Atmospheric CO2 enrichment and drought stress modify root exudation of barley
Abstract
Rising CO2 concentrations associated with drought stress is likely to influence not only aboveground growth, but also belowground plant processes. Little is known about root exudation being influenced by elements of climate change. Therefore, this study wanted to clarify whether barley root exudation responds to drought and CO2 enrichment and whether this reaction differs between an old and a recently released malting barley cultivar. Barley plants were grown in pots filled with sand in controlled climate chambers at ambient (380 ppm) or elevated (550 ppm) atmospheric [CO2] and a normal or reduced water supply. Root exudation patterns were examined at the stem elongation growth stage and when the inflorescences emerged. At both dates, root exudates were analyzed for different compounds such as total free amino acids, proline, potassium, and some phytohormones. Elevated [CO2] decreased the concentrations in root exudates of some compounds such as total free amino acids, proline, and abscisic acid. Moreover, reduced water supply increased proline, potassium, electric conductivity, and hormone concentrations. In general, the modern cultivar showed higher concentrations of proline and abscisic acid than the old one, but the cultivars responded differentially under elevated CO2. Plant developmental stage had also an impact on the root exudation patterns of barley. Generally, we observed significant effects of CO2 enrichment, watering levels, and, to a lesser extent, cultivar on root exudation. However, we did not find any mitigation of the adverse effects of drought by elevated CO2. Understanding the multitude of relationships within the rhizosphere is an important aspect that has to be taken into consideration in the context of crop performance and carbon balance under conditions of climate change.
Introduction
Elevated atmospheric carbon dioxide concentrations (eCO2) represent one of the major drivers of anthropogenic global climate change which is characterized by steady, multidecadal warming of the earth's surface, altered precipitation patterns, and more frequent extreme climate events (Diffenbaugh & Field, 2013).
Due to anthropogenic activities, atmospheric [CO2] has increased by 40% since the preindustrial times and the current [CO2] of 400 ppm will continuously increase to reach 550 ppm by the year 2050 (IPCC, 2013). In the near future, further changes in water availability are anticipated. Discrepancies in precipitation between wet and dry regions and between wet and dry seasons will increase (IPCC, 2013). Stresses induced by soil drying might incorporate water shortage, salinity, and nutrient deficiency (Langridge et al., 2006). These factors could affect 50% of the global crop production area by the year 2050 (Wang et al., 2003).
Changes in [CO2], water, and nutrient availability are known to affect plant performance. The current [CO2] does not yet saturate photosynthetic CO2 fixation in C3 plants. Hence, CO2 enrichment enhances growth and yield of these plants compared to C4 plants (Long et al., 2004). In the short term, under drought conditions, eCO2 may diminish the importance of stomatal limitation for carbon assimilation, inhibit photorespiration, and increase water-use efficiency between 40% and 100% (Fangmeier & Jäger, 2001; Morison, 2001) and therefore improve plant water status (Robredo et al., 2010). These responses vary with water availability (Kimball et al., 2002), between species (Jablonski et al., 2002; Crous et al., 2010) and even between crop cultivars (Pérez-López et al., 2010). In the longer term, a negative acclimation of photosynthesis may occur in many species (Chaves & Pereira, 1992).
Barley (Hordeum vulgare L.) is an economically important cereal worldwide and is used commercially for animal feed, malt production, and for human consumption. Yield quantity of cereals was significantly improved up until the 1990s (FAOSTAT, 2014). However, a slight decline in yields has recently been reported in several regions, for example, in France (Brisson et al., 2010) mainly due to unfavorable climatic conditions such as drought (FAO, 2009). Barley has a C3 photosynthetic pathway and is particularly tolerant to cold, drought, and salinity and can therefore be cultivated under a diverse range of environmental conditions. In spite of this, barley responds to drought stress with a reduction in productivity (Serraj & Sinclair, 2002). To meet yield safety, it is important to know how different cultivars vary in their adaptation to altered environmental conditions. There might also be a correlation between the year of release and the response to CO2 enrichment. Correspondingly, older cultivars of spring wheat showed a greater growth stimulation and aboveground biomass than modern cultivars under eCO2 (Ziska et al., 2004).
