Prey switching as a means of enhancing persistence in predators at the trailing southern edge
Abstract
Understanding the effects of climate change on species’ persistence is a major research interest; however, most studies have focused on responses at the northern or expanding range edge. There is a pressing need to explain how species can persist at their southern range when changing biotic interactions will influence species occurrence. For predators, variation in distribution of primary prey owing to climate change will lead to mismatched distribution and local extinction, unless their diet is altered to more extensively include alternate prey. We assessed whether addition of prey information in climate projections restricted projected habitat of a specialist predator, Canada lynx (Lynx canadensis), and if switching from their primary prey (snowshoe hare; Lepus americanus) to an alternate prey (red squirrel; Tamiasciurus hudsonicus) mitigates range restriction along the southern range edge. Our models projected distributions of each species to 2050 and 2080 to then refine predictions for southern lynx on the basis of varying combinations of prey availability. We found that models that incorporated information on prey substantially reduced the total predicted southern range of lynx in both 2050 and 2080. However, models that emphasized red squirrel as the primary species had 7–24% lower southern range loss than the corresponding snowshoe hare model. These results illustrate that (i) persistence at the southern range may require species to exploit higher portions of alternate food; (ii) selection may act on marginal populations to accommodate phenotypic changes that will allow increased use of alternate resources; and (iii) climate projections based solely on abiotic data can underestimate the severity of future range restriction. In the case of Canada lynx, our results indicate that the southern range likely will be characterized by locally varying levels of mismatch with prey such that the extent of range recession or local adaptation may appear as a geographical mosaic.
Introduction
The consequences of anthropogenic climate change for species include phenological changes (Parmesan & Yohe, 2003), localized extinctions (Sinervo et al., 2010), increased hybridization (Garroway et al., 2010) and latitudinal and altitudinal range shifts (Moritz et al., 2008; Pöyry et al., 2009). Understanding the effects of climate change on species persistence has been the subject of extensive research (Heller & Zavaleta, 2009), although most studies focus on how populations are affected by changing climatic conditions at northern ranges (Pöyry et al., 2009; Angert et al., 2011). Conversely, past extinctions stemming from climate change have typically occurred at the lower latitudinal edge of a species range (Davis & Shaw, 2001). This lower latitudinal edge may define either the leading or trailing edge of a species range depending on the geographical pattern of speciation. While populations at the trailing edge of occurrence often contain important genetic diversity for species (Hewitt, 2004) and potentially represent local adaptations and ecotypes, leading edge populations are often less specialized and potentially demonstrate greater plasticity, and thus can influence a species ability to adapt to differing conditions (Dynesius & Jansson, 2000; Thuiller et al., 2008). Although a few studies have examined range shifts at the lower latitudinal edge of a population (Moritz et al., 2008; Jones et al., 2010), they have not provided insights into the theoretical possibilities of how species will persist at their lower latitudes (but see Morelli et al., 2012). Accordingly, a pressing need exists to understand the effects of climate change on species persistence at the lower latitude edge of their distribution (Hampe & Petit, 2005; Thuiller et al., 2008).
One potential complication in our understanding of how climate change will influence species at lower latitudes relates to biotic interactions. In temperate regions, lower latitude range edges should be more strongly influenced by biotic interactions in comparison to higher latitudinal edges that are constrained by thermal tolerances (MacArthur, 1972; Normand et al., 2009). Therefore, the ability to adapt to differing biotic conditions should influence survival and persistence probability at the lower latitudinal edge of occurrence, perhaps more-so than at the northern range edge (Thuiller et al., 2008). A range of biotic interactions influenced by climate change have been documented at the southern range edge of species distributions. These include, but are not limited to, increased importance of conspecifics on survival (Castro et al., 2004), increased spatial overlap with potential competitors (Bowman et al., 2005), and increased extinction risk due to declining resource abundance (Pearce-Higgins et al., 2010). However, distribution models used to predict future range patterns are almost always based on abiotic data and ignore the importance of biotic influences impacting the suitable habitat of a species (Kissling et al., 2012). This method has been questioned since the introduction of climate modeling (Davis et al., 1998) and the omission of biotic variables is labeled a key uncertainty in species forecasting (Lavergne et al., 2010). Moreover, the failures to account for biotic components have the potential to both over- and underestimate future extinction risk (Bateman et al., 2012; Schwartz, 2012). Despite this, climate projections that incorporate biotic interactions are uncommon (but see Bateman et al., 2012; Hof et al., 2012a), though these variables are typically included in nonclimate change niche and distribution models (Hellmann et al., 2012). Therefore, incorporating biotic interactions in climate envelope projection models should improve climate change predictions.
