Trait-based analysis of decline in plant species ranges during the 20th century: a regional comparison between the UK and Estonia
Abstract
Although the distribution ranges and abundance of many plant species have declined dramatically in recent decades, detailed analysis of these changes and their cause have only become possible following the publication of second- and third-generation national distribution atlases. Decline can now be compared both between species and in different parts of species' ranges. We extracted data from distribution atlases to compare range persistence of 736 plant species common to both the UK and Estonia between survey periods encompassing almost the same years (1969 and 1999 in the UK and 1970 and 2004 in Estonia). We determined which traits were most closely associated with variation in species persistence, whether these were the same in each country, and the extent to which they explained differences in persistence between the countries. Mean range size declined less in Estonia than in the UK (24.3% vs. 30.3%). One-third of species in Estonia (239) maintained >90% of their distribution range compared with one-fifth (141) in the UK. In Estonia, 99 species lost >50% of their range compared with 127 species in the UK. Persistence was very positively related to original range in both countries. Major differences in species persistence between the studied countries were primarily determined by biogeographic (affiliation to floristic element) and ecoevolutionary (plant strategy) factors. In contrast, within-country persistence was most strongly determined by tolerance of anthropogenic activities. Decline of species in the families Orchidaceae and Potamogetonaceae was significantly greater in the UK than in Estonia. Almost all of the 736 common and native European plant species in our study are currently declining in their range due to pressure from anthropogenic activities. Those species with low tolerance of human activity, with biotic pollination vectors and in the families referred to above are the most vulnerable, especially where human population density is high.
Introduction
Anthropogenic activities cause irreversible change to natural and semi-natural communities. It is well established that activities associated with the increasing density of the human population have caused declines in the sizes of populations of many species, in localized species extinctions, and therefore in contractions in species ranges', and in the establishment of invasive aliens (Drayton & Primack, 1996; Hooper et al., 2005; Kull & Hutchings, 2006; Isbell & Wilsey, 2011; Chown, 2012; Dullinger et al., 2013). Loss of biodiversity and of the ecosystem services and benefits provided by species is accelerating, potentially threatening the functional integrity of communities as a consequence of increasing anthropogenic impacts (Aguilar et al., 2006; Brook et al., 2008; Isbell et al., 2013).
Factors causing declines in range and abundance include habitat loss and fragmentation. The smaller sizes and greater isolation of populations following such disruptions place them at greater risk of further decline and local extinction (Joshi et al., 2006; Laanisto et al., 2013). In addition, the impact of invasive species (Powell et al., 2011), the decrease in numbers, or total loss, of pollinating species (Aguilar et al., 2006; Albrecht et al., 2012) and other plant symbionts (Wagg et al., 2011), soil degradation (Verbruggen et al., 2010) and many other factors have been shown to be responsible for local extinctions of plant populations. Furthermore, changes in land use and climate have altered the locations at which species can find both optimum and acceptable conditions for their continued existence (Brook et al., 2008), leading to formerly occupied sites becoming unsuitable, and necessitating the colonization of new locations.
Whereas much biogeographical research has focused on predicting changes in the overall distribution ranges and range boundaries of species and communities (Heikkinen et al., 2006; Peterson, 2011), research in conservation biology has often concentrated more on small scale changes, such as those occurring at the scale of single dots on distribution maps (Kujala et al., 2011). These are often records of species' occurrences at scales of anything from 1 to 10 km2. To date, it has been difficult to connect knowledge between these two approaches due to differences in spatial scale (Guisan et al., 2013) and lack of entailed biological information (Travis et al., 2013), but the recent publication of second- and even third-generation national atlases of species distribution maps (Tamis et al., 2005) now enables analyses of changes in species' ranges between specified dates, and comparison of changes in range within regions or national territories for groups of species categorized according to their evolutionary history, ecological preferences and morphological, physiological and reproductive characteristics. To date, most analyses of changes in distribution ranges over time have focused on single countries or regions (e.g. the Netherlands (Tamis et al., 2005), Northamptonshire in the UK (McCollin et al., 2000), Thiérache in France (Van Calster et al., 2008) and Flanders in Belgium (Van Landuyt et al., 2008). To our knowledge, apart from a comparison of range decline in the orchid species common to Estonia and the United Kingdom (Kull & Hutchings, 2006), only one comparative analysis of changes in species' distributions has been carried out involving species common to the floras of more than one region or country (Powney et al., 2014).
Although understanding of the limitations on species distributions is poor (Peterson, 2011; Araújo et al., 2013; Guisan et al., 2013), much is known about the evolutionary background of species, their ecology and their tolerance of anthropogenic influences. Trait information is, however, rarely taken into account when analyzing species distributions and their changes from a conservation point of view (van Kleunen et al., 2010; Wiens et al., 2010; Chown, 2012; Saar et al., 2012). Although accurate knowledge of the locations of populations has a significant role to play in informing conservation practices, forecasting the future dynamics of those populations from presence/absence data alone might not be reliable. The addition of trait-based information might significantly improve predictions of future behaviour (Peterson, 2011; Chown, 2012).