Plant roots deposit a wide range of compounds in the rhizosphere such as sloughed-off cells, mucilage, volatiles, and exudates. Cereals such as wheat and barley transfer 20–30% of all photosynthetically fixed carbon to the roots. Approximately half of these remain in the roots, and the other half is released into the rhizosphere (Kuzyakov & Domanski, 2000). Root-secreted chemicals are involved in a variety of functions such as enhancing nutrient mobilization (Neumann & Römheld, 1999), detoxifying the rhizosphere (Badri & Vivanco, 2009), supporting the microbial activity in the rhizosphere (Watt et al., 2006), and attracting or suppressing microorganisms (Bais et al., 2006), maintaining a highly specific diversity of microbes in the rhizosphere (Badri et al., 2009). Root exudates contain carbohydrates, amino acids, organic acids, ions, vitamins, enzymes, and signal substances (Badri & Vivanco, 2009). Knowledge of the composition and quantity of organic substances released from roots of different plant species is necessary for understanding the chemical and biological processes in the rhizosphere. However, the quantity and composition of root exudates are determined by different factors, including phenological stage (Neumann & Römheld, 2007), nutritional status (Marschner, 1986), mechanical impedance (Neumann & Römheld, 2007), and the presence of microorganisms (Jones & Darrah, 1992). Root exudate composition varies also between the type of plant metabolism (Vranova et al., 2013), plant species (Lesuffleur et al., 2007), and among cultivars (Cieslinski et al., 1997; Micallef et al., 2009). Moreover, these exudates are modulated by external biotic (Matilla et al., 2010) and abiotic stresses. The mechanisms controlling root secretion are poorly understood. The majority of root exudates are thought to be released passively via diffusion. However, some compounds such as auxins could also be actively transported (Walker et al., 2003; Badri & Vivanco, 2009). Exudation is actually a net efflux, because it involves both efflux and influx components (Phillips et al., 2006).
It has been hypothesized that increases in CO2 will likely produce greater amounts of root exudates, suggesting that associated soil microbial and fungal activities would be stimulated from increases in plant-derived C inputs. However, no indication for a general increase in total root exudation was found in bean plants (Haase et al., 2007). In studies on the composition of exudates as they leave the root, care must be taken to avoid an overestimation due to root damage and microbial degradation. Hence, a suitable sampling technique, which does not alter the root system, is important to understand rhizosphere processes. Most precise studies have been conducted under well-defined conditions such as sand culture, which has mechanical impedance and allows a seminatural root proliferation.
Different hypotheses for the decrease in N and minerals concentration under eCO2 have been discussed. It could be an effect of reduced mass flow due to reduced stomatal conductance and transpiration under eCO2 (Poorter et al., 1997; Taub & Wang, 2008; McGrath & Lobell, 2013). Another supported mechanism for the N decrease is a downregulation of photosynthesis (Fangmeier et al., 1999, 2000). Currently, the inhibition of nitrate reduction under eCO2 is being discussed to be responsible for lower N concentrations and proteins in aboveground tissues of cereals (Bloom et al., 2010). Additional studies should be performed to investigate whether plant N could be lost by root exudation (Pang et al., 2006). A decrease in nutrient concentrations has been detected already at the booting stage, being further enhanced until plant maturity (Manderscheid et al., 1995). In addition, changes in mineral nutrients depend also on the plant species (McGrath & Lobell, 2013). Aboveground plant morphology and physiology are markedly altered under CO2 enrichment. Schmid et al. (2016) have investigated growth, yield, and photosynthetic parameters in the same experiment as ours with barley and found that CO2 fertilization compensated for the negative effect of drought on grain yield and water-use efficiency, especially in one of the two cultivars tested. Here, we present results from root exudation analyses to gain information on responses of the hidden half of barley plants. However, until now, there have been very few studies examining the combined effects of [CO2] and drought stress on root exudation. Based on the assumption that not only aboveground responses but also root exudation will differ largely between cultivars, we therefore compare changes in root exudation of two barley cultivars exposed to elevated CO2 and drought. Specifically, we hypothesized that (i) drought and elevated CO2 do affect root exudation in both cultivars, that (ii) these effects vary depending on the plant developmental stage and cultivar, and that (iii) elevated CO2 will mitigate adverse effects of drought.
Materials and methods
Plant material
Two two-row spring barley cultivars, cv. Golden Promise (GP) and cv. Bambina (BA), were used in the experiment. The older cultivar GP is a mutant generated in Scotland in 1966 from the cultivar ‘Maythorpe’ following gamma ray treatment. The semidwarf spring barley cultivar ‘Golden Promise’ has considerable salt tolerance compared to its parental line (Pakniyat et al., 1997) and was mainly used in Scotland between 1977 and 2001 for whiskey malting purposes (Maluszynski et al., 2009). The new cultivar used in this study is BA, a modern variety released in 2009 by KWS Lochow (Bergen-Wohlde, Germany). Due to the low seed protein content, it is very suitable as malting barley.
Experimental setup
The climate profile (quantity of light, humidity, and temperature) for programming the controlled growth chambers (Vötsch BioLine, Balingen, Germany) was derived from the 1991–2005 climate time series registered at Stuttgart Airport by the German Weather Service (DWD) and was based on daily values for a vegetation period lasting from 15 March to 20 July. Furthermore, temperatures were varied according to the diurnal course.