Projections that have included biotic drivers primarily utilize vegetative cover and focus on positive interactions with plants, providing support for their functional role in future range estimation (Schwartz, 2012). A handful of studies have incorporated prey information into the climate change projections of carnivores, but these tend to be limited in their spatial scale (Hof et al., 2012a). Future climate envelope models should have higher performance if they incorporate the full spectrum of species response to environmental change (Kissling et al., 2012). The distribution and abundance of specialist predators may be particularly affected by biotic interactions along the lower latitude edge, given that the distribution of their primary food source should fundamentally influence their lower latitude range limits in the face of climate change. If future patterns of primary prey suitability and predator suitability diverge (i.e., a climate change-driven ecological mismatch), then future range contractions for specialist predators will be more severe than predicted based on climatic variables alone. However, local adaptation may complicate this pattern in lower latitude populations, with some species potentially demonstrating less reliance on their primary prey (Roth et al., 2007). Yet, it is important to consider that such changes in prey selection may be expressed as a geographical mosaic (sensu Thompson, 2005), in that local prey availability across the southern distributional edge may favor different levels of dietary plasticity and adaptation. Regardless, we expect that the future persistence of specialist predators at their southern range edge will be influenced by both primary and alternate prey species, or the ability to demonstrate less reliance on preferred or primary prey species (Roth et al., 2007). It follows that incorporating future prey estimates into the projections of a specialist predator at the continent-wide scale should improve species specific predictions and provide a refined estimate of the uncertainty in models based solely on climatic variables.
Canada lynx (Lynx canadensis) is a mammalian carnivore in North America with a narrow environmental niche (Peers et al., 2012) despite a broad distribution encompassing Canada, Alaska, and portions of the contiguous United States (McKelvey et al., 2000). Lynx occurrence and abundance is linked both to the presence of boreal forest habitat and the density of snowshoe hares (Lepus americanus), their primary prey species (Mowat et al., 2000). This has led to the study of their decade-long population cycles, which are reported throughout most of their range (Krebs et al., 2001). However, lynx also rely on other prey species (e.g., grouse, ptarmigan, and squirrels, see Krebs, 2011), principally red squirrel (Tamiasciurus hudsonicus), which are an important species that can be temporally prevalent in the lynx diet (up to 72% of prey biomass) for short-time periods when hares are rare in the boreal forest (O'Donoghue et al., 1997) or in the southern range where hare numbers remain consistently low (Roth et al., 2007). This dietary plasticity provides a unique system to determine the effect that primary and alternate prey occurrence can have on predator persistence in the face of climate change.
In this study, we used the program MaxEnt (Phillips et al., 2006) to model current and future suitable habitat of Canada lynx, snowshoe hare and red squirrel in the southern range of the lynx distribution. We determine the change in available habitat using climate scenarios from 2050 and 2080, and then restrict these models to the future suitability of their primary and alternate prey. We predict greater range contraction and reduced habitat suitability for lynx with inclusion of predicted changes in their primary prey (snowshoe hare) than based on climate-only models. We also expect that the predicted range contraction will be further lessened by including information on alternate prey distribution (red squirrel). Given that hare account for less than half of lynx diet in the most southern portions of their range (Roth et al., 2007) and that the range of red squirrel extends beyond the southern limit of hare (Hall, 1981), we predict that increased reliance on squirrel for lynx persistence will be most pronounced closest to the southern limit of their southern range. Moreover, we expect that the percent of the southern range in which alternate prey availability is higher than primary prey availability will increase over time.
