
Influence of hydraulics on the uptake of ammonium by two freshwater plants
Summary
- Macrophytes are important in the biogeochemistry of flowing rivers, although most information so far has relied on measurements of nutrients in plant tissues. This yields only indirect information on the nutrient uptake fluxes by roots and shoots and about nutrient translocation between roots and shoots. Here, we studied nitrogen uptake through experiments with enriched 15N stable isotopes.
- Two macrophytes (Potamogeton natans and Ranunculus fluitans) were grown in a closed race track-shaped flume, allowing us to control the hydraulic conditions in and around the plants. Overall ammonium uptake rates (μmol g−1 dry mass h−1) were higher for R. fluitans than P. natans.
- In addition to differences between the species, the spatial position of individuals within the plant patch and water flow were also important in explaining ammonium uptake. Thus, ammonium uptake was high at the leading edge of the patch and increased with velocity.
- Plant characteristic, such as the angle at which the plants bent in the flow, was also correlated with ammonium uptake. Differences in nutrient uptake associated with hydrodynamic parameters raised the question of how the two are related. For both species, uptake was not correlated with Reynolds stress, indicating the poor effect of turbulent mixing in determining ammonium uptake.
Introduction
Eutrophication of rivers results mainly from pollution by both point (sewage) and diffuse (agriculture, atmospheric deposition) sources of nutrients, particularly N and P (e.g. Hilton et al., 2006). Fluvial processes can strongly reduce nutrient loads to downstream ecosystems, thereby mitigating eutrophication effects. Nitrogen and phosphorus can be removed from the water column by either uptake into plant biomass or benthic processes such as denitrification and sedimentation. However, incorporation into plant biomass is only temporary as the nutrients can be recycled after plant decomposition or through fragmentation. In small, shallow rivers, macrophytes are one of the important mechanisms absorbing nutrients, although many small European rivers now suffer from excessive macrophyte biomass in summer. For example, in the Semois River (a tributary of the Meuse-Rhine), the biomass of Ranunculus fluitans can exceed 500 g m−² dry mass (Gommes & Froment, 1978). While macrophytes can be considered as beneficial in terms of nutrient retention, excess biomass may conflict with other (recreational) ecosystem services and also limit the hydraulic capacity of the channel (Bal & Meire, 2009; De Doncker et al., 2009a,b; Bal et al., 2011a), increasing the risk of flooding.
Both experimental and in situ studies have focussed on N uptake (NH4+ and NO3−) and subsequent assimilation by submerged plants, such as sea grass, freshwater macrophytes and microalgae, to characterise N-nutrient cycling and growth processes in salt marshes, lakes, estuaries, rivers and seas (e.g. Madsen & Breinholt, 1995; Bouma et al., 2002; Cornelisen & Thomas, 2002, 2004; Lepoint et al., 2004; Morris et al., 2008). However, most studies have addressed sea grasses in low nutrient habitats rather than macrophytes from eutrophic rivers (e.g. Vonk et al., 2008). Moreover, whereas the role of macrophytes in the cycling of nitrogen and phosphorus is well understood for standing waters (Weisner et al., 1994), their role on the nutrient dynamics of running waters is less clear. Submerged macrophytes take up nutrients via the roots from the sediments and via the shoots from the water column (Sytsma & Anderson, 1993; Carr & Chambers, 1998). The relative importance of the two depends on the element concerned, its concentration in the interstitial and the water column and on plant species (Carignan & Kalff, 1980). While macrophytes in standing waters may be limited by the availability of nutrients in the water column and rely mainly on nutrients from the sediments, in flowing waters, macrophytes are exposed to a continuous supply of fresh nutrients and species adapted to running water can take up inorganic nitrogen and phosphorus from the water column through their shoots (Madsen & Cedergreen, 2002).