Pots (10.2 cm in diameter and 40 cm tall) were filled with washed sand (4.7 kg). Fertilization was based on Egle et al. (2008) and was added at the beginning of the experiment. Each pot received 150 mL of nutrient solution containing 0.48 g Ca(H2PO4)2·H2O, 0.41 g CaCO3, 1.08 g K2SO4, 1.24 g MgSO4·7H2O, and 0.56 mg FeNa EDTA. In addition, each pot received 150 mL of a nutrient solution containing 0.85 mg MnCl2·4H2O, 1.34 mg H3BO3, 0.22 mg ZnSO4·7H2O, 0.12 mg CuSO4·5H2O, 0.12 mg Co(NO3)2·6H2O, 0.12 mg CoCl2·6H2O, 0.07 mg (NH4)6Mo7O24·4H2O, and 0.03 mg CaCl2·2H2O. Furthermore, nitrogen fertilization was added at sowing (DC0), at stem elongation stage (DC30), and at the 5-node stage during stem elongation (DC35). At each of these stages, every pot received 150 mL of water with 0.18 g NH4NO3, corresponding to 225 kg N ha−1 in total. Saucers placed underneath avoided the possible water loss. Four plants per pot were sown at a depth of 2 cm, and the number of plants was adjusted to two per pot 18 days after sowing (DAS).
Experimental design
Plants were grown in six controlled climate chambers with three replicates per treatment. Each climate chamber contained pots of two water treatments, one subjected to normal rainfall of southwest Germany and the other subjected to a low rainfall treatment. In the normal rainfall treatment, pots received a mean of 20 mL deionized water per day, corresponding to an average precipitation of 2.4 mm day−1. In the low rainfall treatment, watering was reduced by 33% simulating a dry year, such as 2003. At the start of the experiment, all pots received 50 mL of deionized water.
Two CO2 treatments were applied 24 h per day, representing the current concentration (380 ppm) and the higher concentration expected in 2050 (550 ppm). Each CO2 treatment was replicated in three different controlled growth chambers. To avoid putative chamber effects, plant containers and CO2 treatments were shifted weekly among chambers. Moreover, pots were arranged randomly in the respective climate chamber.
Measurements of plant-related parameters
All measurements were made on two different harvest days, corresponding to DC30 (stem elongation stage) and DC49 (booting stage), which were achieved at 71 and 91 DAS, respectively. At maturity, no collection of root exudates was performed as root degradation had already begun. At each harvest, above- and belowground fractions were separated. The different dry biomasses were determined after drying at 80 °C until constant weight. Results of these measurements are given in Schmid et al. (2016). Plant development was reported using the BBCH code (Meier, 2001).
Measurements of root exudate metabolites
Before the collection of root exudates, pots were soaked twice for 1 min in deionized water to remove nutrients and soluble material. Root systems were submerged into 300 mL pure water over a time period of 2 h to avoid microbial degradation, starting exactly 2 h after the onset of the light period, as it has been demonstrated that exudate release from roots depends largely on current photosynthesis (Kuzyakov & Cheng, 2001). Eluates were collected during a 15-min period, filtered through a Whatman Klari-Flex filter (GE Healthcare Bio Sciences Corp., Piscataway, NJ, USA) with a 0.45-μm polyethersulfone membrane to exclude root-border-like cells and much of the microbial population, and then stored at −20 °C in polyethylene bottles for further analyses. Ten-mililitre aliquots of exudates were concentrated tenfold using a SpeedVac concentrator (Savant, GMI, Richmond, CA, USA).
The electric conductivity (EC) of root exudates was determined directly using a conductivity meter with a standard conductivity cell (WTW, Weilheim, Germany). Root exudate samples were analyzed for potassium by means of inductively coupled plasma optical emission spectrometry (ICP-OES, Vista Pro radial; Varian, Mulgrave, Victoria, Australia). The method is based on VDLUFA VII 2.2.2.6 (2011). Total free amino acids (AA) were analyzed by the fluorometric OPA-MET method using 3-mercaptopropionic acid in place of β-mercaptoethanol (Jones et al., 2002), using a spectral fluorometer (Cary Eclipse; Varian, Darmstadt, Germany) with glycine as a standard. Free proline was measured photometrically by the ninhydrin procedure measuring the absorbance at 546 nm (Bates et al., 1973), and the hormones ABA, IAA, and isopentenyladenine (CYT) were determined by radioimmunoassay (Bohner & Bangerth, 1988).
Statistical analyses
All data were analyzed using package version 3.1-113 of the statistical software r (R Core Team, 2014). Data were transformed when model residuals for normal distribution and variance homogeneity failed. Three-way analysis of variance (anova) was carried out to test CO2, cultivar and water effects, and their interactions. Differences were considered significant at P ≤ 0.05, highly significant at P ≤ 0.01, and very highly significant at P ≤ 0.001. Spearman's rank correlation coefficient (r) was used to determine the strength of relationships between different variables.
