
Decryption of Amphipod Cryptic Species: Ecological Differentiation of Syntopic Lineages of a Gammarus Species Complex
Funding: This work was supported by the Charles University project START/SCI/076 within ‘Grant Schemes at CU’ (CZ.02.2.69/0.0/0.0/19_073/0016935) framework.
ABSTRACT
- Cryptic species represent an ever-increasing proportion of discovered biological diversity. Thus, the number of observations of cryptic species co-occurring in syntopy also increases, raising the question of mechanisms facilitating this phenomenon.
- Gammarus fossarum is a widespread, ecologically important, and diversified cryptic species complex with dozens of deeply divergent lineages of Miocene origin, some of which have been observed in syntopy in its European range.
- We used a multifaceted approach to study the ecology of two syntopic, genetically differentiated G. fossarum lineages in the stable environment of a calcareous spring fen. We investigated the effects of spatial distribution of sampling sites and local environmental factors (water chemistry, substrate composition) on the fine-scale distribution of the two lineages, together with their trophic ecology (δ13C, δ15N and gut content analysis). Moreover, we assessed functional morphological differentiation of the lineages, and compared identification based on mitochondrial DNA with variation at three nuclear markers to confirm their reproductive isolation.
- Small-scale syntopy of both lineages prevailed across the studied locality, having been observed at 14 out of 15 sampling spots. Lineage ratios varied within the spring fen; they were significantly affected by spatial proximity between the sampling spots, but not by local environmental conditions. The two lineages significantly differed in body size as well as trophic niche. However, they were essentially morphologically indistinguishable, although significant shifts were observed in a few phenotypic traits mainly related to feeding. The lack of nuclear allele sharing confirmed the existence of a strong reproductive barrier between them.
- The two studied G. fossarum lineages are reproductively isolated species that significantly differ in trophic ecology and body size in syntopy. Their local coexistence may thus be facilitated by trophic niche differentiation due to alternative resource use. Our results add to the growing body of evidence that morphologically cryptic species may not be functionally fully equivalent, indicating that cryptic species complexes may be more ecologically differentiated than currently known.
1 Introduction
Cryptic diversity is increasingly being discovered in many established invertebrate taxa due to the constant development of molecular methods (Pérez-Ponce de Léon and Poulin 2016; Fišer et al. 2018). Cryptic species are phenotypically very similar, and therefore, their distinction is usually based on molecular, behavioural or ecological traits rather than on the traditionally used morphological characteristics (Bickford et al. 2007). The surge of cryptic species discovery within common morphospecies poses a serious challenge for biodiversity assessment (Adams et al. 2014) or conservation (Bickford et al. 2007; Scriven et al. 2015) and highlights the need to integrate taxonomy with molecular markers, reproductive isolation, behaviour and ecology (Padial et al. 2010).
This comprehensive approach is very demanding. Therefore, it is not surprising that even within relatively well-studied groups, most cryptic species are defined only by comparative morphology and a few molecular markers, rarely taking ecology into account (Fišer et al. 2018). Moreover, presumably cryptic species are frequently discovered as a byproduct of phylogeographic studies, in which morphological or ecological variation has not been investigated (Witt et al. 2006; Copilaş-Ciocianu and Petrusek 2015). In many cases, their close phenotypic similarity is thus more an expectation than result of a quantitative assessment. Although there are examples of ecological differentiation among co-occurring cryptic invertebrate species (e.g., Wellborn and Cothran 2007; Scriven et al. 2015; Derycke et al. 2016), their ecology still remains heavily understudied. These gaps of knowledge are usually due to methodological constraints caused by the need of molecular identification of each specimen.
Of particular interest are the mechanisms that lead to the syntopy of two or more very similar species. Cryptic species can achieve stable coexistence through biological mechanisms such as niche differentiation or resource partitioning, which reduce interspecific competition and promote the long-term stability of their populations (Chesson 2000; Valladares et al. 2015; Scriven et al. 2015). However, they may also be ecologically largely equivalent, and their co-occurrence could be facilitated by stochastic processes that slow down competitive exclusion but do not necessarily involve specific mechanisms stabilising the focal species populations (Chesson 2000; Hubbell 2001; Blanchet et al. 2020). This may be achieved, for example, by a recurrent immigration of one or more species from source areas where they are more abundant and/or competitively superior (as discussed in Dionne et al. 2011), or by temporal fluctuations of environmental factors that favour different species (Descamps-Julien and Gonzalez 2005). To assess which mechanism(s) promoting syntopy are relevant, detailed information on the ecology of the species involved is necessary.
Freshwater amphipods are an excellent model system for investigation of secondary contacts and possible ecological differentiation among distinct genetic lineages, that is, potential cryptic species (Fišer et al. 2018). Many species from this group, including members of the genus Gammarus, are common and ecologically important shredders of coarse detritus (Kelly et al. 2002), but are capable of using alternative food sources (e.g., Friberg and Jacobsen 1994), often involving predatory behaviour (Syrovátka et al. 2020) including cannibalism (Dick et al. 1995). This group includes numerous widespread taxa in which a high level of cryptic diversity has been documented, such as Nearctic Hyallela azteca (Witt et al. 2006), Palearctic Gammarus lacustris (Hou et al. 2022), or European G. balcanicus (Copilaş-Ciocianu and Petrusek 2017) and G. fossarum (Wattier et al. 2020).