This study took advantage of the availability of second-generation national plant distribution atlases for the United Kingdom (hereafter the UK) and Estonia to examine the relationships between a wide range of species characteristics and change in species ranges. Our purpose was to identify traits and factors associated with change in distribution ranges and to determine whether their influences have been similar in these two countries. The national floras of the UK and Estonia provide a unique opportunity to gain ecological insights into the factors underlying species range changes for the following reasons. Firstly, the national floras have a large number of species (and a high proportion of their total species) in common, enabling direct comparison of the proportional changes of many species ranges in both countries. Secondly, there have been significant differences in the pressures to which natural and semi-natural vegetation has been exposed in the two countries. In particular, the UK has a very high mean human population density, and the infrastructure supporting this population has resulted in considerable habitat fragmentation and isolation of the remaining patches of natural and semi-natural vegetation, many of which are suffering considerable biodiversity loss (Thomas et al., 2004; Walker et al., 2009). In contrast, Estonia has one of the lowest mean human population densities in Europe, and, after the collapse of the USSR in 1991, agriculture and forestry became less intensive and the rate of wetland drainage declined (Kull & Hutchings, 2006; Kurganova et al., 2014). There was, for example, a 20-fold increase in the area of unused former arable land between 1991 and 1995 (Peterson & Aunap, 1998) (see Table 1 for further comparison of the UK and Estonia). Species range declines and loss of biodiversity to date in Estonia might therefore be expected to be less than in the UK. It is already known that mean percentage decline of the distribution range of orchid species in the UK during the second half of the 20th century was twice as high (49.7%) as for orchid species in Estonia (25.0%; Kull & Hutchings, 2006). The difference in decline for the 30 orchid species common to both countries was also significant (mean for the UK = 52.0%, mean for Estonia = 28.5%) (Kull & Hutchings, 2006).
| UK | Estonia | |
|---|---|---|
| Area (km2) | 243 610 | 45 227 |
| Population (millions) | 63.18 | 1.29 |
| Mean density of human population (km−2) | 662 | 29 |
| Land use (% of total area) | ||
| Agriculture | 80 | 29 |
| Forest | 7 | 50 |
| Wetland | 2 | 13 |
| Urban | 11 | 8 |
| Latitudinal range °N | 49–60 | 57–59 |
| Annual precipitation (mm) | 850 | 650 |
| Nr of rainy days per year | 133 | 115 |
| Annual temperature °C | 9.7 | 5.2 |
| Temp. of warmest month °C | 16.3 | 16.4 |
| Temp. of coldest month °C | 4.1 | −5.7 |
Species distributions depend on a wide variety of factors, ranging from life-history traits to their responses to various abiotic environmental characteristics (Chown, 2012). In social sciences, the standard method of projecting population dynamics (e.g. population projections made by national census bureaus, http://www.census.gov/population/projections/) is based on analysis of a wide variety of information about the individuals in the population, including age, sex, ethnicity, income and education. A similar approach is often used in evolutionary population and community ecology, where evolutionary stable strategies or stable stage distributions are calculated based on the most general traits describing the sample (Williams et al., 2011). As temporal population projections are already available, from successive distribution atlases, from two contrasting countries for a large number of the same species, we can apply the same method to investigate the main factors behind the changes in distribution of those species. For this purpose, a range of plant traits was selected for use in analyses. These were the evolutionary age and origin of the species, their average size, their tolerances towards potential environmental stressors, the extent to which they are dependent upon other species (e.g. pollinators), their reproductive strategy and their tolerance of anthropogenic influences. Comparative analysis of such traits, for all the species in a single model (for details of traits and related hypothesis see Methods), allowed us to determine the main traits and domains of traits associated with variation in species persistence. We use the term ‘domain’ to signify groups of traits that were considered likely to be associated with range decline.
Our study had three main goals: (1) to analyze and compare species range changes during the second half of the 20th century in the UK and Estonia. Besides documenting the overall changes, we examined whether changes in persistence, both for the whole flora and for individual species, were comparable between countries. (2) To determine the traits and domains of traits that are most closely associated with differences in species persistence and whether these are the same in both countries. (3) To analyse and compare persistence at the level of plant families between the countries. We describe the traits and domains of traits selected for analysis, together with the specific hypotheses examined in connection with each of them in the Methods section below.
Materials and methods
The United Kingdom and Estonia are both situated in northern Europe. The UK is about 30 degrees west of Estonia, but there is latitudinal overlap between the two countries, with Estonia situated at similar latitude to northern Scotland and the Orkney Islands. The land area of the UK is more than five times that of Estonia, and its mean human population density is more than twenty times as high (Table 1). About 3000 vascular plant species have been recorded in the UK and Ireland (Preston et al., 2002). The number of native species has been reported to be 1254 (Thomas et al., 2004), 1446 (Pilgrim et al., 2004) and 1515 (Crawley et al., 1996). The Estonian flora consists of 1538 native species (Kukk, 1999; Kull et al., 2002) plus 880 alien species (Ööpik et al., 2013).
The databases used in the study were the New Atlas of the British and Irish Flora (Preston et al., 2002) and the Atlas of the Estonian Flora (Kukk & Kull, 2005). Note that data for the Republic of Ireland were excluded; only the data for the UK were used in all the analysis. In both atlases, the presence of species was recorded on maps in 100 km2 grid squares. The two survey periods for which distribution maps were prepared were 1930–1969, and 1987–1999 in the UK and 1921–1970 and 1971–2004 in Estonia. Thus, there was an interval of 30 and 34 years between the ends of the first and second survey periods in the UK and Estonia, respectively, and the ends of the two survey periods corresponded closely in both countries. For each species in each country, persistence in distribution range was calculated as the percentage increase or decrease in the number of grid squares occupied by the end of the second survey period compared with the number of grid squares occupied by the end of the first survey period.