Results
Total root biomass
Plants were healthy and grew well without presenting any signs of nutrient deficiencies at the first and second harvest, corresponding to stem elongation stage (DC30) and booting stage (DC49), respectively (Fig. S1). At DC30, root biomass was significantly (P ≤ 0.01) higher by 20% at elevated compared to ambient [CO2] (Table 1, Fig. 1a). However, cv. BA did not show this response under reduced water supply. At DC49 under eCO2, cv. GP showed an increase by 9% of root biomass under normal watering conditions, and this effect was doubled at reduced water supply (Fig. 1b). However, the effect of [CO2] was not significant (Table 1).
| Three-way anova | ||||
|---|---|---|---|---|
| Main effects | Interactions | |||
| Cult | CO2 | Wat | ||
| DC31 | ||||
| Root dry weight [g pot−1] | ns | * | ** | ns |
| Root-to-shoot ratio | * | * | * | ns |
| Amino acids | ||||
| Total [μmol gly equ g−1 root DW] | ns | ns | ns | ns |
| Free proline [mg g−1 root DW] | * | *** | ns | ns |
| Electric conductivity [μS cm−1 g−1 root DW] | ns | * | ** | |
| Potassium [mg g−1 root DW] | ns | * | * | ns |
| Hormones | ||||
| ABA [ng g−1 root DW] | ns | ** | * | ns |
| CYT [ng g−1 root DW] | * | ns | * | ns |
| DC49 | ns | ns | ns | |
| Root dry weight [g pot−1] | ns | ns | *** | ns |
| Root-to-shoot ratio | *** | *** | ** | ns |
| Amino acids | ||||
| Total [μmol gly equ g−1 root DW] | ns | * | ns | ns |
| Free proline [mg g−1 root DW] | * | * | * | CO2 × Cult* |
| Electric conductivity [μS cm−1 g−1 root DW] | ns | ns | ** | |
| Potassium [mg g−1 root DW] | ns | ns | ns | ns |
| Hormones | ||||
| ABA [ng g−1 root DW] | ns | ns | *** | ns |
| IAA [ng g−1 root DW] | ns | * | ** | CO2 × Cult** |
- CYT, isopentenyladenine.
- *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, not significant.
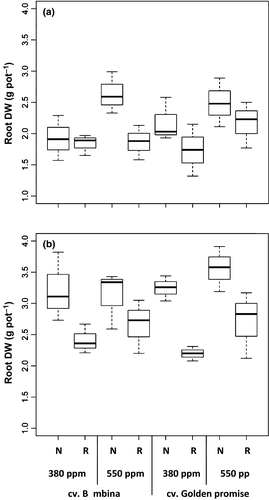
Highly and very highly significant effects of water supply on root biomass of both cultivars were observed at DC30 and DC49, respectively (Table 1). Root biomass under reduced watering conditions was on average 22% (at DC30) and 33% (at DC49) lower than under well-watered conditions (Fig. 1). There were no significant effects of cultivar on root biomass at any phenological stage (Fig. 1).
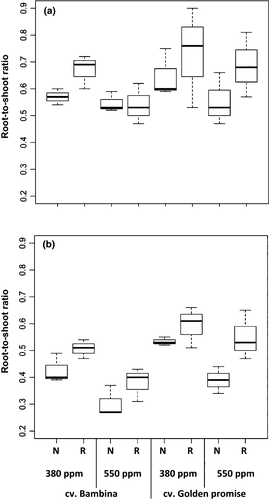
At both developmental stages, significant effects of [CO2] on root-to-shoot ratio (RSR) were observed (Table 1). In all treatments, RSR was reduced in response to eCO2 (Fig. 2). As expected, plants with reduced water supply showed significantly higher RSR than well-watered plants, independently of the developmental stage (Table 1, Fig. 2). The old cultivar GP presented significantly higher RSR than the new cultivar BA across all tested growth conditions (Table 1, Fig. 2).
Electric conductivity and potassium
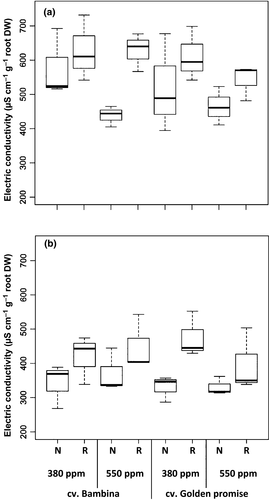
Only at DC30, a significant (P = 0.04) effect of [CO2] on EC of the exudates was observed (Table 1). At ambient [CO2], the EC was higher than at eCO2 (Fig. 3, Table 1). At both growth stages, anova indicated significant (P = 0.003) effects of water supply (Table 1). Plants grown with reduced water supply always showed higher EC values than well-watered plants (Fig. 3). At DC49, this water effect was even doubled, but only when plants were growing under ambient [CO2] (Fig. 3b). EC of exudates was not affected by cultivar (Table 1). At both stages, there were negative correlations between the EC values and aboveground biomass (rs = −0.7, P ≤ 0.001, rs = −0.5, P = 0.02, respectively), root biomass (rs = −0.9, P ≤ 0.001), and ear biomass (rs = −0.5, P ≤ 0.01). Negative correlations between EC and number of leaves were found only at DC30 (rs = −0.5, P = 0.02).
