climate change increase risk to an ectotherm at the Arctic's edge
Abstract
Globally, Arctic and Subarctic regions have experienced the greatest temperature increases during the last 30 years. These extreme changes have amplified threats to the freshwater ecosystems that dominate the landscape in many areas by altering water budgets. Several studies in temperate environments have examined the adaptive capacity of organisms to enhance our understanding of the potential repercussions of warming and associated accelerated drying for freshwater ecosystems. However, few experiments have examined these impacts in Arctic or Subarctic freshwater ecosystems, where the climate is changing most rapidly. To evaluate the capacity of a widespread ectotherm to anticipated environmental changes, we conducted a mesocosm experiment with wood frogs (Rana sylvatica) in the Canadian Subarctic. Three warming treatments were fully crossed with three drying treatments to simulate a range of predicted changes in wetland environments. We predicted wetland warming and drying would act synergistically, with water temperature partially compensating for some of the negative effects of accelerated drying. Across all drying regimes, a 1 °C increase in water temperature increased the odds of survival by 1.79, and tadpoles in 52-day and 64-day hydroperiod mesocosms were 4.1–4.3 times more likely to survive to metamorphosis than tadpoles in 45-day mesocosms. For individuals who survived to metamorphosis, there was only a weak negative effect of temperature on size. As expected, increased temperatures accelerated tadpole growth through day 30 of the experiment. Our results reveal that one of the dominant herbivores in Subarctic wetlands, wood frog tadpoles, are capable of increasing their developmental rates in response to increased temperature and accelerated drying, but only in an additive manner. The strong negative effects of drying on survival, combined with lack of compensation between these two environmental drivers, suggest changes in the aquatic environment that are expected in this ecosystem will reduce mean fitness of populations across the landscape.
Introduction
Climate change is disrupting ecological systems worldwide (IPCC, 2014). These disruptions will likely worsen as changes in temperature and precipitation cascade across ecosystems and disproportionately affect some ecosystems more than others (Walther, 2010). For example, Arctic and Subarctic regions have experienced the greatest increases in temperature during the last 30 years; by 2100, many areas are expected to experience increases in air temperature of ≥7 °C above 1986–2005 temperatures (IPCC, 2014). This pattern of Arctic warming at twice the global rate is called Arctic amplification (Cohen et al., 2014). These rapid, extreme changes have also amplified threats to the high-latitude, freshwater ecosystems that dominate the landscape in some areas (Smith et al., 2005; Avis et al., 2011). Within Arctic and Subarctic environments, shallow lakes and wetlands can occupy up to 50% of the total land area (Petrone et al., 2000) and are one of the primary drivers of local geochemical and ecosystem processes (White et al., 2007). Although the direction, rate, and magnitude of change can be difficult to predict locally, there is widespread evidence of increased variation in water budgets for Arctic and Subarctic wetlands (Smol & Douglas, 2007). In many areas, increased drainage caused by melting permafrost and increased evaporation rates have caused extensive loss of surface water (Smol et al., 2005; Riordan et al., 2006; Carroll et al., 2011; Wolfe et al., 2011). These changes are especially notable because there seems to be comparatively less evidence for change in terrestrial communities of the Arctic (Gauthier et al., 2013).
Ectotherms are particularly susceptible to environmental change, because their metabolic functions are dependent upon local conditions (Walther et al., 2002; Lang et al., 2012). Warm temperatures can increase growth or development rates of ectotherms (Smith-Gill & Berven, 1979; King et al., 1999) either directly by increasing metabolic rates (Dillon et al., 2010; Chang et al., 2014) or indirectly by increasing productivity (Winder & Schindler, 2004; Shurin et al., 2012). Changes in growth or developmental rates could have differential effects on organisms that have ontogenetic shifts in life stages and habitat use (Crozier et al., 2008; Kingsolver et al., 2011). Many ectotherms already rely on these ontogenetic shifts to cope with dynamic environments (e.g., ephemeral wetlands); however, extreme changes may be beyond adaptive capacity and detrimental to populations (Wellborn et al., 1996; Crozier et al., 2008; O'Regan et al., 2014). For example, faster growth or development might allow some life stages to escape the threat of habitat change (e.g., accelerated drying), especially for species with complex life histories. Due to the inherent nature of ponds drying, faster growth and development could be amplified with a greater per-unit impact of warming as wetlands decrease in surface area (O'Regan et al., 2014).