The available data do not include information on the abundance of species in each grid square. For practical purposes, in large-scale comparative analyses of changes in distribution ranges such as this study, it has to be assumed that populations of all species are equally detectable in all habitats and in all grid squares (Kéry, 2004). The number of species available (i.e. species for which there were distribution maps for both survey periods) from the initial data set was 1411 species in the UK and 1115 species in Estonia. The number of species common to both the UK and Estonia, for which data on changes in distribution range were available, was 736 (see Appendix S1 for species list). Pairwise comparisons of changes in distribution range in the two countries were carried out for all of these species.
Persistence, and differences in persistence between the two countries, calculated as persistence in Estonia minus persistence of the same species in the UK, were analysed using Type III analysis of covariance (ancova) in the GLM procedure in statistica 8.0 (StatSoft, Inc., Tulsa, OK, USA). We used this approach because of analytical transparency and lack of bias, and because the results produced can be readily comprehended by the widest possible audience. To examine persistence in plant families, we used two-way anova Type III, with family and country as factors, and we performed post hoc comparisons (Fisher LSD test).
To relate species persistences in Estonia and UK to species traits and variables, we used the redundancy analysis procedure (RDA) of multivariate analysis. RDA is an extension of multivariate linear regression for a multivariate response variable (Lepš & Šmilauer, 2003), with the parametric test replaced by a Monte Carlo permutation test to overcome problems with distributional characteristics. RDA enables visualization of the main trends in the data. Variables and trait states (for qualitative variables) that contributed significantly to explaining persistence were selected by forward selection (based on 499 permutations). The RDA analysis and graphical presentation of the results were carried out using the Canoco 4.56 and CanoDraw programs (Ter-Braak & Šmilauer, 2002).
Our analysis included a wide variety of variables, ranging from historical factors, such as species origin, to functional traits such as plant height. We assigned each variable into one of several larger and more comprehensive domains related to evolutionary history, adaptation, ecological preference and tolerance of anthropogenic activities and disturbance, each of which might play an important role in determining species persistence. In addition, the analysis of larger sets of interconnected variables enables determination of the key factors, both within and between the domains, having the most direct effect on species persistence. This approach also allows the domains causing the greatest changes in distribution, both within and between countries, to be identified. Appendix S2 gives details of each variable, the qualitative or quantitative categories into which each variable was divided, and the number of species that fell within each category. The domains of traits, with descriptions of the individual traits included, their relevance to the analysis of species distribution change and related hypotheses, were as follows:
Evolutionary history domain: Species age and origin
Floristic element
The geographic origin of the species, based on Hultén's distribution atlas (Hultén, 1971). Data were extracted from the List of Estonian Vascular Plants (Kukk, 1999: summary in English: http://www.zbi.ee/~tomkukk/nimestik/english.htm).
Age
The evolutionary age (in millions of years) of the crown group of the family. Data were extracted from the Angiosperm Phylogeny Webpage (http://www.mobot.org/MOBOT/research/APweb/) and Pryer et al. (2004).
The information included in this domain is essentially historical in nature; age and geographic origin of species can be considered the basis for their ecological preferences (Pärtel et al., 2007; Araújo et al., 2013; Marske et al., 2013). Niche conservatism– that is the degree to which taxa retain their ancestral ecological traits and geographical distributions– can influence their current habitat and environmental preferences (Wiens & Graham, 2005; Wiens et al., 2010; Crisp & Cook, 2012), and significantly affect plant species' success at a local scale, whether by limiting their capacity to reproduce (Laanisto et al., 2008), the ranges of species-specific pollen vectors (McLeish et al., 2011), or some other functional property.
Species age and origin do not in themselves provide information about species that will influence their persistence over ecological timescales. These factors, especially age, would be expected to have little influence on species persistence in either country. On the other hand, climate change may not yet have affected persistence significantly, but the evolutionary origin of species and their persistence might be related. For example, it has been shown that the ranges of species from some floristic elements, such as arctic-alpine species, have been in decline for some time (Lesica & McCune, 2004); we therefore hypothesized that the time interval between the end of the two surveys in each country might reveal changes in persistence related to the floristic element to which species belong.
Adaptation domain: Qualitative life-history traits
Strategy
Plant strategies based on their tolerance of stress and disturbance, and their ruderality. Species were classified into three main strategy types – competitors (C), stress tolerators (S) and ruderals (R) and four intermediate strategies (CS, CR, SR and CSR). Data were extracted from Grime et al. (2007).
Diaspore type
Generative diaspores (units of dispersal) may be seeds or seeds either embedded in additional structures or with additional structures attached. Data to describe diaspore type were obtained from the BiolFlor database (http://www2.ufz.de/biolflor/overview/merkmale.jsp).