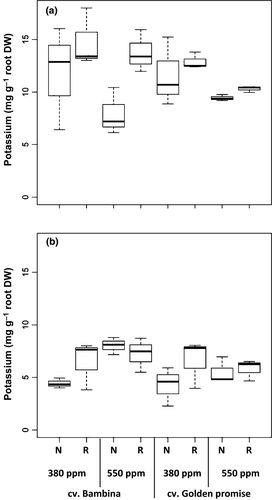
Concerning potassium, a significant [CO2] effect was found only at DC30 (Table 1). Root exudates had lower [K] under elevated than under ambient [CO2] (Table 1, Fig. 4a). Similarly, a significant effect of water supply on K exudation was found only at DC30 (Table 1). Plants grown under reduced water supply showed on average 20% higher [K] levels than well-watered plants (Fig. 4a). At DC30, no significant cultivar effect on potassium exudation was found (Table 1). At DC49, no significant effects of [CO2], water supply, or cultivar were found (Table 1). At both stages, positive correlations between levels of EC and K (rs = 0.8, P ≤ 0.001; rs = 0.5, P = 0.006) and negative correlations between levels of K and number of leaves (rs = −0.5, P = 0.01; rs = −0.4, P = 0.05) were found. At DC30, K was also negatively correlated to the shoot biomass (rs = −0.7, P < 0.001) and root biomass (rs = −0.7, P ≤ 0.001). However, these correlations were no more significant at DC49.
Total free amino acids
An effect of [CO2] on the AA concentration was observed, being significant at DC49 (Table 1). In general, plants grown at eCO2 showed lower levels of AA than the ones grown at ambient [CO2]. However, BA grown under reduced water conditions showed higher levels of AA at eCO2 (Table 1, Fig. 5). We did not detect any significant water or cultivar effect on the concentration of total free amino acids at any developmental stage (Table 1). At both stages, there was a significant positive correlation between AA and EC (rs = 0.5, P = 0.007) and number of leaves (rs = −0.6, P < 0.001; rs = −0.5, P = 0.02). At DC49, AA was negatively correlated to aboveground and root biomass (rs = −0.6, P = 0.002, respectively) and to ear biomass (rs = −0.5, P = 0.01).
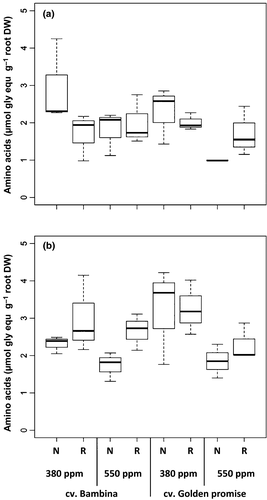
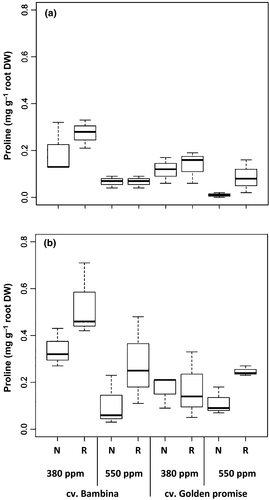
Similar to total free amino acids, at DC30, plants grown under eCO2 showed a very highly significant reduction in proline concentration compared to plants grown at ambient [CO2] (Table 1, Fig. 6). At DC49, plants grown under eCO2 exuded lower amounts of proline than plants grown under ambient [CO2]. However, an interaction between CO2 and cultivar was observed (Table 1). GP plants at eCO2 with reduced water supply exuded 32% more proline than when grown under ambient [CO2]. In contrast to this, BA plants exuded 47% less proline in the same growth conditions (Fig. 6b). As expected, plants grown under a reduced water supply exhibited increased proline concentrations (Fig. 6). Nevertheless, anova showed a significant effect of water only at DC49 (Table 1). At both growth stages, a significant effect of cultivar was observed (Table 1). In general, BA showed significantly higher proline levels than GP, especially when plants grew at ambient [CO2] (Fig. 6). Similar to the amino acids, there were moderate negative correlations between proline and the number of leaves (rs = −0.6, P < 0.001) and to the aboveground and root biomass (rs = −0.6, P = 0.001; rs = −0.5, P = 0.01, respectively), but the correlation between proline and the aboveground biomass disappeared at DC49. At DC30, positive correlations were found between proline and AA (only in GP, rs = 0.9, P < 0.001), EC (rs = 0.5, P = 0.007), and K (rs = 0.5, P < 0.006). However, at DC49, the correlation between proline with AA (rs = 0.7, P = 0.02) was significant only for the cv. BA.