In addition, macrophytes strongly modify the hydrodynamics of rivers. They ‘engineer’ the physical condition of the channel in terms of water velocity, depth, quality, sediment transport and deposition and provide structural habitat diversity (Sand-Jensen & Pedersen, 1999; Franklin, Dunbar & Whitehead, 2008; Schoelynck et al., 2012). The dynamics of riverine plants have been studied by Riis & Biggs (2003) and Hilton et al. (2006), who suggested that some important factors for macrophytes, such as light availability, nutrient status and biological interactions, are over-ridden by the influence of water velocity. Biggs et al. (2005) have shown that the physical and biological processes, such as individual growth and plant nutrient uptake, are controlled by short-term hydraulic fluctuations (over minutes to milliseconds). More recent experimental studies in an artificial flume by Peralta et al. (2008) and Hendriks et al. (2010) have also identified the effects of shoot flexibility and density on the flow patterns in the canopies of submerged plants.
The rates of nutrient uptake by submerged plants are mediated by water flow, and flume experiments have been used to isolate the effects of water velocity on uptake rates by seagrasses measured using 15N-labelled NH4+ (Cornelisen & Thomas, 2002, 2004, 2006; Morris et al., 2008). For instance, Morris et al. (2008) demonstrated the effect of unidirectional flow and spatial position of shoots on ammonium uptake by two seagrass species with differing morphology. Further, Cornelisen & Thomas (2004) showed that under unidirectional flow, the rate of NH4+ uptake by seagrass leaves was influenced by the rate at which NH4+ was delivered to the leaf surface and thus depends on factors such as near-bed shear stress and energy dissipations, both influencing the thickness of the diffusive boundary layers. No flume experiments using 15N-labelled nutrients have been carried out on river macrophytes to date.
In this article, we describe flume experiments to investigate the concomitant effects of: (i) leaf morphology and shape, (ii) water flow (bulk and high-resolution velocities) and (iii) patch configuration (including plant density) on the rate of 15NH4+ uptake by two macrophytes, Potamogeton natans and Ranunculus fluitans. Incubation experiments using 15N-labelled ammonium were performed, in conjunction with high-resolution measurements of water velocity, to test the hypotheses that ammonium uptake accelerates with increased water velocity and that the morphological characteristics of plants influence ammonium uptake. Ammonium uptake was chosen as this is the preferred source of nitrogen when available in excess. For example, Boedeltje, Smolders & Roelofs (2005) tested the uptake preference of Potamogeton alpinus for NO3− and NH4+. As for most aquatic plants, both forms of nitrogen can be taken up, but NO3− is energetically less preferable because it has to be reduced by nitrate reductase before assimilation (Duff & Triska, 2000). This preference for ammonium over nitrate holds for species adapted to dystrophic waters in which NH4+ is at high concentration, but may not be true for species that prefer NO3− (e.g. Groenlandia densa; Schuurkes, Kok & Den Hartog, 1986).
Methods
Plant material
Shoots of two freshwater macrophyte species, P. natans and R. fluitans, were collected by hand in September 2008 in the Zwarte Nete and the Semois River (Belgium), both eutrophic lowland streams. Their specific N content (mean ± SD) was 3.0 ± 0.4% dry mass for P. natans (n = 198) and 2.6 ± 0.4% dry mass for R. fluitans (n = 239). Batches of plants were stored in 40-L plastic containers half-filled with the river water and transported to the flume laboratory in Yerseke (the Netherlands), where they were kept outside in the shade and at natural light until transplanting into the flume. Time of storage before use is shown in Table 1. Before transfer to the flume, plant roots were removed to prevent ammonium uptake by that means. In this way, the inevitable penetration of labelled water in the sand and uptake by the roots was prevented. The two species were selected for their widespread occurrence in eutrophic Belgian rivers and for their differing morphology. Potamogeton natans has loose bunches of long flexible stems ending in oval or elliptical floating leaves, whereas R. fluitans has dense, flexible, dissected and threadlike leaves.