Increased water temperatures caused by rapid warming of cold environments may be beneficial for growth and development of many ectotherms; however, accelerated drying of surface waters that is often commensurate with increased temperatures could negate any benefits of a warmer environment (Somero, 2010; Duarte et al., 2012). Larvae of some species with complex life histories, such as invertebrates and amphibians, might compensate for changing conditions by accelerating growth or development, but often at the expense of reduced size at metamorphosis and lower fitness (Berven, 1990; Denver 1997). Furthermore, plasticity in growth and development may already be near maximum physiological capacities for many species that breed in temporary environments (Newman, 1992; Richter-Boix et al., 2011). Thus, there is a potential trade-off for larval ectotherms in which enhanced growth and development can allow an earlier exit from wetland habitats and increase survival in the short-term, but with potential long-term reductions in growth potential, future reproduction, and survival that are typically associated with early metamorphosis and small size (Semlitsch et al., 1988; Berven, 1990).
Several studies in temperate environments have examined repercussions of warming and associated accelerated drying for freshwater communities (Kratina et al., 2012; Shurin et al., 2012; O'Regan et al., 2014), but we are unaware of any published experiments that have examined these impacts in the Arctic or Subarctic, where the climate is changing most rapidly. To evaluate the capacity of an ectotherm that occupies cold environments to respond to wetland warming and drying, we conducted a mesocosm experiment with the wood frog (Rana sylvatica Leconte). The wood frog inhabits much of the North American Arctic and Subarctic, where it is the most northerly distributed vertebrate ectotherm in the world (Martof & Humphries, 1959), and wood frog tadpoles are often the only herbivores in high-latitude wetland ecosystems. We used an experiment with three levels of wetland warming crossed with three levels of wetland drying, which allowed us to test hypotheses related to additive and interactive effects of projected climate changes on wood frogs. We expected accelerated drying would reduce survival and size at metamorphosis compared to long hydroperiod environments. However, we predicted wetland warming and drying would act synergistically, such that water temperature would have a greater per-unit effect on growth and development in fast-drying compared to slow-drying mesocosms, partially compensating for some of the negative effects of accelerated drying.
Materials and methods
Study system
We conducted the experiment at the Churchill Northern Studies Centre, near Churchill, Manitoba, Canada (58.7381° N, −93.8225° W). Churchill is at the northern end of the Hudson Bay Lowlands, one of the world's most extensive Subarctic wetland landscapes (Fraser & Keddy, 2009). Wetlands in the Hudson Bay Lowlands typically range between 400 and 50,000 m2 in surface area with mean water depths ranging from ~0.1 to 0.6 m and account for 25–40% of the land cover (Duguay & Lafleur, 2002; Macrae et al., 2004). The northern Hudson Bay Lowlands region near our field site is poorly drained and underlain by continuous permafrost.
The wood frog is the predominant amphibian that breeds in wetlands in the Churchill area and elsewhere in the North American Subarctic. The wood frog has a geographic range of >10.5 million km2 that extends from humid, subtropical forests in the southern Appalachian Mountains, includes much of the midwestern USA, and continues into tundra habitats north of the Arctic Circle in Canada and Alaska (Dodd, 2013). Throughout their range, wood frogs are primarily associated with wooded, temporary wetlands (Redmer & Trauth, 2005). The larval period of wood frogs ranges from 44 to 130 days (Wright & Wright, 1949; Redmer & Trauth, 2005) and depends greatly upon biotic interactions and environmental characteristics (Dodd, 2013). As with other amphibians, local selective forces (e.g., canopy cover, predators, competitors) can also strongly shape life-history traits (Berven & Gill, 1983; Berven, 1995; Skelly, 2004).