Pollen vector
Pollen vector or mode of pollen transfer. Data were obtained from the BiolFlor database (http://www2.ufz.de/biolflor/overview/merkmale.jsp) (Mode of fertilization, as opposed to mode of pollen transfer, is considered within Ecological preferences domain).
A key requirement for understanding the processes behind vegetation change is the availability of functional autecological data about the species involved (Hodgson et al., 1999). Qualitative life-history traits and trade-offs, as reflected in CSR plant strategy categories, are useful reflections of species fundamental niches (Tilman, 1994; Kneitel & Chase, 2004; Hubbell, 2005; de Bello et al., 2013), and determine, in broad terms, species′ competition strategy.
All factors in this domain are qualitative in nature and they are essentially trade-offs. Plant strategy reflects behaviour in the established phase of the life history of the species and is based on a trade-off between tolerance of stress and tolerance of disturbance (Hodgson et al., 1999; Grime et al., 2007). Selection of strategy is based on long-term presence in, and adaptation to, particular habitat types. Plant strategies can therefore indicate whether local vegetation composition conforms with expectation under current land use and climatic conditions, and enable us to predict changes in vegetation if conditions alter (Lepš et al., 1982; Hodgson et al., 1999; Vicente et al., 2013). We hypothesized that fragmentation and destabilization of habitats due to anthropogenic activities will be reflected in different plant strategies influencing species persistence, especially in the UK, where human density is far greater than in Estonia.
Similarly, diaspore type and pollen vector are determined by the long-term dynamics of the habitats species occupy and can provide information on traits that have been successful in promoting persistence during the interval between the two surveys in each country. For example, populations of plant species that use a limited range of animal species for pollination and seed dispersal could be at greater danger of extinction as a result of habitat fragmentation and disturbance, than species that use wind to disperse their pollen and diaspores (Schleicher et al., 2011). On the assumption that disturbance due to human impact has been greater in the UK than in Estonia due to contrasting human population densities, we hypothesized that both diaspore type and pollen vector will have had a greater impact on persistence in the UK than in Estonia.
Ecological preferences domain: Quantitative and plastic traits
Height
Mean heights of species (cm) were obtained from the Flora of Estonia (Flora of Estonia SSR 1953–1984). The height of trees (38 species) and aquatic plants (37 species) was not included in this analysis because the inclusion of very tall species and floating species raised variance to an unacceptable level in the analysis.
Flowering phenology
This variable records the start, end and duration of the flowering period of species (in months). Data were obtained from the BiolFlor database (http://www2.ufz.de/biolflor/overview/merkmale.jsp).
Type of reproduction
Reproduction can be by seed, by vegetative propagation, or include both of these possibilities. Data were obtained from the BiolFlor database (http://www2.ufz.de/biolflor/overview/merkmale.jsp).
Plasticity
The capacity to alter phenotype in response to environmental conditions (e.g. being able to alter height, flowering for different periods and propagate either generatively or vegetatively) has been shown to have a significant positive impact on species persistence in the face of fluctuating environmental conditions (Lande, 2009), increasing human impact (Crispo et al., 2010) and changing climate (Nicotra et al., 2010).
Plant height is considered one of the strongest predictors of fitness (Falster & Westoby, 2003; Nicotra et al., 2010). It is one of the most plastic traits in response to changes in habitat conditions (Pan et al., 2013). Alteration of height can change many aspects of plant behaviour, including the balance between sexual and vegetative propagation, water use and light harvesting efficiency. It can also determine successional status (Falster & Westoby, 2003).
Climate change is strongly affecting plant phenology patterns worldwide (Anderson et al., 2012; Wolkovich et al., 2012). Shifts in the phenology of flowering caused by climate change can benefit species with long flowering periods, whereas species with shorter flowering periods may suffer, for example by losing synchrony with the critical periods of activity of their pollinators.
Type of reproduction affects local persistence of species in various ways, for example by altering competition (Zobel, 2008). While the ability to propagate vegetatively can sustain populations through adverse conditions, or allow species to re-establish quickly after severe disturbance, such effects of clonality normally manifest themselves only at a local scale (Laanisto et al., 2008). Wind-pollinated species usually have wider dispersal potential and are less pollen-limited than species that are pollinated by animals, yet their pollination success rate depends more on plant size (Friedman & Barrett, 2009). Self-pollination can be a very effective strategy over a short time frame, but, like vegetative propagation, it is inferior to sexual reproduction for genetic recombination, wide dispersal and resistance to parasites and viruses (Barrett, 1998). However, if the abiotic environment changes, self-pollination rates in plant communities tend to increase (Jones et al., 2013).
We hypothesized that these quantitative traits would play an important role in determining changes in species distribution ranges in both the UK and Estonia during the period under investigation, because species with greater phenotypic plasticity are expected to be better competitors in unstable and fragmented habitats (Callaway et al., 2003; Laanisto et al., 2008; Anderson et al., 2012).
Human influence domain
Urbanity
Urbanity reflects the ability of plant species to persist in urban areas. Species are categorized from urbanophile (species recorded mainly in cities) to urbanophobe (species recorded rarely in cities). Data were obtained from the BiolFlor database (http://www2.ufz.de/biolflor/overview/merkmale.jsp).