| Incubation name | Date | Side of channel | Plant species | Plant density Spec. m−2 | Age plants days | NH4 μm | Temperature °C | Velocity ms−1 |
|---|---|---|---|---|---|---|---|---|
| Incubation 1.1 | 17/09/2008 | Right | P. natans | 278 | 3 | 24.5 | 16.1 | 0.3 |
| Left | Empty | 0 | ||||||
| Incubation 1.2 | 18/09/2008 | Right | P. natans | 278 | 4 | 20.6 | 15.6 | 0.3 |
| Left | Empty | 0 | ||||||
| Incubation 2.1 | 17/09/2008 | Right | P. natans | 278 | 3 | 26.2 | 16.1 | 0.1 |
| Left | Empty | 0 | ||||||
| Incubation 2.2 | 18/09/2008 | Right | P. natans | 278 | 4 | 22.3 | 15.6 | 0.1 |
| Left | Empty | 0 | ||||||
| Incubation 3.1 | 01/10/2008 | Right | R. fluitans | >400 | 9 | 30.5 | 15.8 | 0.3 |
| Left | P. natans | 278 | 1 | |||||
| Incubation 3.2 | 02/10/2008 | Right | R. fluitans | >400 | 0 | 19.9 | 15.8 | 0.3 |
| Left | P. natans | 278 | 2 | |||||
| Incubation 4.1 | 01/10/2008 | Right | R. fluitans | >400 | 9 | 28.7 | 15.8 | 0.1 |
| Left | P. natans | 278 | 1 | |||||
| Incubation 4.2 | 03/10/2008 | Right | R. fluitans | >400 | 1 | 12.5 | 15.8 | 0.1 |
| Left | P. natans | 278 | 3 | |||||
| Incubation 5.1 | 23/09/2008 | Right | R. fluitans | >400 | 1 | 25.6 | 15.8 | 0.3 |
| Left | Empty | 0 | ||||||
| Incubation 5.2 | 24/09/2008 | Right | R. fluitans | >400 | 2 | 27.2 | 15.8 | 0.3 |
| Left | Empty | 0 | ||||||
| Incubation 6.1 | 23/09/2008 | Right | R. fluitans | >400 | 1 | 27.4 | 15.8 | 0.1 |
| Left | Empty | 0 | ||||||
| Incubation 6.2 | 24/09/2008 | Right | R. fluitans | >400 | 2 | 22.0 | 15.8 | 0.1 |
| Left | Empty | 0 |
Flume characteristics and deployment
Flume incubation experiments were conducted from 16 September to 8 October, 2008 in a unidirectional race track flume tank at the laboratory facilities of the NIOO-CEME (Nederlands Instituut voor Ecologisch Onderzoek-Centrum voor Estuariene en Marine Ecologie). This flume is located in a climate-controlled laboratory and consists of a large race track-shaped channel of total length of 17.55 m, a straight working section of 10.8 m and a total capacity of about 10 m3. The channel is 60 cm wide, and water depth is maintained at 40 cm. Water flow is generated by a conveyor belt system, acting as a paddle wheel. Flow velocity is controlled through the regulation of the speed of the conveyor belt. Within the working section, a 3-m test section has a glass wall for visual observations and the bottom consists of a 15-cm-deep pit filled with sand to secure the plants. A carriage with a 3D positioning system can be placed anywhere along the length of the working section. The 3D positioning system can move across the total width and depth of the flume and over a maximum length of 70 cm in the direction of the main flow. The latter axis is defined as the x-axis, the y-axis is horizontally across the main flow, and the z-axis is the vertical axis. For a more detailed flume description, see Bouma et al. (2005).
The whole flume was filled up to a depth of 40 cm with tap water containing 20 μm NO3−, 0.2 μm PO43− and 0.4 μm NH4+. Greenhouse lamps were attached above the flume tank to generate a homogeneous irradiance over the test section. The photoperiod and photosynthetic photon flux density were constant during 14 h and 280 mmol photon m−2 s−1, see Morris et al. (2008) for further details.
Incubation experiments in the flume tank and determination 15N-NH4+ uptake
Six series of duplicate incubation experiments were performed within the flume using P. natans and/or R. fluitans at three different patch configurations and at two water velocities (0.3 m s−1 and 0.1 m s−1). All the conditions and characteristics of these experiments are summarised in Table 1.
In a first configuration (series 1 and 2), a patch of P. natans of 0.3 m × 2.1 m, with a plant density of 278 plant stems m−², was established in the flume test section along the right bank of the channel (Fig. 1a). This is within the range of densities found in rivers in Flanders for P. natans (K. D. Bal and J. Schoelynck, unpublished data). The average length of P. natans used was 0.89 m (n = 198 and standard deviation = 0.18 m). In this configuration, the flume section was half-filled with plants, while the other half was left open.