Hormone levels
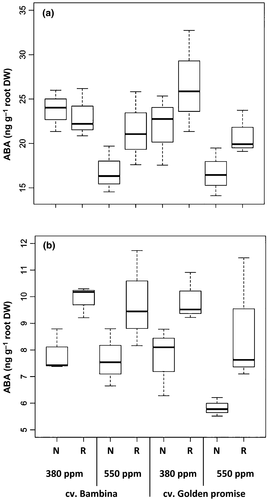
Plants grown at ambient [CO2] showed higher ABA concentrations than plants grown under eCO2 (Fig. 7). This CO2 effect was significant (P = 0.002) at DC30, but it disappeared at the later growth stage (Table 1). Under reduced water supply, both cultivars exuded significantly higher ABA concentrations than under well-watered conditions (Fig. 7). This water effect was significant at DC30 (P = 0.002) and at DC49 (P ≤ 0.001) (Table 1). No significant cultivar differences in [ABA] were observed at any growth stage (Table 1). At DC30, ABA showed correlation with K (rs = 0.6, P < 0.001), and a negative correlation with number of leaves (rs = −0.5, P = 0.02). Furthermore, at both developmental stages, ABA showed moderate correlations to AA (rs = 0.4, P = 0.05; rs = 0.7, P < 0.001), to proline (rs = 0.6, P = 0.001; rs = 0.5, P = 0.005), and to EC (both rs = 0.8, P < 0.001) and negative correlations to aboveground biomass (rs = −0.7, P < 0.001; rs = −0.6, P = 0.001), to root biomass (both rs = −0.9, P < 0.001), and to ear biomass (rs = −0.6, P < 0.001).
The most abundant form of cytokinins is trans-zeatin, but the less abundant isopentenyladenine is also very active. We have only detected isopentenyladenine (CYT) in our analyses. This cytokinin was found at DC30, but not at DC49. In contrast, the auxin IAA was only detected at DC49 (Table 1). Root exudation of CYT was not affected by different levels of CO2 (Table 1). In contrast, different watering levels significantly (P < 0.05) affected this hormonal level. In general, plants with reduced water supply exuded more CYT than well-watered plants (Fig. 8a). A significant (P < 0.03) difference between both cultivars was observed (Table 1). The old cultivar GP exuded more CYT than the new cultivar BA (Fig. 8a). Significant moderate correlations were found between levels of CYT with EC (rs = 0.5, P = 0.01) and with ABA (rs = 0.4, P = 0.03). Negative correlations between CYT and aboveground (rs = −0.4, P = 0.04) and root biomass (rs = −0.5, P = 0.02) were observed.
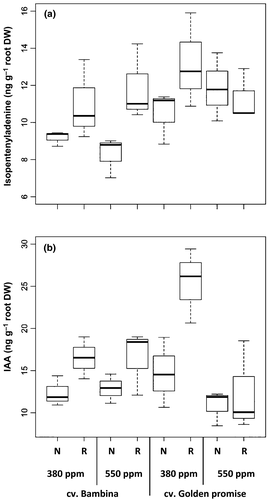
Concerning root exudation of auxins, a highly significant interaction between CO2 and cultivar was observed (Table 1). While under ambient [CO2], GP exuded more IAA than BA, the contrary was observed at eCO2 (Table 1, Fig. 8b). Similar to the CYT, the effect of water supply on the level of IAA was highly significant (P < 0.004) (Table 1). Under reduced water supply, plants exuded higher [IAA] than well-watered plants (Fig. 8b). No significant differences of cultivar on [IAA] were observed.
Auxins showed strong correlations with EC (rs = 0.8, P < 0.001), AA (rs = 0.8, P < 0.001), and ABA (rs = 0.8, P < 0.001) and similar to CYT, negative correlations with aboveground (rs = −0.5, P = 0.006), root (rs = -0.8, P < 0.001), and ear biomasses (rs = −0.5, P = 0.02).
Discussion
Effect of CO2 enrichment on root exudation in barley over time
At the early developmental stage (DC30), root biomass was significantly higher at elevated than at ambient [CO2]. Similarly, many studies have reported that root dry mass increases under eCO2 regardless of species or study conditions (Rogers et al., 1994). Nevertheless, the initial positive effect of CO2 enrichment on root biomass disappeared as the experiment progressed. Similar responses have also been observed in Phaseolus vulgaris (Haase et al., 2007). In barley, a rapid transition takes place from a vegetative green plant acquiring CO2 and nutrients from the soil, to a generative plant which mainly redistributes these assimilates from vegetative to generative tissues (Fangmeier et al., 2000). The effects of CO2 enrichment on RSR reported in literature are contradictory due to the complexity of accurately estimating underground biomass under diverse conditions (Madhu & Hatfield, 2013). Some studies have shown an increase in RSR due to CO2 enrichment (Day et al., 2013; Madhu & Hatfield, 2013), whereas this study observed a decrease in RSR at both developmental stages. The explanation for these discrepancies has been suggested to be related with crop type, resource supply, and other experimental factors (Rogers et al., 1996).