Materials and methods
Species data
We collected information on Canada lynx, snowshoe hare and red squirrel presence across North America using museum records from freely accessible databases (MaNIS; www.Manisnet.org) as well as from smaller museums that provided data through individual contact (see Table S1 for complete list). Specimen records from museums provided a locality description of the specimen along with the date of collection, which was converted to X/Y coordinates with an associated uncertainty, using the programs Biogeomancer (Guralnick et al., 2006) and Google Earth. During this step, localities with large uncertainty (ca. >13 km radius of uncertainty) were removed, as were those collected before 1940, to improve accuracy of the distribution models (Lütolf et al., 2006) and better match the time frame of our environmental data (see below). Presence records for species were supplemented with provincial harvest records (see Table S1 for list). Harvest records had varying levels of uncertainty, with some localities providing fine resolution for location (i.e., registered trapline), while others offered coarser resolution (i.e., township or county). As with the museum records, we excluded from further consideration all harvest records that had >13 km uncertainty and those that were collected before 1940. We also included a data set on lynx occurrence in the United States based on sightings, museum records, and other sources (see McKelvey et al., 2000; see 2.3 for details on correcting bias in presence records). To address minimal (<25) outliers, presence records that fell outside 100 km from the current species range were removed. This step was conducted to exclude data points that fell in habitats lost to urbanization or intensive agriculture, or data points with potentially spurious identifications. Species geographic ranges were determined using Natureserve (Patterson et al., 2007), which is an open access organization that provides information on distribution and abundance of species. Ranges were adjusted for the presence records that fell within 100 km outside of the range provided by this data source.
Environmental data
Climatic variables were obtained from the WorldClim database (Hijmans et al., 2005), which provides a variety of climatic data averaged over the years 1950–2000. An elevation layer was also obtained from the WorldClim database. We also included information regarding the ecoregion of each grid cell (United States Environmental Protection Agency, see Omernik, 1987). All environmental data were resampled to 10 km grid cell size to correspond to the accuracy of our species records. Given the large number of potential environmental variables (particularly, the bioclimatic variables), we included sets of bioclimatic variables that we thought a priori would be biologically relevant. In total, eight bioclimatic variables were included in the final MaxEnt modeling (annual mean temperature, maximum temperature of the warmest month, minimum temperature of the coldest month, temperature seasonality, annual precipitation, precipitation seasonality, precipitation of the wettest quarter and precipitation of the driest quarter), as well as ecoregion and elevation.
To model future environmental suitability, we used general circulation models (GCM): the Canadian Centre for Climate Modelling and Analysis model CGCM3, and the Commonwealth Scientific and Industrial Research Organization model CSIRO mk3.5. Under each GCM we used the A2 and B1 scenarios which represent the most and least severe atmospheric carbon content of all scenarios, respectively. Both scenarios revealed similar trends in model output and we used the A2 scenario to run our analysis (see below). Downscaled climate grids in bioclim format for the 10 year periods 2050 and 2080 for each GCM were downloaded from the Climate Change, Agriculture and Food Security (CCAFS) website (www.ccafs-climate.org). All future projections were resampled to 10 km grid cell size to match the current environmental data.
Model development
The program MaxEnt was used to create SDMs for each species. MaxEnt compares presence records with randomly selected points from the background to create maps of habitat suitability and determine the effect of environmental variables on species presence. MaxEnt assumes that sampling of presence locations is unbiased; biased sampling promotes model inaccuracy (Phillips et al., 2006). Presence records obtained from museum samples can be biased given collection patterns favoring areas near roads, higher human density and known areas of occurrence (Phillips et al., 2006). Use of harvest records adds additional spatial bias owing to jurisdictional differences in spatial extent of traplines and therefore location uncertainty. Provinces with very high resolution harvest records contributed more samples to our database of presence records (i.e., uncertainty in the exact location of the presence record could be assumed to be low, and therefore these records could be included in our analysis). To account for the spatial bias in presence records, we first reduced the unevenness in density of presence records by randomly subsampling our presence records so that a single record was included for every 900 km2 area. Bias in presence records was further addressed by creating a bias grid for use in MaxEnt modeling, following procedures outlined in Elith et al. (2010). The bias grid is used to down-weigh the importance of presence records from areas with more intense sampling (i.e., areas with a high density of presence records, Elith et al., 2010), and has been used previously in large-scale distribution modeling (Peers et al., 2012; Pickles et al., 2013). The weighting surface is calculated based on the number of presence records within a neighborhood around any given cell (weighted by a Gaussian kernel with a standard deviation of 200 km). The weighting surface was then scaled to a maximum of 20 and minimum of one to avoid extreme down-weighting of highly sampled cells (Elith et al., 2010). We developed MaxEnt models for all species using background records selected from Canada and the United States to include the full environmental range of the species, including areas reachable by the species (Phillips et al., 2009). The models were run without the threshold feature (which allows abrupt step-like relationships between response and predictor), to reduce the number of estimated parameters and to allow better understanding of the variable response curves for each environmental layer. Given the recent criticisms regarding the use of elevation as a predictor variable in MaxEnt modeling (Hof et al., 2012b), we ran current, 2050, and 2080 models for Canada lynx with and without elevation. Model outputs between the two approaches were qualitatively similar for all three models (see Fig. S1), and further climate projections were performed with models including elevation.