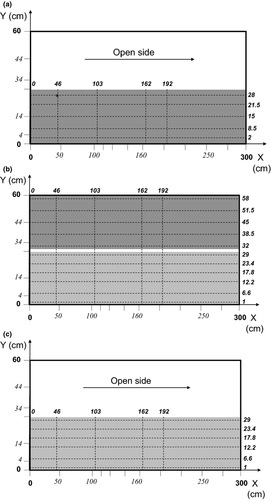
For the second configuration (series 3 and 4), a R. fluitans patch was placed alongside a P. natans patch as described before. In this configuration, the whole flume channel was filled with plants (Fig. 1b). The final configuration (series 5 and 6) was the same as the first using R. fluitans at a plant density of >400 specimens m−2 (Fig. 1c). The mean length of these specimens was 0.71 m (n = 238 and standard deviation = 0.21 m). The dimensions of the patches so created were within the range found by Schoelynck et al. (2012) and follow the typical patch distribution caused by spatial self-organisation of macrophytes, that is, an empty zone besides a patch due to increased stress (in this case velocity). In Flanders, this kind of vegetation patch is also frequently seen, due to management of lowland rivers (Bal et al., 2011a,b).
Practically, for all three patch configurations, the test section was filled with plants as described above, prior to all incubations. However, the determination of NH4+ uptake rates by plants within the patch was performed only on specimens at selected positions. The selected test positions were located along five lines perpendicular to the direction of flow placed at x = 0, 46, 103, 162 and 192 cm from the leading edge of the patch and with n number of specimens placed on each line, with n = 5 for P. natans and n = 6 for R. fluitans (Fig. 1b). Between successive incubations, these selected test positions were left open.
For all incubations, 15N-NH4+ was added to reach a final concentration between 20 and 30 μm with 30% of the N as 15N abundance. The NH4+ concentration was measured at the start, middle and end of the incubation at six to 12 positions inside the plant patch and the coefficient of variation between measurements never exceeded 15%, demonstrating homogenous labelling and negligible depletion during the incubation. The same labelled flume water was used to perform the four experiments for a given patch configuration, before being replaced with fresh water and label for the next configuration.
At the start of an incubation, the five lines of test positions in the macrophyte patch were planted with the selected specimens. The flow was then set at the desired velocity, and after 30 min, photographs were taken in front of each test position line, through the transparent wall, to measure the bending of plants. The selected and transplanted specimens were incubated for about 6 h.
At the end of each incubation, shoots of P. natans and R. fluitans located at the five × n test positions were removed one by one from the labelled water and rinsed with tap water. Prior to being packed in aluminium packets, each R. fluitans specimen was measured (length), while each P. natans specimen was photographed against a gridded white background. In addition to the samples collected (i.e. 25 shoots of P. natans and 30 shoots of R. fluitans), six specimens of each species were also collected at random over the course of the experiments from our various species stocks to determine the background 15N signal. All were packed individually in aluminium packets, dried for at least 48 h at 60 °C, weighed and ground/homogenised to fine powder using an analytical mill (IKA) equipped with a cutting blade (for fibrous material). About 3 mg of powder from each sample was analysed for total N content and 15N-atomic percentage [as (15N/total N) × 100] by combustion with an Elemental Analyser (Flash series 1112) and subsequent isotope ratio mass spectrometry (Thermo Delta V – IRMS). An acetanilide internal standard was used to calibrate the measured N contents. IAEA-N1 and IAEA-311 were used as natural abundance and 15N enriched reference materials, respectively, for the isotopic analysis.
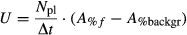
Hydrodynamic measurements in the flume tank
To evaluate the effects of shoot flexibility and shoot density of submerged plants on hydrodynamics, velocity measurements were performed within the flume inside the vegetated patches of P. natans and/or R. fluitans. The velocity components (u, v and w) in the downstream (x), cross-stream (y) and vertical (z) directions, respectively, were measured with an acoustic Doppler velocimeter (ADV, Nortek field version) mounted on a 3D positioning system.