Hemeroby
Hemeroby is a measure of departure from naturalness of the habitats in which a species is recorded. Habitats and vegetation types are classified on a scale from ahemerobe (species occurring exclusively in natural habitats) to polyhemerobe (species occurring exclusively in non-natural habitats). Data were obtained from the BiolFlor database (http://www2.ufz.de/biolflor/overview/merkmale.jsp).
Nitrogen requirement
Ellenberg indicator value for species' nitrogen requirement was obtained from Ellenberg et al. (1992).
The ability to tolerate pollution, land use changes, habitat disturbance and fragmentation, and other stresses resulting from human activities, has become increasingly important for plant and other species (Garay-Narvaez et al., 2013). The formation of novel ecosystems and communities with altered soil conditions and invasive biota influences biodiversity and species composition at a variety of spatial scales (Tamis et al., 2005).
Some species tolerate proximity to human populations better than others (Bernhardt-Romermann et al., 2011), and such species are also more likely to be successful aliens (Dawson et al., 2012). Generating a suitable measure of species' tolerance of anthropogenic influences is difficult (Hill et al., 2002), and therefore, we also used an indirect indicator. The last glacial period destroyed almost all the high-nutrient habitats from northern parts of the northern hemisphere (Graham et al., 2003), including the UK and Estonia, and this caused the extinction of many species with high-nutrient requirements (Birks & Birks, 2004; Pärtel et al., 2007). Current human activities both generate nutrient-rich novel ecosystems and enrich existing habitats with nutrients (Hoover et al., 2012), so that plant that are adapted to grow on substrates with higher nutrient content are favoured, and plants with lower nutrient preferences are more prone to local extinction (Saar et al., 2012; Powney et al., 2014). Among the essential plant macronutrients, a low availability of nitrogen is generally associated with high plant diversity (Bobbink et al., 2010).
We hypothesized that all three traits in this domain would play significant roles in determining local plant population persistence both in the UK and Estonia. As the traits influence vegetation primarily at a local level, we also hypothesized that they would have a similar impact on species persistence in the two countries.
Results
Mean decline of the 736 species common to both the UK and Estonia during the period between the two surveys (approximately 30 years in both countries) was significantly lower (t = 5.2874; P < 0.0001) in Estonia than in the UK: average persistence (±SE) was 75.7 ± 0.008% for species in Estonia compared to 69.7 ± 0.008% in the UK.
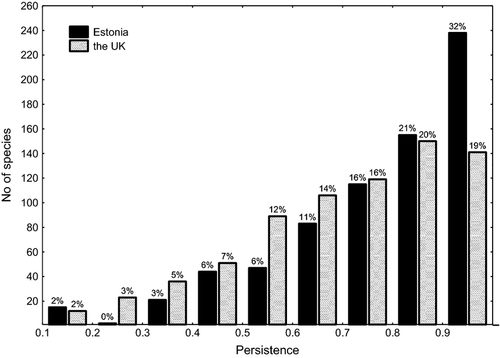
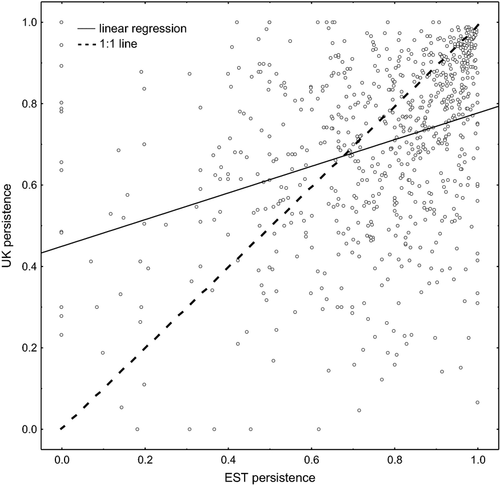
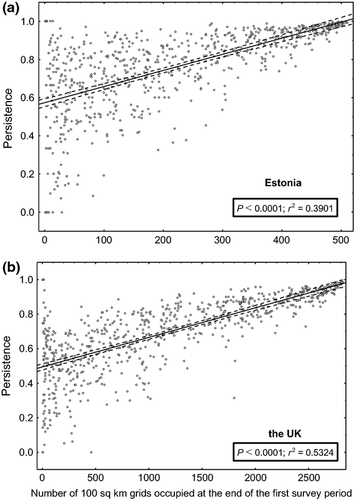
At the end of the second survey, nearly one-third of the 736 species in Estonia (239 species) were still recorded in at least 90% as many grid squares as they had been recorded in at the end of the first survey, whereas less than one-fifth of the species in the UK (141) had such high persistence (Fig. 1). In Estonia, 99 species (13.5% of the 736 species) had lost more than half of their local populations compared with 127 (17.3%) in the UK (Fig. 1); 13 species had apparently become nationally extinct in Estonia; and 5 had suffered the same fate in the UK. Comparing species persistence between the countries showed that 258 species had higher persistence in the UK, while 478 species had higher persistence in Estonia (Fig. 2). Persistence in both countries was strongly positively correlated with the number of grid squares occupied at the end of the first survey (Fig. 3).