Performance of MaxEnt models was calculated based on Receiver Operating Characteristic Plots (ROC) and area under the curve (AUC) statistics. AUC values range from 0 to 1, with the value indicating the probability that a randomly selected presence point will have a higher suitability value from the model than a randomly selected location in the background. We performed a 10-fold cross-validation procedure (where 10% of presence records are set aside to test the model, and this process is repeated 10 times) to create the MaxEnt models and calculate AUC statistics. The average of the 10 models produced during the cross-validation was used to calculate model performance.
In each future projection, we included the environmental variables mentioned above, as well as ecoregion and elevation as static variables. To account for variation among GCMs we created a final projection environmental suitability map for each species by averaging habitat suitability estimates over all GCMs. This was repeated for 2050 and 2080.
Testing the predictions
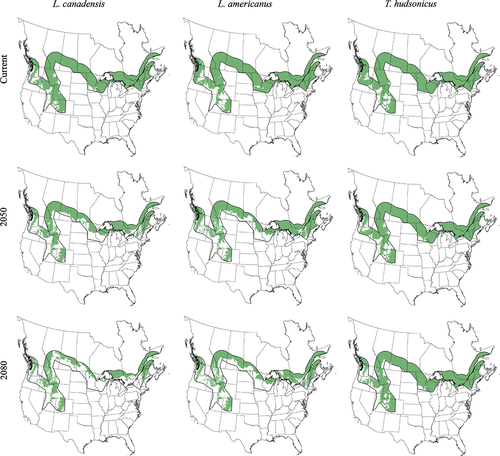
From the climate change projections, we used only suitable habitat projected in the southern range of Canada lynx. The southern range was considered to extend 300 km from the southern edge of the adjusted natureserve range map (see Fig 1), which corresponds to two times the average dispersal distance of Canada lynx (Poole, 1997). We assumed greater than 0.25 probability of presence was considered occupied, and anything lower was assumed unoccupied. This value relates closely to the lowest suitability value of the test data for all three species, which is a common method for changing continuous distributions into a binary presence/absence map (Phillips et al., 2006), and relates to the values used in further analysis (see below). We ran models for both future decades and calculated the total reduction in suitable environment for each species in the southern range of the lynx and the % of range lost from the current model.
We developed 10 additional models for Canada lynx for the years 2050 and 2080 that restricted lynx presence to varying combinations of prey occurrence. First, we divided the future prey ranges into two models representing the full range and core range of the species based on logistic output. For this, 0.25 probability of presence is considered the species full distribution, while 0.5 probability of presence is the range core. Although densities and habitat suitability values have only been partially correlated (Tôrres et al., 2012), these values should relate to substantial differences in abundance of each prey species and therefore core prey ranges should more greatly restrict lynx presence than the full prey ranges. We then restricted lynx presence to only those areas that were suitable for lynx and various combinations of prey full and core ranges. We calculated the total % of range loss from models that incorporate information on prey occurrence from the projections based only on climate data. We calculated these values for each of the 10 replicates in the 10-fold cross-validation to obtain an average with confidence intervals.
Predator–prey mismatch models
To help further test our predictions about the effects of prey on future lynx habitat, we developed a model of predator–prey mismatch for present conditions, 2050 and 2080 using the logistic output provided by MaxEnt for each species in the southern range of Canada lynx. This was accomplished by subtracting the logistic output values of the prey species model from that of Canada lynx. This creates a map of the southern lynx range that reveals areas where prey is saturated (occupancy values higher than lynx) and limiting (occupancy values lower than lynx). For each projection period, we calculated the percentage of the southern range where each prey species is saturated for lynx (i.e., areas of the mismatch model that have a negative value). We acknowledge that our measures of saturation and limitation are based on long-term averages of habitat suitability, and that this is an imperfect reflection of year-to-year variability or density differences between areas and species.