Experimental methods
To assess the capacity for wood frogs to respond to climate change, we measured growth and survival of tadpoles across nine experimental treatments applied in 36, 416-L cattle mesocosms that were designed to mimic natural wetlands. Three wetland warming treatments (ambient, 50W, 100W) were fully crossed with three wetland drying treatments (45, 52, 64 days) to simulate a range of predicted changes. We used 45 days as the shortest hydroperiod based on growth and development data reported for northern populations with short growing seasons, including interior Alaska and northern Manitoba (Herreid & Kinney, 1967; Berven & Gill, 1983). To apply warming treatments, we used 50W and 100W aquarium heaters (Aqueon, Franklin, WI, USA) set to their maximum temperature; these heaters produced approximate 1 °C and 2 °C increases in mean temperature, respectively. These methods are similar to other published warming experiments in aquatic mesocosms (Kratina et al., 2012; Shurin et al., 2012; O'Regan et al., 2014). The ambient warming treatments received dummy heaters (black plastic pipe) of the same size and shape of the aquarium heaters. Temperature was logged hourly for all mesocosms (Onset Hobo, Bourne, MA, USA). The mean summer temperatures of 20 wetlands near Churchill ranged from 6 °C to 22 °C and were similar to temperatures in the mesocosms (Fig. 1).
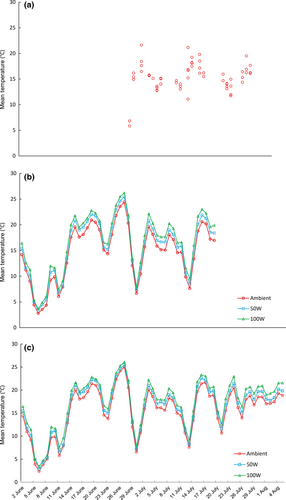
At the start of the experiment, all mesocosms were 45 cm deep, but they diverged through the study as the result of the experimentally imposed drying regime (following the procedure of Wilbur, 1987). To simulate the three rates of drying, we checked water levels daily and manually removed water with buckets to the desired level (Wilbur, 1987; O'Regan et al., 2014). If a mesocosm did not require adjustment of its water level, we simulated water removal by scooping water and then replacing it immediately. To avoid mortality associated with heat stress, mesocosms were considered dry when only 10 cm of water remained. Drying rates are representative of published and projected hydroperiods for Subarctic wetlands (Herreid & Kinney, 1967; Wolfe et al., 2011; Macrae et al., 2014).
We arranged the 36 mesocosms into two spatial blocks of 18 at the Churchill Northern Studies Centre and performed all field procedures described below on a block-by-block basis. Each of the nine treatments was randomly assigned to two mesocosms within each spatial block. Mesocosms were filled with water from the same lake on May 26, 2014‒May 27, 2014 and each received 50 g of dried sedge (Carex spp.) on June 01, 2014 to provide a natural refuge and nutrient source. A screen on the hose used to extract water from the source lake prevented colonization of predators, but allowed inoculations of natural zooplankton and algae. Nine wood frog egg masses were collected from four ponds on May 28, 2014‒May 29, 2014 for the experiment. After hatching on June 2, 2014‒June 3, 2014, 25 tadpoles were randomly assigned to each mesocosm on June 03, 2014. This density of tadpoles is within the range of densities observed locally (Davenport et al., 2013).