Although we had predicted that species age and origin (the variables within the Evolutionary history domain) would not play a significant role in determining persistence, especially at a local (i.e. within countries) scale, affiliation to floristic element was a significant factor both at local and regional (i.e. between the two countries) scales (Tables 2-4).
| SS | MS | F | P | |
|---|---|---|---|---|
| Evolutionary history domain | ||||
| Age | 0.055 | 0.055 | 1.311 | 0.253 |
| Floristic element | 0.533 | 0.107 | 2.563 | 0.026 |
| Adaptation domain | ||||
| Strategy | 2.311 | 0.083 | 1.982 | 0.002 |
| Diaspore type | 0.014 | 0.014 | 0.343 | 0.558 |
| Pollen vector | 0.006 | 0.006 | 0.148 | 0.700 |
| Ecological preferences domain | ||||
| Type of reproduction | 0.048 | 0.048 | 1.153 | 0.283 |
| Height | 0.174 | 0.174 | 4.168 | 0.042 |
| Duration of flowering | 0.055 | 0.055 | 1.333 | 0.249 |
| Human influence domain | ||||
| N (Ellenberg) | 0.029 | 0.0289 | 0.693 | 0.406 |
| Hemeroby | 0.309 | 0.309 | 7.428 | 0.007 |
| Urbanity | 0.549 | 0.549 | 13.17 | 0.000 |
| Error | 25.272 | 0.0416 | ||
| SS | MS | F | P | |
|---|---|---|---|---|
| Evolutionary history domain | ||||
| Age | 0.014 | 0.014 | 0.469 | 0.494 |
| Floristic element | 0.342 | 0.068 | 2.371 | 0.038 |
| Adaptation domain | ||||
| Strategy | 7.793 | 0.278 | 9.647 | 0.000 |
| Diaspore type | 0.040 | 0.040 | 1.382 | 0.240 |
| Pollen vector | 0.069 | 0.069 | 2.387 | 0.123 |
| Ecological preferences domain | ||||
| Type of reproduction | 0.007 | 0.007 | 0.241 | 0.624 |
| Height | 0.004 | 0.004 | 0.139 | 0.709 |
| Duration of flowering | 0.013 | 0.013 | 0.458 | 0.499 |
| Human influence domain | ||||
| N (Ellenberg) | 0.002 | 0.002 | 0.063 | 0.802 |
| Hemeroby | 0.336 | 0.336 | 11.650 | 0.001 |
| Urbanity | 0.719 | 0.719 | 24.936 | 0.000 |
| Error | 17.484 | 0.029 | ||
| SS | MS | F | P | |
|---|---|---|---|---|
| Evolutionary history domain | ||||
| Age | 0.013 | 0.013 | 0.221 | 0.638 |
| Floristic element | 1.219 | 0.244 | 4.301 | 0.001 |
| Adaptation domain | ||||
| Strategy | 4.151 | 0.148 | 2.616 | 0.000 |
| Diaspore type | 0.006 | 0.006 | 0.110 | 0.740 |
| Pollen vector | 0.125 | 0.125 | 2.204 | 0.138 |
| Ecological preferences domain | ||||
| Type of reproduction | 0.015 | 0.015 | 0.265 | 0.607 |
| Height | 0.177 | 0.177 | 3.120 | 0.078 |
| Duration of flowering | 0.015 | 0.015 | 0.264 | 0.608 |
| Human influence domain | ||||
| N (Ellenberg) | 0.044 | 0.044 | 0.785 | 0.376 |
| Hemeroby | 0.001 | 0.001 | 0.012 | 0.916 |
| Urbanity | 0.012 | 0.012 | 0.220 | 0.640 |
| Error | 34.346 | 0.057 | ||
Of the variables in the Adaptation domain, plant strategy had a significant effect on both within- and between-country differences in persistence (Tables 2-4). Contrary to our prediction, neither diaspore type nor pollen vector had a significant impact on persistence in any analysis. Neither the set of variables associated with plasticity in the ecological preferences domain nor those reflecting the sensitivity of species to human impact in the human influence domain were associated with differences in persistence between the two countries (Table 4). Of the individual variables within these domains, plant height had a significant impact on persistence in Estonia (Table 2), but not in the UK. Hemeroby and urbanity had significant effects on persistence both in Estonia (Table 2) and in the UK (Table 3). The Ellenberg number for nitrogen was not significantly associated with persistence in any analysis.
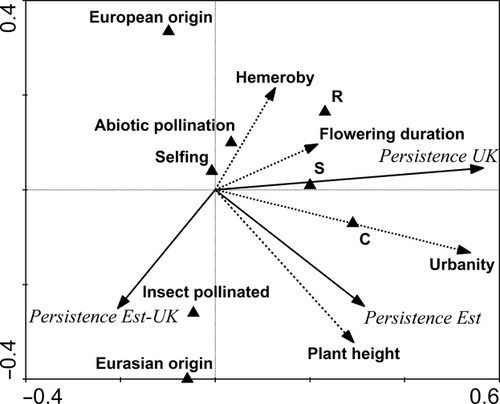
Ten variables and trait states were selected by RDA as predictors of species persistence (Fig. 4). A substantial proportion of the variability remained unexplained by the variables we selected for study. The first axis explained 15.4% of the variation in the data (F = 131.6, P = 0.002), while the second axis explained an additional 4.2% of the variation. The first axis was associated with variables related to persistence as reflected by the complete set of data, whereas the second axis revealed variables associated with differences in persistence between the two countries. Persistence in both countries was positively related to urbanity, with more persistent species tending to be C-strategists, or to have intermediate strategies involving competitiveness. Taller species also displayed greater persistence, especially in Estonia. Differences in persistence between the countries were linked to hemeroby, ruderality, pollination type and floristic origin. Species associated with disturbed habitat persisted better in the UK than in Estonia. Species with pollination syndromes not involving other species (i.e. species with wind, water or self-pollination) had higher persistence in the UK, whereas insect-pollinated species had higher persistence in Estonia. Species of European origin were more persistent in the UK, whereas species of Eurasian origin were more persistent in Estonia.