Gammarus fossarum (sensu lato) is a common stream macroinvertebrate in Western, Central and Southeastern Europe (Wattier et al. 2020) that often reaches very high population densities (Goedmakers 1981). It is a hyper-diversified complex with dozens of deeply divergent mitochondrial lineages, many of which date back to the Miocene (Copilaş-Ciocianu and Petrusek 2015; Wattier et al. 2020). A recent study on another diversified gammarid amphipod from Southern Europe (Hupalo et al. 2023) has, however, demonstrated that the sole use of mitochondrial barcoding markers may lead to substantial overestimation of the actual species diversity within such cryptic complexes. A combination of multiple marker types is thus recommended to appropriately assess the species status of unique mitochondrial lineages (Adams et al. 2014; Mamos et al. 2021). For a number of G. fossarum lineages studied in detail, differentiation at several studied nuclear markers was observed (Copilaş-Ciocianu and Petrusek 2015; Copilaş-Ciocianu et al. 2017; Bystřický et al. 2022). This, in combination with a strong prezygotic reproductive barrier documented for some lineage pairs (Lagrue et al. 2014; Galipaud et al. 2015; Bystřický et al. 2022), supports the interpretation that most lineages recognised within G. fossarum are indeed reproductively isolated species. Among those, some apparently show a high degree of morphological stasis (Müller et al. 2000) supporting their cryptic status, while others may be sufficiently differentiated to allow phenotype-based identification, which recently led to the formal description of a new morphospecies belonging to this complex (Rudolph et al. 2018).
Range size of G. fossarum lineages varies greatly, some being locally endemic and others widespread (Wattier et al. 2020). They are regularly found in syntopy in the regions where their ranges overlap (Eisenring et al. 2016; Bystřický et al. 2022), and such coexistence seems stable at least over several years (Bystřický et al. 2022). Potential ecological differentiation among some of these lineages was suggested based on their regional distributions (Eisenring et al. 2016; Bystřický et al. 2022) as well as experiments investigating tolerance to chemical pollutants (Feckler et al. 2012, 2014). When not considered, significant differences in lineage-specific responses to stressors may pose a particular problem, as G. fossarum (sensu lato) is commonly used in ecotoxicology and as a bioindicator (Jourdan et al. 2023).
In this study, we applied a multidisciplinary approach to evaluate potential ecological differences between two syntopic G. fossarum lineages in a stable environment of a spring fen, focusing on a much finer spatial scale than in previous studies investigating lineage coexistence in amphipod cryptic species. In the Carpathian spring fens, Gammarus is usually the dominant macroinvertebrate, frequently exceeding the abundances of other taxa by an order of magnitude (Georgievová et al. 2020; Zhai et al. 2020). These habitats are fishless, with limited influence from other predators (Zhai et al. 2020), and their location in spring areas excludes effects of passive drift or active downstream colonisation on the community composition. Therefore, local Gammarus populations are particularly suitable for testing ecological mechanisms that may facilitate the syntopy of cryptic species.
We employed a multifaceted approach by: (i) investigating the effects of spatial distribution of sampling sites and their environmental characteristics (water chemistry, substrate composition) on lineage distribution; (ii) analysing stable carbon and nitrogen isotope composition, gut content and relevant morphological traits of Gammarus individuals to examine a potential trophic niche differentiation of the lineages; and (iii) comparing lineage identification based on mitochondrial DNA with variation of three nuclear markers to check for reproductive isolation. We hypothesised that the syntopy of Gammarus lineages in a spring fen may be facilitated by ecological differentiation (either trophic or environmental). Alternatively, we wanted to obtain robust data supporting their ecological equivalence.
2 Methods
2.1 Studied Taxa and Habitat
Based on our previous analyses of samples collected in 2017–2018, we originally selected three calcareous spring fens located in the Western Carpathian mountain range near the Czechia–Slovakia border with an anticipated syntopy of at least two G. fossarum lineages. Calcareous spring fens in the area are dominated by bryophyte and low sedge vegetation, and characterised by shallow water, high amounts of tufa, Mg2+ and Ca2+ ions (Hájek et al. 2002), and thermal buffering caused by stable groundwater supply (Horsák et al. 2021). After the pilot investigation, we further focused solely on samples from the Hrubý Mechnáč spring fen (48.9422N, 17.7982E; alt. 630–640 m) where we confirmed the presence of two Gammarus lineages, EE Q and EE R sensu Copilaş-Ciocianu et al. (2017), at the time of sampling (6 June 2021).
Both focal lineages are Central European regional endemics, so far only documented from streams in eastern Czechia and western Slovakia (Copilaş-Ciocianu et al. 2017). Known localities with EE R presence are restricted to the Western Carpathians, where its range partly overlaps with EE Q; the range of the latter extends further westwards to the Bohemian Massif. Each lineage belongs to different major clades of the G. fossarum complex (Wattier et al. 2020), which likely diverged from each other in the early to middle Miocene (Copilaş-Ciocianu et al. 2017). EE R belongs to a highly diversified central to eastern European clade, EE Q is highly distinct, with no close relatives known so far (Wattier et al. 2020).