At DC30, total amino acid concentration in root exudates tended to decrease at eCO2, but this response was not significant. Concentration of AA in barley plants at eCO2 has been shown to be reduced in shoots but not in roots (Wang et al., 2013). Similarly, it has been demonstrated that root exudation of AA at early stages remains unchanged under different CO2 levels in P. vulgaris (Haase et al., 2007) and in rye grass (Phillips et al., 2006). At the later developmental stage (DC49), plants grown at eCO2 showed significantly lower levels of exuded AA than at ambient CO2. It has been reported that AA are lost from plant cells due to passive diffusion (Jones & Darrah, 1994). However, some studies show that AA composition of root extracts does not match with the composition in root exudates (Paynel et al., 2001; Lesuffleur et al., 2007). This could be due to differences in polarity determining membrane permeability. Moreover, plants have retrieval mechanisms which could be modified also by microorganisms (Neumann & Römheld, 2007). Furthermore, it has been suggested that AA export systems in roots can export a broad range of AA but with some selectivity for certain AA (Okumoto & Pilot, 2011). Similarly, a significant reduction in proline concentrations under eCO2 has been observed. Due to the lack of correlation between the total free amino acids and proline, other amino acids should additionally be analyzed.
Root exudates exhibited significantly reduced K concentrations at the early stage when plants grew under eCO2. Changes in mineral nutrients depend on the mineral and plant species (McGrath & Lobell, 2013). However, we have not observed a significant cultivar effect. At DC30, EC and ABA concentrations were significantly reduced in response to eCO2. It has been reported that hormones such as ABA can affect the membrane permeability (Vranova et al., 2013). Accordingly, EC and ABA have a highly significant positive correlation. However, at DC49, the effect of CO2 was no longer significant.
At DC30, no effect of CO2 on the concentration of cytokinins was observed. In contrast, at DC49, a significant effect of eCO2 on auxins was observed. At eCO2 and in both water treatments, higher ratios of CYT/ABA were found in both cultivars. However, higher ratios of IAA/ABA were observed only in BA.
In general, our results on the various exudates support the hypothesis that CO2 enrichment will affect the exudation of both cultivars. Furthermore, the effects of CO2 were more pronounced at DC30 and decreasing or disappearing at DC49. Accordingly, maize and pea have been reported to have very active exudation at early growth stages followed by a reduction with increasing age (Gransee & Wittenmayer, 2000). These results support the hypothesis that the CO2 effect will vary depending on the plant developmental stage.
Responses of root exudation to water deficit over time
At both stages, root biomass of both cultivars was significantly lower at reduced water supply than in well-watered conditions. However, plants with reduced water supply showed significantly higher RSR than well-watered plants. In our study, both shoot and root growths were retarded due to water deficit but the decrease in shoot biomass was higher (Schmid et al., 2016). There is a consensus that shoot growth is more sensitive to soil drying than root growth (Sharp et al., 1989).
There is growing evidence for crosstalk between different plant hormones resulting in different responses to biotic and abiotic stresses (Peleg & Blumwald, 2011). In well-watered conditions, auxins and cytokinins are present mainly in aboveground tissues, promoting stem growth and inhibiting root growth (Taiz & Zeiger, 2002). Auxins also play a role in drought tolerance: higher auxin concentrations promote the activity of the outward K channels in guard cells promoting stomatal closing (Acharya & Assmann, 2009).
Drought affects the hormone balance in plants, activating ABA synthesis and reducing CYT levels (Davies & Zhang, 1991). Similarly, in our results, the effect of ABA was more significant at the later harvest (DC49) where IAA was measurable and CYTs were no longer detectable. It is long-known that an increase in auxin concentration leads to a decrease in the CYT level (Benkova & Hejatko, 2009). Indeed, ABA and IAA were positively correlated in the present study. The observed high RSR under reduced water supply could be related to the accumulation of ABA in the roots, preventing the ethylene-induced inhibition of root growth, and to an insufficient ABA concentration within the shoots, reducing growth (Sharp & LeNoble, 2002). Moreover, it has been reported that higher levels of CYT promote survival under drought-stressed conditions but exert a negative effect on root growth (Werner et al., 2010). This is consistent with the higher CYT concentrations found under water-deficient conditions at DC30 and the higher significances in RSR at DC49 when CYTs were no longer detectable. ABA acts as a signal which roots send to leaves as the soil dries and causes stomatal closure reducing transpiration (Peleg & Blumwald, 2011) by means of releasing K ions from the guard cells (Acharya & Assmann, 2009). In our study, plants with reduced water supply exuded higher ABA and K than well-watered plants. This increase in K goes along with the increase in CYT concentration released by the roots of reduced-watered plants. Cytokinins are known to promote movement of nutrients (Taiz & Zeiger, 2002). Similarly, Shone et al. (1983) found higher K levels in root extracts of barley seedlings grown in nutrient solution with an osmoticum creating drought stress.
At both stages, no significant effects of water supply on AA were observed. Levels of exuded proline increased under reduced water supply, being significant only at DC49. Similarly, it has been reported that proline levels in roots differed at different developmental stages (Ober & Sharp, 1994; Chaparro et al., 2013). Moreover, an increment of ABA is required for high rates of proline accumulation in roots. This is consistent with the observed positive correlation of the two parameters at both developmental stages.