Results
SDMs and climate projections
After processing presence locations according to the appropriate uncertainty level and excluding points within the same 900 km2 grid, we had 997, 362, and 806 observations to model lynx, snowshoe hare and red squirrel distributions, respectively. The AUCpo (Yackulic et al., 2012) value for the lynx model was high (test: 0.851, train: 0.859), indicating the model successfully discriminated presence from background locations. AUC values were marginally lower for hare (test: 0.796, train: 0.827) and red squirrel (test: 0.817, train: 0.831), respectively, which is consistent with that expected for more generalized species with larger ranges (Elith et al., 2006). Maximum probability range was high for all three species with the largest values for hare and squirrel (lynx: 0.855 ± 0.007, hare: 0.971 ± 0.008, squirrel: 0.955 ± 0.003; average over 10 runs ± 95% CI). Based on jackknife estimates, annual mean temperature was the most influential variable for the Canada lynx model when considered alone, with the ecoregion variable also being highly influential. Annual mean temperature decreased the performance the most when omitted, indicating it had the most information not contained within the other predictor variables (Fig. S2a). Maximum temperature of the warmest month and ecoregion were most influential for the hare model, with elevation decreasing the performance the most when omitted (Fig. S2b). Ecoregion and maximum temperature of the warmest month were also the most influential variables for modeling red squirrel, with ecoregion reducing performance the most when removed (Fig. S2c).
The estimated current geographic suitability for lynx along their southern range shows high probability of occupancy throughout the Rocky Mountains, the northeastern United States and Canada and in the north central States and provinces (Fig. 1). The estimated range for snowshoe hare and red squirrel along the southern range edge is similar to that of Canada lynx in the Rocky Mountains and eastern United States. However, both prey species are projected further south in all areas except the Rocky Mountains (Fig. 1).
Single species models
Projections of the Canada lynx range at the A2 scenario for both 2050 and 2080 demonstrate a large reduction of habitat in the southern extent of their range (Fig. 1). Total southern range area was reduced by 29.14% and 50.98% in 2050 and 2080, respectively, when using the climate-only models (Table S2). Both snowshoe hare and red squirrel ranges were restricted similarly to lynx range under future climate scenarios (Fig. 1). However, red squirrel range was reduced the least with occupancy projected to persist beyond the Rocky Mountains and throughout Canada. Snowshoe hare range was reduced 26.12% and 37.48% for the years 2050 and 2080, respectively, in the southern lynx range. Red squirrel range experienced a reduction of 6.68% and 9.35% for 2050 and 2080, respectively (Table S2).
Testing the Predictions: predator with prey models
Consistent with our prediction that incorporating prey information into climate change projections reduces the estimated future range of a predator, projected range estimates for lynx were substantially reduced when restricted to suitable habitat for snowshoe hare and red squirrel. For the 2050 projections, lynx climate-only models were most limited when occupancy was restricted to areas that consisted of the core range for both prey species (−66.1 ± 1.1%; average percent range reduction ± 95% CI, see Fig. 2a or Table S3 for full result set). The least reduction in lynx range from the climate-only model happened when lynx were allowed to persist in the presence of either prey species’ full range (−0.56 ± 0.23%). The 2080 models demonstrated similar results. When compared to the climate-only model, the range was reduced the most when lynx occurrence was restricted to the core range of both species (−61.0 ± 1.4%; see Fig. 2b). Moreover, the least reduction occurred when the climate-only model was restricted to the full range of either species (0.036 ± 0.029%).
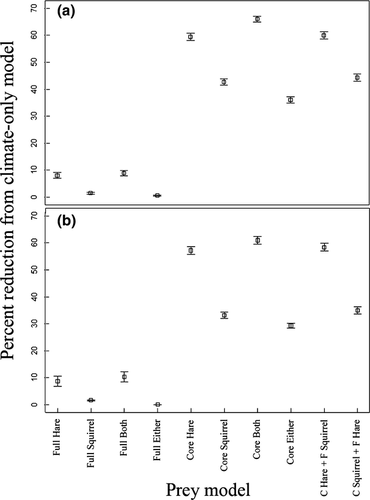
The results also support the prediction that lynx range loss can be alleviated by increasing their reliance on red squirrel. For 2050, all models that were restricted by red squirrel had a lower percentage range loss than the corresponding snowshoe hare model. Total range loss for lynx when restricted to full prey ranges (hare: −8.5 ± 1.0%, squirrel: −1.5 ± 0.4%), core prey ranges (hare: −59.4 ± 1.4%, squirrel: −42.7 ± 1.2%) and the core range of one species supplemented with the full range of the other (hare: −60.0 ± 1.4%, squirrel: −44.3 ± 1.3%) consistently demonstrated lower values with red squirrel as a main prey. For 2080, suitable environment for lynx when restricted to full prey ranges (hare: −8.7 ± 1.9%, squirrel: −1.7 ± 1.2%), core prey ranges (hare: −57.2 ± 1.4%, squirrel: −33.1 ± 1.2%) and the core range of one species supplemented with the full range of the other (hare: −58.4 ± 1.4, squirrel: −35.0 ± 1.3%) showed similar patterns. The values for all 10 models used in the study are provided in Table S3.