The studied spring fen (helocrene) is located on a hill slope where groundwater water seeps diffusely to the surface, with several places of a stable water runoff. Most of the area is largely overgrown by vegetation (sedges and grasses, wetland plants, mosses), with varying proportions of the surface covered by rocks and fine sediment matter consisting of tufa and organic matter (Figure 1A,B). Most of the area is saturated by water, providing suitable conditions for aquatic macroinvertebrates; the fen is drained to a larger stream ca 80 m away by very shallow rivulets, in which pools a few centimetres deep can occasionally form. Fish are completely absent in this environment, other potential vertebrate predators play a minor role, and predatory invertebrates that can feed on adult or juvenile Gammarus (e.g., dragonfly larvae, leeches, some aquatic beetles, trichopteran and dipteran larvae) are present in much lower densities (Zhai et al. 2020; pers. obs.).
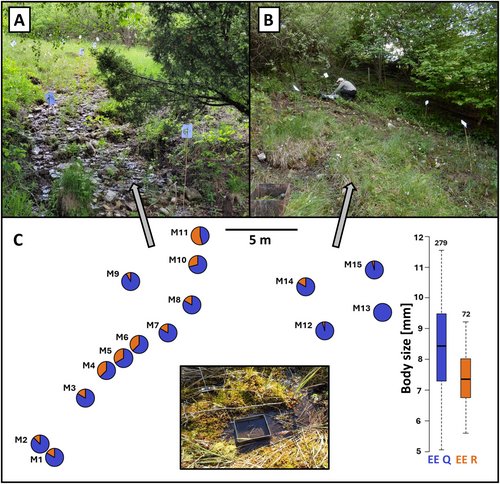
2.2 Sampling
Within the core area of the Hrubý Mechnáč fen of approximately 25 × 25 m, we selected 15 representative sampling spots where we collected samples of Gammarus, their potential food sources, sampled water for chemistry analyses and recorded substrate characteristics. Each sampling spot was delineated by a 25 × 25 cm metal frame (Figure 1C, inset), from which the substrate containing benthic animals was collected. From this material, we randomly picked several tens of adult-sized Gammarus individuals (> 5.5 mm body size). We stored them in liquid nitrogen for isotopic and molecular analyses, along with separately collected potential food sources for the isotopic analyses. These included fine organic-rich mud, coarse plant detritus and fresh plants, and other invertebrates in a size range potentially suitable to become Gammarus prey (including juvenile Gammarus, annelids and chironomid, trichopteran, odonate and simuliid larvae). If the sampling spot was dominated by Gammarus subadult and juvenile individuals, we preserved all the largest specimens from the sample. The spring fen was revisited two years later in late summer (27 August 2023), and additional Gammarus individuals were collected from three previously studied sampling spots (M4, M5 and M11; Figure 1C) and stored in 96% ethanol for evaluation of temporal stability of coexistence and body size trends.
The substrate composition at each sampling spot (expressed as the proportion of the surface area covered by course gravel and stones, fine tufa, macrovegetation and coarse organic matter) was assessed by an experienced member of the team (J.B.). Physical and chemical water characteristics (conductivity, pH, oxygen concentration and water temperature) were measured in situ by a portable multiparameter probe Hach HQ40d (Hach Company, Loveland, CO, USA), and local water depth and flow intensity was recorded. Due to very shallow depth even where open water was present, the flow at each sampling spot was quantified on an ordinal scale (1: stagnant water, 2: noticeable water movement, 3: slow flow and 4: fast flowing water). Water samples collected from each sampling spot were cooled in a refrigerator, stored in the dark and analysed within the next day after sampling for total organic carbon, inorganic carbon and concentrations of Mg2+, Ca2+, PO43−, NH4+ and NO3−.
2.3 Processing of the Gammarus Individuals
Each individual animal was defrosted on a glass plate at room temperature and photographed in a standardised position (Worsham et al. 2017) along with a scale for subsequent body size measurements (Figure S1) in ImageJ v. 1.50e (Schneider et al. 2012). Then, muscle tissue with adjacent cuticle was dissected from the dorso-abdominal part of the body (above the gut) for isotopic analyses, transferred into a pre-weighted tin capsule, and deep-frozen (−80 °C). The dissection was performed by a single person (P.K.B.) to ensure standardisation of the process. In parallel, one to three slightly shredded pereiopods were placed into a proteinase K buffer for DNA isolation, following Schwenk et al. (1998). The rest of the body was conserved in 96% ethanol for further gut content investigation and morphological analyses.
2.4 Molecular Determination and Species Delimitation
We assigned 24 Gammarus individuals from each sampling spot to a mitochondrial lineage, based on the variation of a ca. 323 bp long fragment of the mitochondrial gene for the large ribosomal subunit (16S rRNA). We used the primer pair 16STf (Macdonald III et al. 2005) and 16Sbr (Palumbi et al. 2002), following the protocol from Copilaş-Ciocianu and Petrusek (2015). Lineages were identified by aligning obtained sequences to already available reference sequences from the study region (Copilaş-Ciocianu et al. 2017).