We determined growth rates by measuring five tadpoles from each mesocosm on days 15 and 30 of the experiment. Each tadpole was lightly blotted and weighed (ScienTech Model SA 210D, Boulder, CO, USA) in a beaker. Within 10 min, each tadpole was returned to the appropriate mesocosm. No mortality due to handling and measuring was observed during this process. We monitored mesocosms daily for any metamorphosing frogs. Metamorphosed frogs (defined by emergence of at least one forelimb) were captured and returned to the laboratory where we recorded day of emergence, snout-vent length (SVL), and date of collection. Snout-vent length was measured for all fully metamorphosed individuals because it is correlated with future reproductive success and fitness (Semlitsch et al., 1988; Berven, 1990). Individuals were not considered fully metamorphosed until total absorption of the tail (Gosner stage 46). Size at metamorphosis was represented by the mean SVL of all frogs that successfully metamorphosed from a particular mesocosm (i.e., before the water level was down to 10 cm).
We drained mesocosms on July 20, 2014 for the 45-day hydroperiod, on July 27 for the 52-day hydroperiod, and on August 8 for the 64-day hydroperiod and searched thoroughly for any surviving tadpoles. Wet weight (g) of all remaining tadpoles was recorded before they were preserved in ethanol. All animals were sacrificed at Gosner stage 46 in accordance with American Society of Ichthyologists and Herpetologists standards.
Data analysis
To test hypotheses related to how survival and size responded to variation in temperature and drying regime, we fit a small set of models that we expected would provide reasonable descriptions of the systems. For data on survival (proportion that metamorphosed) and size (defined as SVL) of survivors, we fit five models: (1) a model with a two-way interaction of drying regime × water temperature; (2) a model with additive effects of drying and temperature; models that included only the linear effect of (3) drying or (4) temperature; and (5) a block-only model. For size at metamorphosis, we incorporated the experimental block as a random effect (described below), so the only fixed effect in the fifth model was the intercept. For the survival data, we used a generalized linear model (GLM) with a quasibinomial link fitted in program r v 3.1.0 (R Core Team, 2014) to account for the overdispersed data (McCullagh & Nelder, 1997). This type of model (GLM) does not allow random effects, so we included experimental block as a fixed effect in all survival models. For the size at metamorphosis and growth data, we used linear mixed-effects models (Pinheiro et al., 2016), with mesocosm nested in block as a random effect. All models were fitted in program r using maximum-likelihood methods and ranked with QAICc (survival) or AICc (size at metamorphosis and growth). Models that included interactions also included all main effects.
To measure variation in growth (weights of tadpoles during development) across treatments, we also fit five models: (1) a model with a three-way interaction between measurement date × drying regime × water temperature; (2) a model with two-way interactions of date × drying regime and date × water temperature; (3) a model with a linear effect of temperature and a date × water temperature interaction; (4) a model with an additive effect of drying regime and a date × drying interaction; and (5) an intercept-only model. Main effects were estimated in all models with interactions. We did not consider models with just a linear effect of date because, due to changes in resource allocation as development progresses, we knew growth of tadpoles would be nonlinear.
For all models, we used the mean temperature recorded in each mesocosm as a continuous predictor rather than treat the heat application (ambient, 50W, 100W) as a factor. We used this approach because there was significant variation in the heat gained by each size of heater (Table 1). For example, within drying regimes, mean temperatures varied up to 2.4 °C for the same-sized heater, with the largest difference in the moderate-drying regime (Table 1). Also, treating temperature as a continuous covariate in models provides more useful estimates of effects compared to a factorial approach (e.g., Inouye, 2001; Cottingham & Lennon, 2005). For three mesocosms whose temperature loggers failed, we used the mean temperature of the other mesocosms with the same combination of heat and drying regime (e.g., 45-day hydroperiod × 100W).