Most pairwise comparisons of persistence made at a family level showed similar patterns in both countries (Fig. 5). In most families with 15 or more species representatives in both countries, persistence was close to the overall mean persistence for all species. However, species in the Orchidaceae and Potamogetonaceae had significantly greater mean persistence in Estonia compared to the persistence in the UK (Fig. 5).

Discussion
Our results confirm widespread net reduction in plant species distribution ranges. Of the 736 species common to both countries on which our analyses are based, there was on average nearly 25% loss of national range in Estonia and more than 30% loss in the UK during approximately 30 years. The distribution range of all other species declined in both countries (Figs 1 and 2). The latter result is of particular concern because our data set comprises the core of the native flora of both countries. The species represented have wide regional distributions (the distance between the UK and Estonia is roughly 2000 km). While several previous studies (e.g. Lavergne et al., 2005; Kull & Hutchings, 2006) have shown that rare species, including many for which conservation management plans have been developed, have lost territorial range, this study demonstrates that the ranges of more common, regionally widespread plant species are suffering from the changes in climate and human impact that have taken place during the 20th century.
On average, species with small national distribution ranges had higher persistence in Estonia than in the UK (Fig. 3). While a considerable number of species had high persistence in Estonia, irrespective of the number of grid cells they occupied at the end of the first survey period (Fig. 3 upper graph), examples of such species were virtually absent in the UK (Fig. 3 lower graph). This difference between the countries suggests that suitable habitats for populations of species to survive in are more scarce in the UK and that human influence on vegetation composition is more direct in the UK than in Estonia.
Contrary to our expectations, one of the variables in the Evolutionary history domain – namely species affiliation to floristic element – played a significant role in explaining differences in persistence both within and between the countries (Tables 2-4). Significance of the effect of floristic affiliation confirms the importance of large-scale processes in affecting vegetation (Pärtel et al., 2007). The often intangible processes operating across large spatial and temporal scales can provide valuable information for predicting the effects of global change on vegetation and enable a distinction to be made between anthropogenic and natural causes of changes in local and regional distributions (Huston, 1999). In agreement with results obtained by Lesica & McCune (2004), our data showed that species from the arctic-alpine floristic element had lost the highest proportion of their range in both countries (mean persistence for both the UK and Estonia was only ~62%). RDA analysis further indicated that whereas species with Eurasian origin have greater persistence in Estonia, species originating from Europe have greater persistence in the UK (Fig. 4). This may be because the range limits of Eurasian species are more commonly reached in the UK, whereas those of European species are more often reached in Estonia.
The effects of functional traits, both qualitative and quantitative (i.e. the Adaptation and ecological preferences domain), on persistence agreed with our expectations (Tables 2-4; Fig. 4). In recent years, functional trait diversity has been strongly linked with plant distribution and is regarded as a key factor regulating diversity dynamics (Suding et al., 2005; Flynn et al., 2011). Decline in the abundance of specialist species and expansion of the ranges of generalists has been described as the most crucial reason for loss of plant and animal diversity in terrestrial and marine ecosystems (Helm et al., 2009; Clavel et al., 2011; Potts et al., 2010; Ozinga et al., 2013). While plant height was positively related to persistence in Estonia (Table 2; Fig. 4), species with longer flowering periods displayed greater persistence in the UK (Fig. 4). Moreover, whereas insect-pollinated species had higher persistence in Estonia, species with abiotic pollination had higher persistence in the UK (Fig. 4). These results reflect clear differences in the impact of anthropogenic influences between the two countries. In Estonia, taller species have higher persistence. Such species are more successful at attracting pollinators in fallow habitat (Janeček et al., 2013; Otsus et al., 2014), the abundance of which increased dramatically after the collapse of the USSR (Peterson & Aunap, 1998). In the far more densely populated UK (see Table 1), the relative scarcity of pollinator species is reflected in species with abiotic pollination, and those with long flowering periods that maximize the probability of insect pollination, having greater persistence (Pickering & Hill, 2002; Potts et al., 2010).
There were significant effects of plant strategy on persistence, both within and between countries (Tables 2-4; Fig. 4). Although some studies have shown that plant CSR strategy types (sensu Grime et al., 2007) carry signals of niche conservatism (Knapp et al., 2008), relationships between phylogeny and strategy are rather weak (Pavoine & Ricotta, 2013). As predicted, the RDA model showed that both stress tolerators and ruderals had higher persistence in the UK, where anthropogenic disturbance is higher, than in Estonia. In addition, the ability to propagate vegetatively did not influence persistence, even though clonal species are known to have higher survival in disturbed and anthropogenically influenced habitats (Fahrig et al., 1994; Saar et al., 2012).