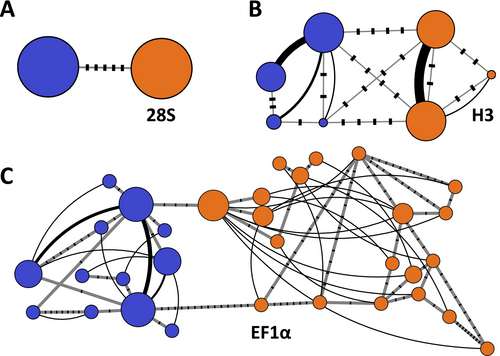
Moreover, we amplified three nuclear markers from 32 co-occurring individuals (16 per lineage) to investigate potential gene flow between studied lineages. These markers included a partial gene for 28S rRNA (28S; 921 bp) and a fragment of a gene encoding the elongation factor 1α (EF1α; 508 bp), both amplified using the protocols in Hou et al. (2011), and a gene for the histone H3 (H3; 292 bp), following Fišer et al. (2013) and using the primer pair from Colgan et al. (2000). Obtained heterozygous EF1α and H3 sequences were first phased into alleles using PHASE (Stephens et al. 2001) in DNA Sequence Polymorphism v. 6.12.03 (Rozas et al. 2017). Then, we reconstructed for those nuclear markers haplowebs at a dedicated domain HaplowebMaker (Spöri and Flot 2020) to check for potential allele sharing among lineages that would indicate hybridisation or introgression. The same online service was used to create a haplotype network for homozygous 28S data. Obtained unique sequences have been deposited to GenBank (accession numbers PQ893182 to PQ893186 for ribosomal markers, and PV020751 to PV020781 for protein-coding genes).
Based on the previous molecular data (Copilaş-Ciocianu et al. 2017) as well as experience with other highly divergent lineages of the G. fossarum complex in the region (Bystřický et al. 2022), we anticipated that the distinct mitochondrial lineages we found (EE Q and EE R sensu Copilaş-Ciocianu et al. 2017) are in fact reproductively isolated biological species. To test this hypothesis, we used a Bayesian coalescent species tree approach (closely following Bystřický et al. 2022). We combined all three nuclear markers (28S, and both phased alleles of EF1α and H3). We used BEAST 1.8.2 (Drummond et al. 2012) with the package *BEAST (Heled and Drummond 2009) to test which dataset partition (two vs. one species) fits best to the data, based on Bayes factors species delimitation (Grummer et al. 2014).
2.5 Stable Isotopes and Gut Content Analyses
Samples with G. fossarum muscle and cuticle tissue were freeze-dried, weighed, and stored in a desiccator until further processing. Putative invertebrate food sources were grouped by morphotype similarity (usually pooling the same order or family), placed in 2 mL Eppendorf tubes, freeze-dried and homogenised using stainless-steel beads. After the homogenization, samples were divided into two groups: (i) those for nitrogen stable isotope analysis were weighed and transferred to tin capsules without any further preparation; (ii) those for carbon stable isotope analysis were, due to presence of CaCO3 incrustations in samples, decarbonized in silver capsules according to Brodie et al. (2011) with the modification after Vindušková et al. (2019) and kept in a desiccator for at least 10 days.
Fine and coarse detritus samples were homogenised and oven-dried at 45 °C. For analyses of δ15N, the dry homogenate was transferred to tin capsules and weighed. For analyses of δ13C, carbonates were removed as described above. All samples were prepared in three replicates and stored in a desiccator.
Both carbon and nitrogen stable isotope composition was analysed using a Flash 2000 elemental analyser, Delta V Advantage isotope-ratio mass spectrometer, and a Continuous Flow IV system (all Thermo Fisher Scientific, Bremen, Germany), as described in detail by Novotná Jaroměřská et al. (2021). The results were expressed in standard delta notation (δ) relative to Pee Dee Belemnite for carbon isotopes and to atmospheric N2 for nitrogen isotopes and normalised based on international standards.
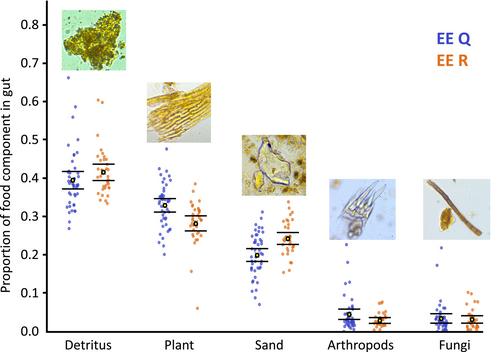
The gut content analysis followed Copilaș-Ciocianu et al. (2021). Briefly, the guts were dissected in glycerine under a stereomicroscope and their content was evenly spread onto a microscope slide with a 10 × 10 counting grid (area 1 cm2). In each square, we noted the presence of each of six recognisable gut content categories: detritus (fine undistinguishable organic material), plant matter (with apparent cell structure), sand (mineral particles), invertebrates (fragments with obvious cuticular structures or remains of various appendages), fungi (mycelial structures), and filamentous algae. In total, we investigated 80 individuals representing both lineages (45 EE Q and 35 EE R) randomly picked from different sampling spots, with body size distribution corresponding to that observed at the locality.
2.6 Morphometry
Forty specimens (10 of each lineage and sex) were used for morphometric analyses. We measured 38 functional morphological traits (Figure S2) related to feeding ecology (mouthparts and stomach: 12 measurements; both pairs of gnathopods: 12), sensory perception (eyes and antennae: 7), and locomotion (coxae of the 2nd and 3rd pereiopods: 4, length of 3rd pereiopod: 1). Processing of specimens, dissection and photography followed Copilaș-Ciocianu et al. (2021) and Hupalo et al. (2023). Specimens were placed overnight into a 1.5% lactic acid solution and subsequently transferred for 3 h to a 3:1 mixture of 70% ethanol and glycerol for softening and partially clearing the cuticle. Dissected appendages were temporarily mounted on slides and photographed with a Pixelink M15C-CYL digital camera attached to a Nikon SMZ1000 stereomicroscope or a Nikon Si microscope. Measurements were done with Digimizer (MedCalc Software, Ostend, Belgium). Appendages from either left or right side of the body were measured, depending on specimen completeness, except the mouthparts that are asymmetric, so only right-side ones were considered.