| Drying regime | Temperature treatment | ||
|---|---|---|---|
| Ambient | 50W | 100W | |
| 45 day | 14.65 (6.53) | 15.71 (6.62) | 16.61 (6.75) |
| 52 day | 14.75 (6.31) | 15.94 (6.75) | 17.17 (6.56) |
| 64 day | 15.52 (6.19) | 16.42 (6.11) | 17.36 (6.26) |
Results
Survival
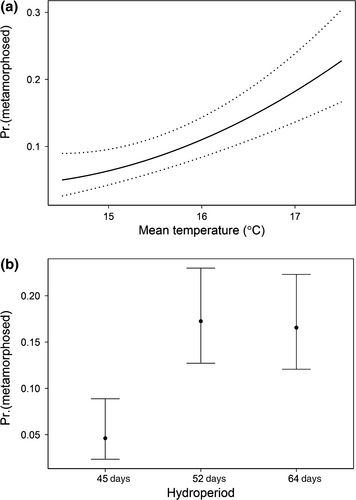
Across all mesocosms, 18.6% of tadpoles metamorphosed by the end of their drying treatment (i.e., they survived). The model with additive effects of water temperature and drying regime was the best supported of the four we fit (Table 2). Across all drying regimes, a 1 °C increase in water temperature increased the odds of survival by 1.79 (95% CI = 1.39 to 2.34; Fig. 2). Estimated odds ratios from this model indicated that tadpoles in the 52-day and 64-day hydroperiod mesocosms were 4.1–4.3 times more likely to survive to metamorphosis than tadpoles in the fast-drying mesocosms (Fig. 2). Surprisingly, the point estimate for survival was slightly higher in the moderate-drying mesocosms than in slow-drying mesocosms, although the confidence intervals for these estimates overlapped broadly. Survival in the fast-drying treatment averaged only 5%, with no survivors in the mesocosms at ambient temperatures. Although emergence from short hydroperiod environments occurred only with supplement heat, the drying regime × water temperature interaction term did not differ from zero (F = 1.40, P = 0.263).
| Model | K | Residual deviance | QAICc |
|---|---|---|---|
| Survival ~ hydro. + mean temp. | 5 | 51.638 | 47.982 |
| Survival ~ hydro. + mean temp. + hydro. × mean temp. | 7 | 47.266 | 51.618 |
| Survival ~ mean temp. | 3 | 84.014 | 63.120 |
| Survival ~ hydro. | 3 | 84.052 | 63.145 |
| Survival ~ 1 | 2 | 136.440 | 94.170 |
Size at metamorphosis
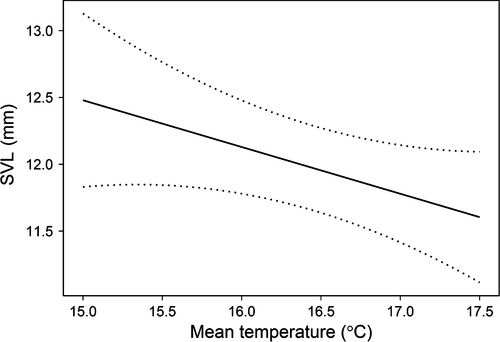
The model with only a linear effect of water temperature provided the best description of variation in size of metamorphs (Table 3). For individuals who survived to metamorphosis, there was a weak negative effect of temperature on size: for every 1 °C increase in water temperature, metamorph SVL decreased by 0.350 mm (SE = 0.194) (Fig. 3). This effect was estimated imprecisely, however, and the 95% confidence interval for the effect included zero. As a result, the model that only included an intercept received nearly as much support (ΔAICc = 1.06) as the top-ranked model (Table 3). The model that only included mesocosm hydroperiod received less support than the intercept-only model (ΔAICc = 2.85), which indicates size did not vary with drying rate. The model with the drying regime × water temperature interaction (χ2 = 0.68, P = 0.711) was also much less likely than the model based on temperature (ΔAICc = 6.64).