The variables hemeroby and urbanity in the human influence domain significantly influenced persistence in the UK and Estonia (Tables 2-4). The RDA model indicates that hemeroby is linked with differences in persistence between the two countries, while urbanity is positively related to persistence in both the UK and Estonia (Fig. 4). As predicted, species that are intolerant of human influence are less persistent, especially in the UK. The contrasting effects of hemeroby between the countries are probably caused by differences in persistence in two families, Orchidaceae and Potamogetonaceae, with large numbers of species common to both countries (Fig. 4). Species in both families have very low values of hemeroby.
Our data showed that the evolutionary age of lineage of descent is not only negatively related to species hemeroby (r = −0.148; P < 0.0001) and urbanity (r = −0.165; P < 0.0001) indices, but also negatively related to Ellenberg nitrogen number (r = −0.084; P = 0.024). Despite this, evolutionary age itself was not a significant explanatory variable in any analyses, implying that while species belonging to younger lineages tolerate human activities better than those of older lineages, the relatively recent increase in anthropogenic activities is yet to have a detectable influence on plant species persistence at a phylogenetic level.
Both the UK and Estonia have ancient traditions of herding domestic animals in pastures, and this type of historical human influence, and the historical connectivity between pastures, has had a lasting positive effect on local plant species diversity (Helm et al., 2006). Even when traditional land use has changed and the habitats associated with these activities have declined in abundance, local species extinctions are often not seen for a considerable time. For example, the payment of extinction debts in temperate habitats can take anything from 50 to 100 years or more (Vellend et al., 2006; Kuussaari et al., 2009). Despite current conservation measures and subsidy of traditional land use methods, the general trend of local population extinction might be irreversible, and communities may become more and more species poor and similar (Clavel et al., 2011), both taxonomically and functionally. Given that plant species extinction debt has been clearly demonstrated in regions where landscape changes are taking place (Körner & Jeltsch, 2008), the decline of species ranges reported in this study may be only the start of a process that has a long way to run before completion.
In addition to the roles of human and climatic impacts, the fact that almost all the species examined in this study are declining in range can be partly explained by the fact that only native and archaeophyte species were included in the sample. The influx of many alien species into Europe has taken place only since the 1970s (Pyšek et al., 2009). Consequently, we were unable to analyse the effect of the arrival and range expansion of alien species on the persistence of common native species in the UK and Estonia. Other studies have shown that the effect of invading alien species on the range of native species can be highly negative, especially because traits such as size, fitness and growth rate of invasive species tend to be significantly higher than those of native species (van Kleunen et al., 2010). Nevertheless, even the distribution range of Elodea canadensis, an invasive aquatic species that qualified for inclusion in our data set, has declined by 13% in Estonia and by 20% in the UK over the studied period.
For the Orchidaceae (cf. Kull & Hutchings, 2006) and Potamogetonaceae, mean local extinction was significantly higher in the UK than in Estonia (P ≤ 0.01); similar trends for Apiaceae, Asteraceae, Caryophyllaceae and Fabaceae were significant at a greater P-level (P ≤ 0.1; Fig. 5). Mean persistence in each of the other families with 15 or more species in our study sample was very similar in the UK and Estonia. Apart from the Orchidaceae, many species of which are rare and protected in Europe, and the Potamogetonaceae, of which many species have specific habitat requirements, persistence varied considerably in these well-represented families. Most of these families include species capable of occupying a wide range of habitat types, but with the exception of Juncaceae, higher family level persistence was found in Estonia (Fig. 5).
Our examination of persistence of plant species in the UK and Estonia excluded rare, specialized and narrowly distributed species that occur only in one or the other country. Most of the species included in our analysis are rather widely distributed throughout Europe, and they are therefore probably more persistent over ecological time scales. This may explain why evolutionary adaptations and ecological preferences did not make a significant contribution to explanation of differences in persistence between the two countries.
Comparisons of plant distributions during the second half of the 20th century demonstrated that widespread and common European plant species are declining in range by, on average, 30% in the UK and 25% in Estonia. Species’ affinity for urban habitats (urbanity) and tolerance of non-natural conditions (hemeroby) were both significant factors explaining species persistence within the countries. However, despite very large differences in mean human population density and land use between the two studied countries, differences in persistence between the UK and Estonia were not primarily affected by species' tolerance towards human activities, or by their mode of propagation or height. Floristic element affiliation and CSR strategy type both influenced persistence. A competitive strategy, a more local floristic elemental origin (Eurasian in Estonia and European in the UK) and tolerance of human influences promote local persistence of plant populations. More detailed studies are now needed to evaluate in greater depth the way in which these key factors affect local and regional persistence, their inter-relationships, and the persistence of more narrowly distributed and rarer species in the face of changes in vegetation, climate and human activities.
Acknowledgements
The work of LL, TK and MS was supported by institutional research funding IUT 21-1 of the Estonian Ministry of Education and Research; we also acknowledge the support of the herbarium TAA. Additional funding for LL was provided by the Estonian Research Council grant PUT (607) and the European Commission through the European Regional Fund (the Center of Excellence in Environmental Adaptation). PM was supported by MSMT LM2010009 CzechPolar. We thank Meelis Pärtel and two anonymous referees for valuable comments. The authors declare no conflict of interest.