2.7 Data Analyses
2.7.1 Spatial and Environmental Determinants of Lineage Distribution
All statistical analyses were performed in R 4.3.2 (R Core Team 2023). Whenever relevant, the significance values were adjusted for multiple testing by Bonferroni correction. To identify potential environmental factors impacting the Gammarus lineage distribution, we built a GLM model with the AIC backward selection. All continuous variables, with the exception of pH, were log-transformed. Consequently, we performed a Moran's I test to analyse the similarity between the lineage distribution (expressed as ratio) and Euclidean distances between the sampling sites expressed as a list of nearest neighbours. Both analyses were calculated with the R package spdep (Bivand and Wong 2018).
2.7.2 Trophic Niche of Syntopic Lineages of G. fossarum
Differences in δ13C and δ15N between lineages were tested using two separate ANCOVA tests with body size as a covariate. The trophic niche of both lineages of G. fossarum based on the δ13C and δ15N data was estimated by the standard ellipse area (SEA) in the R package SIBER (Jackson et al. 2011). We also calculated the Bayesian standard ellipse area (SEAb; encompassing 95% of the data points) and the corrected standard ellipse area (SEAc; considering 40% of central data points), the latter less sensitive to small sample sizes (Jackson et al. 2011). The trophic niche overlap was expressed as the ratio between the area of the intersection and the total area (union) of SEAb ellipses representing trophic niches of both lineages (i.e., EE Q ∩ EE R ÷ EE Q ∪ EE R; Jackson et al. 2011).
To assess the contribution of the different putative food sources to the isotopic signature of G. fossarum lineages, separate Bayesian stable isotope mixing models (Moore and Semmens 2008) were run using the R package MixSiar (Stock and Semmens 2016). For both lineages, a three-source model was used including fine organic matter, plant matter (both fresh and coarse detritus), and pooled locally common small-bodied macroinvertebrate groups (juvenile G. fossarum, dipteran larvae, annelids) as potential prey of G. fossarum.
For analyses of the gut content composition, we divided the value of each food category (ranging from 0 to 100) by the sum of values for all categories (including also the sand particles) observed within the gut of a given individual; the resulting ratio is thus a proxy for a proportion of the given material in the gut. The results were visualised as means and their 95% confidence intervals, which were then compared between both lineages. Algae were excluded from the final comparisons because their recognisable remains were usually absent or extremely low in abundance. The differences in relative proportion of gut content between lineages were then tested using permutational multivariate analysis of variance (PERMANOVA) with 999 permutations and a Euclidean similarity.
2.7.3 Body Size and Morphometry
We tested for potential differences in total body size between lineages by linear mixed models with sampling spots as a random effect, using the R packages lme4 (Bates et al. 2015) and lmerTest (Kuznetsova et al. 2017). Then, we used the PERMANOVA with 9999 permutations to test for differences in other morphological characteristics between lineages and sexes. The effect of body size was removed by regressing raw measurements against body length.
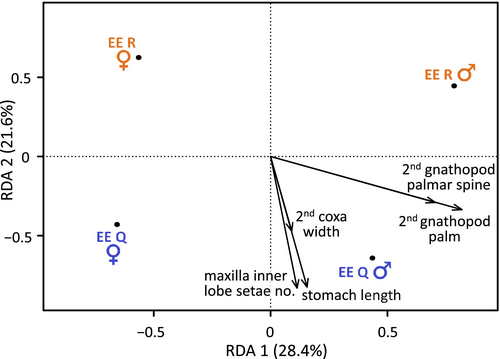
Due to the high number of measured traits and relatively small number of individuals, we decided to narrow the pool of closely investigated traits. We used the redundancy analyses (RDAs) with original non-transformed data, including body size as one of the traits. The analysis was performed using the R package vegan v. 2.5–6 (Oksanen et al. 2020). The best model was found by repeated search for a model with the lowest AIC using step function, with one-by-one dropping of the traits with Variance Inflation Factor above 7, using the function vif.caa. The significance of each RDA was assessed by permutation with 999 replications. The traits presumably differing between lineages, which were retained in the final model, were tested for lineage effect using analysis of covariance (ANCOVA) with sex and body size as possible covariates. For each analysed trait, we first built a full model including interactions, and then reduced its complexity using the lowest AIC approach with a function step.
3 Results
3.1 Small Scale Distribution
We confirmed the syntopy of two lineages of the G. fossarum complex (EE Q and EE R) at the Hrubý Mechnáč spring fen (Figure 1). The two studied lineages co-occurred at 14 out of 15 sampling spots, but the lineage ratios varied among them (Figure 1C). EE Q was dominant at all sampling spots except M11, representing 66% or more of determined Gammarus individuals at 12 spots. The rarer EE R lineage was more abundant in the central and the topmost part of the spring fen (Figure 1C). We did not find any significant effect of the measured environmental variables or substrate type on the local proportions of Gammarus lineages. However, the distribution of the lineages was significantly positively spatially autocorrelated across the study area (Figure 1C; Moran's I = 0.51; p = 0.04), that is, sampling spots located closer to each other tended to have more similar lineage composition.