| Model | K | −2(LL) | AICc |
|---|---|---|---|
| SVL ~ mean temp. | 5 | 495.399 | 505.813 |
| SVL ~ 1 | 4 | 498.601 | 506.875 |
| SVL ~ hydro. + mean temp. | 7 | 493.862 | 508.645 |
| SVL ~ hydro. | 6 | 497.146 | 509.730 |
| SVL ~ hydro. + mean temp + hydro. × mean temp. | 9 | 493.179 | 512.455 |
Larval growth
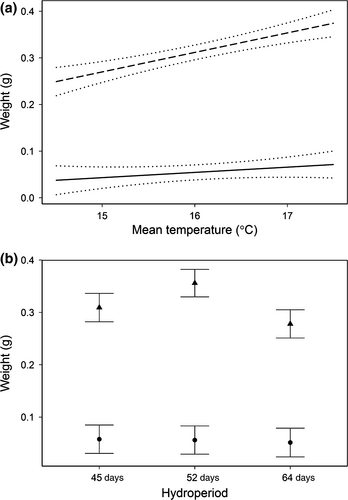
The model with two-way interactions of date × water temperature and date × drying regime provided the best description of variation of larval growth (Table 4). As expected, increased temperatures accelerated tadpole growth, at least through day 30 of the experiment (Fig. 4). On day 15, tadpole weights ranged from 0.036 g (95% CI: −0.001 to 0.074) at 14.5 °C to 0.072 g (95% CI: 0.036 to 0.107) at 17.5 °C. On day 30, tadpole weights ranged from 0.250 g (95% CI: 0.213 to 0.286) at 14.5 °C to 0.375 g (95% CI: 0.339 to 0.410) at 17.5 °C. Drying regime had weaker effects than temperature on larval growth rates. There were no differences in tadpole weight among drying treatments on day 15. But by day 30, tadpoles in moderate-drying mesocosms were larger than those in slow-drying mesocosms, with fast-drying mesocosms in between (Fig. 4). The model with the three-way interaction of date × drying regime × water temperature was the second-ranked of the four models, but the interaction effect did not differ from zero (F = 0.941, P = 0.440), which indicates the effect of temperature on growth did not depend upon drying regime.
| Model | K | −2(LL) | AICc |
|---|---|---|---|
| g ~ hydro. + mean temp. + date + hydro. × date + mean temp. × date | 15 | −809.984 | −779.043 |
| g ~ hydro. + mean temp. + date + hydro. × mean temp. × date | 21 | −815.713 | −771.880 |
| g ~ mean temp. + date + mean temp. × date | 9 | −787.011 | −768.662 |
| g ~ hydro. + date + hydro. × date | 12 | −769.003 | −744.395 |
| g ~ 1 | 4 | −252.840 | −244.763 |
Discussion
The ecological impacts of climate change are widespread, with freshwater ecosystems particularly vulnerable across multiple trophic levels (Schindler, 1997; Parmesan & Yohe, 2003; Woodward et al., 2010). To provide insight into the potential repercussions of climate change on biotic communities in Subarctic wetlands, we conducted an experiment to determine how projected changes in temperature and hydroperiod are likely to affect survival and growth of wood frogs. Our results revealed that wood frogs have some capacity to respond to environmental changes individually (e.g., increased drying rate or temperature), but that anticipated changes in climate could represent significant threats to future fitness and population growth.
The effect of the interactions between drying regime × water temperature did not differ from zero for survival to metamorphosis, size at metamorphosis, or growth rate of tadpoles. Warm temperatures increased survival and growth rate of tadpoles: a 1 °C increase in water temperature increased the odds of survival by a factor of 1.8 and increased size of tadpoles on Day 30 by 16%. Furthermore, tadpoles in the 52-day and 64-day hydroperiod treatments were 4.1–4.3 times more likely to survive to metamorphosis than tadpoles in the 45-day hydroperiod. Hydroperiod also affected growth rate, although the pattern appeared less related to drying rate, and ultimately, it did not affect size at metamorphosis. This lack of an interactive response underscores how some species may simply be incapable of mounting a large enough response to overcome environmental change, especially if changes occur more rapidly than new environments become available at the range periphery.