Predator–prey mismatch models
Examining differences in the mismatch models of hare and squirrel (Fig. 3) provides insight into the areas of the southern range that will require expression of plasticity or actual adaptation favoring reliance on squirrel, if lynx are to persist under climate change. Currently, areas saturated with hare are at the northern reaches of the southern range, caused by high hare suitability as opposed to low suitability for lynx. Squirrels are currently saturated in the more southern portions of the lynx range. In central areas like Wisconsin and Minnesota, the environment will be highly suitable for red squirrel in 2050 and 2080 in comparison to hare, and therefore dietary plasticity and possibly adaptation will be needed to ensure lynx persistence. However, although the south-eastern range (Maritime provinces, New England states) will be climatically unfavorable for lynx, both hare and squirrel will maintain suitable habitat and thus lynx will not be limited by prey availability in either 2050 or 2080. Most areas saturated with hare rather than squirrel under future scenarios occur in the northern regions of the southern lynx range. However, persistence close to the southern edge is increasingly dependent on squirrel. Furthermore, there is pronounced spatial variability in the degree of mismatch between lynx and hare occupancy across the southern distribution of lynx. Currently, 4.2% more of the southern lynx range is saturated with hares than squirrel. In 2050 and 2080, however, there is 35% and 33.6% larger range area saturated with squirrels than hare, respectively. This demonstrates that increased amounts of the southern lynx range will be predominated by squirrels than hare in comparison to present conditions.

Discussion
By focusing on climate change impacts along the southern range extent, we gain insight into how species will persist in their receding range edge. This topic has received limited attention to date, but is a focal area of interest given that strong biotic interactions at lower latitudes (Normand et al., 2009), and an inability to express plasticity or adapt to higher extreme temperatures (Araújo et al., 2013), may result in complex and unpredictable shifts in distribution. Our results demonstrate the strong influence of biotic interactions in determining future patterns and extent of a specialist predator at the southern range boundary. This effect is driven by an ecological mismatch between the predator and its primary prey that becomes aggravated with extended climate projections. Such mismatches may be a common phenomena for species that interact strongly across trophic levels (Post & Forchhammer, 2008; Saino et al., 2011; Pickles et al., 2013), and suggest that climate-only predictions of future range shifts may be inadequate. However, our results also reveal the complexities of incorporating prey information into future predictions for predators. In each scenario where lynx presence was restricted by red squirrel (see Fig. 2), range reduction was less severe under climate change than the corresponding hare scenarios. With changes in prey densities already being linked to future extinction risk of southern populations (Pearce-Higgins et al., 2010), these results demonstrate that species persistence in the southern range is dependent on their ability to utilize higher proportions of alternative prey. This is especially evident in models where lynx occurrence was restricted to core range of one prey species and full range of the other species. In the 2050 and 2080 scenarios, a 16% and 23% improvement in the hare model occurred when squirrel represented the core prey range.