The continuous presence of both lineages was further confirmed two years later (in August 2023) at all three re-sampled sampling spots (M4, M5 and M11). The lineage ratio in randomly selected individuals was comparable at the two centrally located spots (proportion of EE R at M4: 33% in 2021, 45% in 2023; at M5: 37.5% in both years). However, it shifted substantially at the M11 spot (proportion of EE R decreased from 54% in 2022 to 8% in 2023). At that spot, however, adult-sized individuals were very scarce, and the sample was dominated by EE Q individuals below 5.5 mm (Figure S4).
3.2 Body Size
The individuals belonging to the EE Q lineage had a significantly larger body size than EE R individuals (LMM SE = 0.16, t339 = 7.4, p < 0.0001), on average by 13%. Specifically, the mean body size (± SD) was 8.4 ± 1.4 mm for EE Q and 7.3 ± 0.9 mm for EE R (Figure 1C). This trend for body size differentiation was consistent across all 14 sampling spots with the presence of both lineages (Figure S3A). A similar pattern was also observed in samples from late summer 2023; the mean body size at M4 and M5 was 14% larger for EE Q (7.1 ± 1.4 mm) than in EE R (6.1 ± 0.8 mm) individuals (Figure S4).
3.3 Food Preferences and Trophic Niche of G. fossarum Lineages
We observed significant differences in stable carbon isotope composition between the two lineages (Figure 2A; Figure S5A). A significant positive correlation of the body size and δ13C was found in both lineages (Figure S5A), but the same-sized EE Q individuals tended to have higher δ13C than those of EE R (ANCOVA adj. R2 = 0.41, F2,344 = 123.5, both p < 0.0001). These differences were consistent across all sampling spots where the lineages coexisted (Figure S3B). For δ15N, the body size effect was significant (ANCOVA adj. R2 = 0.14, F2,344 = 28.95, p < 0.0001; Figure S5B) but no shift in δ15N between EE Q and EE R individuals was observed (Figure 2A, Figures S3C and S5B).
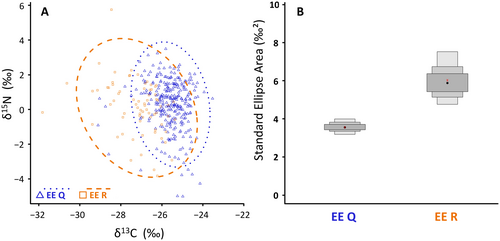
The trophic niche width analyses (Figure 2A) confirmed only a limited overlap (39%) between the lineages. Despite a substantial scatter of individuals, the calculated trophic niche width of EE Q lineage was substantially smaller than that of EE R (Figure 2B). Bayesian mixing models (BMMs) suggested a difference in food source use between the lineages. Interestingly, for EE Q, a higher contribution of macroinvertebrates was indicated (53% ± 20%; values reported as means with standard deviation), with lower proportion of fine organic matter (33% ± 23%) and coarse detritus (14% ± 10%). For EE R, the estimated contributions of these sources were more balanced, with slightly higher proportions of fine organic matter (39% ± 30%) and coarse detritus (35% ± 18%) than of macroinvertebrates (26% ± 13%).
Significant differences between lineages were also observed in gut content (PERMANOVA, F1,78 = 6.08, p = 0.002) (Figure 3), although not corresponding to BMM results. The most abundant material found in guts of both lineages was undifferentiated fine matter pooled under the ‘detritus’ category, followed by coarse plant tissues with visible cell structure. These were significantly more abundant in the guts of the larger and more frequent EE Q lineage, while the indigestible sand particles were more frequently found in EE R (Figure 3). Identifiable remains of invertebrates (i.e., visible arthropod cuticular structures) and fungi were observed in most dissected specimens (95% of guts for both categories) but less frequently than the other gut content categories (Figure 3). Filamentous algae were mostly absent from the guts (in 56% of specimens) and only very rarely (in 5% of specimens) exceeded two observations per gut.
3.4 Morphometry
Apart from the significant difference in body size, most of the investigated morphological traits did not reveal differences between the lineages. Sexual dimorphism was pronounced in both EE Q and EE R, with 30 out of 38 measured traits consistently differing between males and females. Consequently, PERMANOVA did not reveal significant differences between lineages (F1,39 = 0.089, p = 0.89), only between males and females (F3,37 = 33.97, p = 0.0001); all pairwise comparisons between males and females regardless of the lineage were highly significant (p = 0.0006), while the comparison of either males or females between lineages were not.
However, when traits most relevant for distinguishing the sexes and lineages were selected using the RDA by sequential elimination (Figure 4), we identified three traits linked to lineage differentiation: stomach length, the number of setae on the maxilla I inner lobe, and second coxa width. In addition, two traits on the second gnathopods were retained in the model as those most contributing to the sexual dimorphism. Between-lineage differences in the three above-mentioned traits also turned out to be significant in independent ANCOVA tests, together with a significant effect of body size (Figures S6–S8, Table S1). The equally sized EE Q individuals had a significantly longer stomach (Figure S6) and tended to have more setae on the inner lobe of maxilla I (Figure S7) than EE R ones, independently of sex. The between-lineage difference in the second coxa width (Figure S8) was primarily driven by a strong sexual dimorphism within one of them: equally sized EE R females had wider coxae than EE R males and both EE Q sexes. Due to the overlap between lineages, however, none of these phenotypic traits alone would be useful for reliable morphological identification of the individuals.