Collectively, our results indicate more rapid growth and development at warm temperatures could help ensure there are at least some survivors during years with limited water supply or if climate change reduces water availability in breeding sites. However, contrary to results from a similar experiment in southwest Canada that found that two of three amphibian species accelerated development in response to reduced hydroperiod (O'Regan et al., 2014), we found little evidence that more rapid development from warmer environments is likely to compensate for the risk of accelerated drying. The one species in that study suffered higher mortality in fast-drying wetlands, the Northern red-legged frog (Rana aurora), is closely related to wood frogs (O'Regan et al., 2014). This finding along with ours matches phylogenetic predictions that some anuran families may be more restricted in their responses to changes in abiotic factors like wetland drying (Richter-Boix et al., 2011).
One of the risks of climate change is the prediction for warming-related reductions in body size and fitness (Gardner et al., 2011; Bowden et al., 2015), because temperature–size rules predict an inverse relationship between that rearing temperature and size (Atkinson, 1994). Size is often the single greatest predictor of fitness (Morrison & Hero, 2003; Kingsolver et al., 2011), and evidence from long-term data sets indicates that global warming has caused a widespread shift toward younger age classes and smaller size at age for aquatic organisms (Daufresne et al., 2009). While warmer temperatures increased the odds that wood frogs survived to metamorphosis in our experiment, there was only a small cost. Size at metamorphosis decreased by only ~0.35 g per 1 °C increase in water temperature, which equates to ~5.5% reduction in size of metamorphs from unheated mesocosms (mean = 12.52 g) compared to those with 100W heaters (mean = 11.88 g). Similarly, O'Regan et al. (2014) found that 3 °C warming produced only small reductions (<10%) in size at metamorphosis for three species of amphibians, even when combined with reduced hydroperiod. This small cost suggests responses to environmental changes that produce more rapid development are likely not a primary threat to fitness of wood frogs and other amphibians at northern latitudes. The disadvantage of reduced size might be further negated if earlier metamorphosis allows for greater growth in the terrestrial environment before their first winter brumation (Laugen et al., 2005; Earl & Whiteman, 2015). A larger size at metamorphosis, however, does not always confer advantage across life stages. For example, wood frog tadpoles from environments exposed to road salts had greater growth rates and size at metamorphosis, but as terrestrial juveniles they had lower survival than compared to individuals from low-salt environments (Dananay et al., 2015). Future climate change research on amphibians and other organisms that experience ontogenetic niche shifts should consider the consequences of larval development on postmetamorphic vital rates.
While the warming treatments we applied were conservative in comparison with predicted changes for the Subarctic (IPCC 2014), there is greater uncertainty about changes in hydroperiod (Riordan et al., 2006; Carroll et al., 2011; Wolfe et al., 2011). We were surprised that only 5% of tadpoles metamorphosed from the 45-day hydroperiod treatment. Forty-five days (posthatching) seem too short for development in local populations; however, tadpoles from near Fairbanks, Alaska—which has slightly longer growing season than Churchill (Davenport & Hossack, 2016)—have been reported to metamorphose in as little as 46 days (Herreid & Kinney, 1967). Thus, we expected even faster development for populations near Churchill compared to Fairbanks populations. But not all species with complex life histories respond similarly to changes in temperature or water availability. Also, the negative effect of temperature on size in our experiment was highly variable, which is consistent with Berven & Gill's (1983) conclusion that selection in local populations of wood frogs has been stronger for developmental time than for body size.