Although lynx populations should be expressing increased phenotypic plasticity or adaptation in regards to diet breadth in the southern range, it remains uncertain the extent to which southern lynx populations can respond quickly to accommodate more alternate prey in response to declining primary prey (Murray et al., 2008). Recent research suggests that southern lynx populations have a more generalized diet than their northern counterparts, with less reliance on snowshoe hares (Roth et al., 2007), while northern lynx exhibit increased reliance on red squirrels during low hare densities (O'Donoghue et al., 1997); these findings further support the contention that lynx may respond dynamically to shifting availability of primary prey. Elsewhere (Elmhagen & Tannerfeldt, 2000; Hanski et al., 2001), dietary switching occurs in predator species with narrow niches, which can then translate to changes in individual fitness and population dynamics. Although we note that projections consisting of only core squirrel range may not be realistically habitable by lynx, any degree of shift should result in more suitable area under future global change. Indeed, to persist in changing climates, species can either express phenotypic plasticity or else show actual adaptation (Chevin et al., 2010), and southern lynx populations are known to be more generalized in their habitat selection behavior than previously thought (M.L. Hornseth, unpublished results). Likewise, habitat shifts have already been documented at the southern range edge of other species (Pulido et al., 2008), and studies focusing on the future status of southern range populations should address not only habitat change but also whether regional variability owes to plasticity or adaptation. Because studies have shown that climate change rates can exceed adaptive potential (Visser, 2008), species with low inherent plasticity may face high extinction risk and rapid range recession.
Differing environmental tolerance between species that interact across trophic levels may result in variable degrees of mismatch across the southern range (Schweiger et al., 2012; Pickles et al., 2013). It is important to understand areas where this mismatch will occur between predators and prey, allowing predictions of where persistence will require adaptation to an alternate food source. For example, in parts of the southern Rocky Mountains as well as Wisconsin and Minnesota, higher suitability exists for red squirrel than for lynx and hare. Accordingly, lynx persistence in these regions may require intensified response to red squirrels under changing climate. These regions should be examined for genetic adaptation or plasticity to understand species persistence potential at the southern range. However, in areas where hare-only or hare and squirrel suitability is higher, persistence will depend on lynx ability to tolerate unfavorable climate conditions, as prey availability is not limiting. The general mismatch between the availability of both prey species suggests lynx dependence on squirrel will be strongest along the southern limits of their range, as expected. In general, the amount of the southern range that is squirrel-saturated increases, and will be larger than that for hare. However, we note considerable geographic variability in these models and the ability for local adaptation in the southern range for species may, however, be impeded by additional factors such as small spatial scale of the localized differences in prey, or increased overlap with competitors. Indeed, recent evidence from environmental niche factor models suggests that the niche of southern lynx is restricted by the presence of bobcats (Peers et al., 2013), which could potentially limit their ability to exploit areas where squirrel densities are greater than those of hare. Therefore, increased numerical abundance of warm-adapted competitors, or the introduction of new competitors due to climatic warming, could limit lynx ability to persist in their future southern edge.
Our results demonstrate a substantial level of uncertainty in future climate projections that omit biotic interactions, with the total amount of future lynx habitat declining substantially when models were restricted to projected distributions of primary and alternate prey species. Not surprisingly, the largest reduction occurred when core distribution of both prey was used as a restriction. Although lynx may be able to persist beyond this restriction, biologically relevant combinations of both prey species resulted in significant reduction in available habitat from the climate-only model (see Fig. 2a, b). Ultimately, these results add to a recent body of evidence emphasizing the importance of biotic interactions in large-scale ecological niche models (Bateman et al., 2012; Kissling et al., 2012; Wisz et al., 2013), and we must highlight that to some extent the large difference in our study between abiotic-only and biotic models may stem from the more specialized diet of Canada lynx and the notable importance of hare in their diet, relative to other predator species. This has been concluded elsewhere for specialist feeders (Bateman et al., 2012), but does not discount the point that many species will need to exhibit dynamic changes in their biotic interactions to ensure persistence across much of their current southern range.
Our work extends previous research by showing the importance of alternate prey use on future distribution patterns of a specialist predator at the southern edge of their distribution. This highlights the complexity of climate change projections, particularly when dynamic biotic interactions are considered. Our work also highlights the vulnerability of trailing southern range edges to potential mismatch in climate responses between predators and prey, and between primary and alternate prey species. We demonstrate that prey switching will be critical for persistence of predators and point to a need for a greater understanding of diet flexibility in lynx and other specialist predators. Ultimately, these findings provide insight into the uncertainty of projecting species responses to climate change without including biotic interactions, as well as the potential importance of phenotypic plasticity and adaptation toward increasing the likelihood of persistence of specialist predators at their trailing southern range.
Acknowledgements
We thank all of the museums listed for the use of their data as well as the provinces and states that provided access to their harvest records. We also thank J. Bowman and K. McKelvey for datasets on lynx occurrence in Ontario and the United States, respectively. We also thank three anonymous reviewers for their helpful comments that improved this manuscript.