3.5 Species Delimitation
We did not observe allele sharing within the three investigated nuclear markers between any of the 32 individuals identified by the mitochondrial 16S marker (Figure 5). Correspondingly, the Bayes factor delimitation of species strongly favoured the two-species model against the null hypothesis of a single species (2 species lnL = −4900.53 vs. 1 species lnL = −4926.41; 2lnBF = 51.76).
4 Discussion
We confirmed that the two studied lineages of G. fossarum (EE Q and EE R sensu Copilaş-Ciocianu et al. 2017) co-occurred on a very small spatial scale. Phenotypically similar Gammarus congeners can coexist (Goedmakers 1981; Lagrue et al. 2014), however, close documentation of contact of two or more cryptic species of crustacean macrozoobenthos is still rare. Previous studies on syntopy within this and other freshwater amphipod complexes have focused on much larger spatial scales, such as streams segments (Müller et al. 2000; Westram et al. 2011; Eisenring et al. 2016; Bystřický et al. 2022) or different zones within lake littorals (Wellborn and Cothran 2007; Dionne et al. 2011). The distribution of adult-sized EE Q and EE R individuals did not seem to be influenced by any of the studied environmental variables. Nevertheless, some degree of microhabitat partitioning may possibly exist between Gammarus juveniles (which were not specifically studied), for example to avoid intraguild predation or cannibalism (Luštrik et al. 2011).
We observed the presence of both G. fossarum lineages at the Hrubý Mechnáč spring fen over several years (between 2017 and 2023) and confirmed their small-scale syntopy in both 2021 and 2023. The studied helocrene spring with continuous groundwater supply is a temporally stable aquatic habitat (Hájková et al. 2004; Horsák et al. 2018). The local conditions are suitable for the year-round survival of aquatic biota, at least in the central part of the fen with spring source and the rivulets draining it, which are buffered from temperature extremes by the groundwater (Horsák et al. 2021). Marginal spots, more prone to freezing in winter or desiccation in summer, are likely to be swiftly recolonised by individuals from the more stable central areas of the spring fen.
Although we lack information on the local densities of Gammarus at the end of winter, when some population declines may be expected (Goedmakers 1981), it seems unlikely that seasonal fluctuations lead to severe population bottlenecks, which would reset the competitive interactions between the two co-occurring lineages in the Hrubý Mechnáč spring fen. G. fossarum tends to be a prevalent and stable member of small stream communities year-round (Goedmakers 1981; Beracko et al. 2012), and we presume that both lineages find suitable conditions for continual persistence in the stable, thermally buffered groundwater-fed areas.
The location of our study site in the river network rules out any downstream colonisation by Gammarus. Unless we assume a continuous upstream immigration of one of the lineages from the nearby stream, the source-sink dynamics, discussed for another amphipod species complex (Dionne et al. 2011), does not seem a likely explanation for the local G. fossarum lineage co-occurrence, nor do environmental fluctuations. Given the differences in several characteristics, coexistence facilitated by niche differentiation seems the most plausible explanation for their syntopy.
The most prominent phenotypic difference between the two studied G. fossarum lineages was the body size. Larger amphipod individuals may have an advantage when competing for resources, such as food or shelter, and larger species tend to outcompete and even feed on the smaller ones (MacNeil and Platvoet 2005; Luštrik et al. 2011), unless countered by higher aggressiveness (Dick et al. 1995). In some situations, substrate particle size has been shown to correlate with Gammarus body size at the microhabitat scale (Pringle 1982; Adams et al. 1987). Although our results did not reveal a relationship between substrate composition at the sampling spots and lineage ratios, it is possible that a more detailed quantitative assessment of the substrate structure might have revealed some subtle effects on lineage distribution. On the other hand, no discrimination between microhabitat patches by different size cohorts of G. pulex was found in standing water (Adams et al. 1987), a condition prevailing across most of the spring fen.
Body size differences between coexisting lineages were also observed within the Hyallela azteca complex (Wellborn and Cothran 2004; Wellborn and Broughton 2008), in which different lineages evolved repeatedly into either small or large forms, likely in response to fish predation (Wellborn and Broughton 2008). We assume no significant size-selective predation in the studied habitat, due to the absence of fish and scarcity of invertebrate predators other than Gammarus, too rare to have a significant impact on their populations (Zhai et al. 2020; personal observation). The observed differences in G. fossarum body size are therefore more likely related to resource partitioning (Basset and Angelis 2007).
Multiple lines of evidence suggest that the two lineages differ in their trophic ecology. Alternative foraging strategies between cryptic species are a plausible explanation for their coexistence, as described for example in bumblebees (Scriven et al. 2015) or marine nematodes (Derycke et al. 2016; Guden et al. 2021). We observed significant between-lineage shifts in δ13C, generally used as an indicator of food sources (reviewed, e.g., in Wada 2009), and the stable-isotope-based trophic niche estimates overlapped only partially between the two lineages. Higher δ13C in EE Q may indicate increased consumption of carbon originating from autochthonous organic matter (e.g., vegetation, biofilm) which is expected to be higher in δ13C than allochthonous sources (e.g., Post 2002). A higher average proportion of coarse plant particles was also observed in the guts of EE Q individuals. The increased trophic niche width of the smaller-sized EE R lineage, with a higher proportion of inorganic sand particles in their guts, may suggest that this smaller lineage is more of a generalist, and gets pushed towards less favourable food sources within microhabitats.