A recent review suggests that plasticity and genetic variation may allow some ectothermic species to mediate the risk of climate change (Urban et al., 2014). Wood frogs can adapt to environmental conditions across short distances and short time intervals, even with high gene flow (Berven, 1982a,b). For example, in forests of Connecticut, there was strong evidence of local adaptation in the critical thermal maxima of wood frogs across just a few decades (Skelly & Freidenburg, 2000; Freidenburg & Skelly, 2004). Similarly, the population-level thermal performance of a cold-water stream amphibian (Ascaphus montanus) was positively associated with local environmental heterogeneity and varied across small geographic scales (Hossack et al., 2013). We are uncertain if there is similar local adaptation in our study area because it has weaker environmental gradients, such as variation in canopy cover, compared to most areas that wood frogs inhabit. Also, in a prior experiment, wood frog eggs from ephemeral and permanent wetlands around Churchill did not differ in their responses to drying or warming (J. Davenport et al., unpublished data). Local adaptation can still occur with high gene flow at microgeographic scales (Richardson et al., 2014; Fitzpatrick et al., 2015); thus, future work should evaluate the potential for local adaptation by performing reciprocal transplant experiments across a latitudinal gradient with this widespread species.
Amphibian larvae are important herbivores in many freshwater ecosystems and can substantially alter nutrient cycling (Seale, 1980; Kupferberg, 1997). Indeed, disease-related decline of amphibians from tropical streams reduced nutrient turnover rates and whole stream respiration (Whiles et al., 2006, 2013). Therefore, the implications of our experiment and other recent warming experiments provide an opportunity to hypothesize how our results could affect Subarctic wetland ecosystems. If wood frog tadpoles and other consumers respond positively to warming-associated increases in aquatic productivity (Shurin et al., 2012; MacDonald et al., 2015), climate change could accelerate growth and development, further offsetting some of the negative consequences of drying and warming (O'Regan et al., 2014; Stoks et al., 2014). Increased temperatures of experimentally warmed environments can increase growth and development rates (Smith-Gill & Berven, 1979; King et al., 1999) and is likely a product of increased metabolic function and aquatic productivity (Winder & Schindler, 2004; Dillon et al., 2010; Shurin et al., 2012; Chang et al., 2014). We were unable to measure productivity in our experiment because our chlorophyll samples were contaminated, but climate warming in the previous century increased productivity in shallow lakes near our study area and elsewhere in the Subarctic (Smol et al., 2005; Rühland et al., 2013; MacDonald et al., 2015). Fish predators are also important components of wetland food webs as they can influence both the impacts of experimental warming (Kratina et al., 2012; Shurin et al., 2012) and tadpole growth and survival in Subarctic wetlands (Davenport et al., 2013, 2016).
The effects of climate change will manifest in many different forms and will likely interact in complex ways that are difficult to predict. How organisms in dynamic aquatic environments such as shallow wetlands are likely to respond to climate change is still largely unknown (Greig et al., 2012; Kratina et al., 2012). This is especially true in colder environments (Post et al., 2009; Pithan & Mauritsen, 2014). Our results reveal that one of the dominant herbivores in Subarctic wetlands, wood frog tadpoles, are capable of increasing their developmental rates in response to temperature and accelerated drying, but only in an additive manner. The strong negative effects of drying on survival, combined with weak compensation between these two environmental drivers, suggest severe changes in the aquatic environment that are forecasted in this ecosystem could reduce mean fitness of populations. If these responses are broadly representative of ectotherms in Subarctic environments, then the pace and magnitude of climate change may be too great for many species to adapt. This could also lead to further alteration of these ecosystems as vital members of the food web decline in abundance (Gilman et al., 2010).
Acknowledgements
We thank D. Gibson, J. Verstege, A. Goodman, and A. Winegardner for field assistance and data entry, and K. Honeycutt, J. Weber for help with figures. We are also grateful to all Earthwatch participants who helped maintain experiments and collect data. W. Lowe, B. Addis, C. Bayer, L. Schwartz, and two anonymous reviewers all provided helpful comments on the manuscript. J.M. Davenport would like to thank J.K. Davenport, L.O. Davenport, and C.E. Davenport for support during the research. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This is contribution number 563 of the U.S. Geological Survey Amphibian Research and Monitoring Initiative (ARMI). All authors have no conflict of interests to declare.