At both developmental stages, plants with reduced water supply showed significantly higher EC values than well-watered plants. This could probably be caused by membrane damage due to drought stress-induced lipid peroxidation and the consequent release of cell content (Jain et al., 2001). EC values were significantly correlated with hormone levels. Moreover, at DC30, EC was significantly correlated with K.
Our results support the hypothesis that watering level affects the root exudation pattern differently. The effects have been qualitatively similar between both developmental stages but more pronounced at DC49 than at DC30.
Effect of barley cultivar on root exudation
Genotypic variability in response to CO2 has been demonstrated in numerous studies. In our study, the old cv. GP presented higher CYTs (DC30) and auxin levels (DC49) than the modern cv. BA. These hormonal differences could be related to the higher RSR of GP than BA across all growth conditions and developmental stages in this study. CYTs were negatively correlated with root DW in BA, but not in GP. Indeed, a high density of roots at depth is a drought adaptation strategy (Volaire et al., 2014), and the RSR was higher in the dry treatment in the present study.
Drought resistance has been associated more with intraspecific than interspecific variability (Pérez-López et al., 2010; Poirier et al., 2012). However, no differences between cultivars were observed for root biomass, EC, K, AA, or ABA at any developmental stage. On the other hand, at both developmental stages, BA exuded significantly higher proline levels than GP. However at eCO2, no clear differences were observed between cultivars. In the literature, similar values of free proline in root exudates of two barley cultivars have been reported (Bandurska & Stroinski, 2003). At eCO2, BA showed higher K concentrations than GP. Although these results are not significant, they are in line with the lower stomatal conductance of BA found in the same experiment performed by Schmid et al. (2016).
Combined effects of elevated CO2, water level, and cultivar on root exudation
It has been reported that plants grown at eCO2 may in part compensate the negative effects of drought due to increased water-use efficiency. Although this has been shown in some studies (Chaudhuri et al., 1990; Chaves & Pereira, 1992; Morgan et al., 2004; Manderscheid et al., 2014), these interactions were absent in many other studies (Mousseau & Enoch, 1989; Grünzweig & Körner, 2001; Derner et al., 2003; Vaz et al., 2012; Medeiros & Ward, 2013; Perry et al., 2013). In our experiment, CO2 fertilization compensated for the negative effect of drought on grain yield and water-use efficiency at maturity, especially in GP (Schmid et al., 2016). However, we did not observe a mitigation of eCO2 of adverse drought effects on root exudation at DC30 or DC49. Nevertheless, more severe alterations in rainfall patterns (Hovenden et al., 2014) or drought may lead to eCO2–drought interactions.
Significant cultivar by CO2 interactions was observed only at the appearance of ears (DC49). The modern cultivar showed higher proline and lower auxin exudation levels at ambient CO2. The different concentration of phytohormones could affect the plant architecture. Similarly, cultivar by CO2 interaction was found in different wheat cultivars concerning tillering (Ziska, 2008). Therefore, these results support the hypothesis that the effect of CO2 level on root exudation differs depending on the cultivar. On the other hand, we have not found clear interactions between cultivar and water levels; therefore, we need to reject the hypothesis that the effect of water deficit will vary between cultivars. As only two cultivars and a mild drought stress were applied in present study, we cannot rule out that interactions between CO2 and water will be present in other cultivars or under extreme drought conditions.
With this study, we have gained deeper knowledge on the belowground responses of plants to global climate change, in particular rising [CO2] and drought, as there are significant effects of CO2 and water levels and, to a lesser extent, cultivar effects on root exudation. These effects vary with plant developmental stage. Aboveground plant responses may be translated to belowground responses, which can be altered by external stresses (Bais et al., 2006; Lakshmanan et al., 2014). Although it is difficult to draw generalizations from a comparison of only two cultivars, varieties responded differently in several root exudation traits to elevated CO2 in this study, but no differences between cultivars were observed under different water levels. We have not observed a strong mitigation of the adverse effects of drought by elevated CO2. We should thus be cautious in drawing conclusions about root exudation under changed climatic conditions. It has been suggested that the effects of certain stress combinations could depend on the genotype, and/or timing and intensity of the different stresses (Suzuki et al., 2014). Experiments with more cultivars, different soils, and more combinations of abiotic and biotic stresses should be performed. Furthermore, our exudate profiling was not exhaustive, and other root exudate constituents, such as sugars, phenolic compounds, and other phytohormones, should be analyzed. By understanding how biotic and abiotic factors affect root exudation processes, we may be able to predict whether plant–microbe interactions will enhance plant fitness and increase crop yields in a sustainable way under environmental changes.
Acknowledgements
The authors would like to thank Mrs. Sprich from Institute of Crop Science (Hohenheim University) for performing the hormonal analysis and the Institute of Plant Physiology and Biotechnology (Hohenheim University) for helping in the total free amino acid analysis. The authors declare no conflict of interest.