Bayesian stable isotope mixing models indicated that the larger EE Q consumes more macroinvertebrates than the smaller EE R, which would be consistent with the observed body size differences. The level of uncertainty of the models, however, is very high, and we did not observe significant differences between lineages in the frequency of arthropod cuticle fragments in guts or in δ15N, commonly used as an indicator of trophic level (Wada 2009). Consumption of soft-bodied prey, preferred by Gammarus in spring fens, may be, however, hard or even impossible to detect by light microscopy (Syrovátka et al. 2020). Although lineages might have different tendencies for predatory behaviour and δ15N of EE R may be shifted to higher values by consumption of N-limited fine and coarse organic matter (Adams and Sterner 2000), we assume that their overall trophic level is similar. Apart from stable carbon isotopes and gut content, differences in stomach length and number of setae on maxilla I inner lobe between the lineages also seem to support some differentiation in feeding strategies (Coleman 1991; Mayer et al. 2012; Copilaș-Ciocianu et al. 2021).
Overall, knowledge about phenotypic divergence of lineages is scarce within the G. fossarum complex. Apart from this study, only three lineages have been morphologically investigated (Müller et al. 2000; Rudolph et al. 2018). Specifically, Müller et al. (2000) demonstrated, on a subset of traits known to vary among G. fossarum populations (Goedmakers 1972), that Western European lineages A and B (CWE A and CWE B sensu Copilaş-Ciocianu et al. 2017) differ in characters on third uropods (in both sexes), and second antennae (in males), although not enough to allow morphological identification. A formal description of Gammarus jazdzewskii (EE T sensu Copilaş-Ciocianu et al. 2017) by Rudolph et al. (2018) indicated numerous minor morphological differences from the G. fossarum neotype designated by Goedmakers (1972). However, differential diagnosis against other lineages of the complex that may get into contact with G. jazdzewskii is not available. Due to high diversity within the complex, with dozens of anticipated biological species whose ranges frequently overlap (Wattier et al. 2020), future descriptions of new morphospecies should be thus accompanied with a more thorough comparison of locally relevant clades.
The absence of nuclear allele sharing between studied lineages, supporting the lack of gene flow despite syntopy and limited phenotypic differentiation, is congruent with other studies that documented reproductive isolation between co-occurring G. fossarum lineages (Lagrue et al. 2014; Galipaud et al. 2015; Bystřický et al. 2022). This confirms that the coexistence of largely cryptic biological species in this complex might be a rule rather than an exception. They regularly co-occur in unbalanced ratios (Westram et al. 2011; Bystřický et al. 2022), which could lead to reproductive interference in case of imperfect mate choice. This seems not to be the case, however, as strong assortative mating has been documented in G. fossarum (Westram et al. 2011; Lagrue et al. 2014; Bystřický et al. 2022), and reproductive interference has not been supported within the Hyalella azteca complex either (Cothran et al. 2013).
In conclusion, the two reproductively isolated G. fossarum lineages from our study occurred in syntopy at a very small spatial scale and over multiple years, while differing in their trophic ecology and body size. This indicates that at least in this case, syntopic cryptic gammarids are not ecologically equivalent, but tend to differentiate their niches, which may support long-term coexistence. Whether these differences are fixed or phenotypically plastic, and whether species syntopy leads to character displacement, remains open. Potential body size or trophic niche differentiation should be tested in other similar cases of coexisting, presumably cryptic Gammarus lineages in small streams, the dominant habitat for G. fossarum sensu lato (Eisenring et al. 2016; Bystřický et al. 2022). Our study adds further evidence for ecological differentiation of cryptic species of aquatic invertebrates. However, the role of stochastic vs. deterministic processes in structuring the spatial distribution of the dozens of recently discovered crustacean lineages warrants further study.
Author Contributions
Conceptualisation: P.K.B., A.P., T.N.J. and D.C.C. Developing methods: P.K.B., T.N.J., J.B., M.G., D.C.C. and M.H. Conducting the research: P.K.B., T.N.J., L.V., J.B., M.G., V.H., N.K., D.N., D.C.C., M.H. and A.P. Data analysis: P.K.B., T.N.J., P.J.J., L.V. and D.C.C. Data interpretation: P.K.B., T.N.J., P.J.J., L.V. and D.C.C. Preparation of figures and tables: P.K.B., T.N.J., L.V. and D.C.C. Writing: P.K.B., T.N.J., L.V., J.B., M.G., V.H., P.J.J., N.K., D.N., L.V., D.C.C., M.H. and A.P.
Acknowledgements
We thank Marie Zhai and Jonatán Novotný for cooperation during sampling, Lukáš Kratochvíl and Vojtěch Brlík for advice on data analysis, and Jaromír Novák for sharing equipment. The project was funded by Charles University project START/SCI/076 within ‘Grant Schemes at CU’ (CZ.02.2.69/0.0/0.0/19_073/0016935) framework, and by the Czech Science Foundation (project P505/23-05268S). Open access publishing facilitated by Univerzita Karlova, as part of the Wiley - CzechELib agreement.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data underlying the present study are available from the Zenodo repository (doi: 10.5281/zenodo.14604922). Newly obtained sequences have been deposited to GenBank (accession numbers PQ893182 to PQ893186 and PV020751 to PV020781).