Vertical hydrodynamic profiles were measured in the downstream flow direction (x) from outside to inside of the patch using a grid design consisting of 200 individual measuring points. The grid was made up of 10 x-locations, regularly spaced within the patch, from 50 to 280 cm from the front; four cross-stream (y) locations at 4, 14, 34 and 44 cm from the right bank; and five vertical z-locations at 3, 7, 13, 21 and 32 cm from surface (Fig. 1b).
Reynolds stress (τxz, cm2 s−2) at the top of the patch and turbulent kinetic energy (TKE, mm2 s−2) within the patch at each location were calculated according to Jonsson et al. (2006). Turbulent kinetic energy (TKE) is defined as the mean kinetic energy per unit mass associated with eddies in a turbulent flow. Reynolds stress is a stress tensor accounting for turbulent fluctuations in the fluid momentum. Finally, velocity gradients were used to calculate the volumetric flow rate of water through the patches (Qp, m3 s−1), according to equations in Morris et al. (2008).
The bending angle of each specimen was measured from photographs taken during the experiments. The maximum height of each specimen was calculated taking into account the height of the water column, the bending angle and the length of the specimen removed from the patch after the experiment. Plant length and dry mass were measured (stems and peduncles) after removal. Plants were also examined for their morphological characteristics (projected leaf area by taking photographs, see below for explanation), and their stems and peduncles were measured.
To summarise the vertical profiles of the hydrodynamic parameters (to facilitate the investigation of horizontal variation in relation to the leading edge of the patch), two definitions of patch regions were used: (i) ‘top of the patch’, defined as patch height (Zp) ± 10%, and (ii) ‘within the patch’, defined as Zp + 10% to z = 0. Patch height (Zp) was the mean maximum height of the specimens removed from the patch after the experiments (25 for P. natans and 30 for R. fluitans).
Estimation of the surface area for P. natans and R. fluitans specimens
For P. natans, all specimens analysed (n = 198) were photographed in front of a grid. Each photograph was digitised and images analysed individually to determine leave surface area, peduncle length and stem length. The surface area of the peduncles and stems was calculated by multiplying length by circumference (1.5 mm on average). Total surface area was then calculated by summing up the leave surface areas × 2 (both side of the leaves are considered) and stem + peduncle surface areas. A similar procedure could not be applied for R. fluitans due to their complex morphological structure. An estimate of the surface area (S) was calculated from the dry biomass (B) using the equation of Sher-Kaul et al. (1995) for a macrophyte species that has a similar leaf structure to R. fluitans (P. pectinatus): S(cm²) = 158.1861 + 25.5342*B(g)*10.
Statistics
A t-test (for normal distributions) and Mann–Whitney rank-sum test were used to examine statistical differences between the 15NH4+ uptake rates of each species incubated under two velocity treatments and two flume configurations.
Correlations between 15NH4+ uptake rates (μmol g−1 dry mass h−1), hydrodynamic parameters (i.e. mean water column and patch velocity Ū (m s−1); Reynolds stress txz (cm2 s−2); turbulent kinetic energy TKE (mm2 s−2); and patch water flow Qp (m3 s−1) and patch properties [i.e. patch height (m), bending angle (°)] were assessed using Pearson's correlation coefficient. An alpha value of 0.05 was considered to be significant. Statistical procedures were carried out in Statistica 6.0 (StatSoft; Tulsa, OK, U.S.A., Tables 2 & 3).
| Height | Angle | Ū | τxz | TKE | |
|---|---|---|---|---|---|
| Angle | 0.78 | 1.00 | |||
| Ū | −0.72 | −0.86 | 1.00 | ||
| τ xz | 0.03 | −0.15 | −0.12 | 1.00 | |
| TKE | 0.31 | 0.41 | −0.31 | 0.59 | 1.00 |
| Q p | −0.60 | −0.85 | 0.97 | −0.08 | −0.32 |
| P. natans (n = 28) | R. fluitans (n = 27) | All (n = 55) | |
|---|---|---|---|
| Height | −0.19 | −0.36 | −0.60 |
| Angle | −0.69 | −0.51 | −0.55 |
| Ū | 0.70 | 0.53 | 0.39 |
| τ xz | −0.13 | 0.09 | −0.23 |
| TKE | −0.32 | −0.48 | −0.21 |
| Q p | 0.71 | 0.51 | 0.32 |
Results
Influence of species on hydraulics (velocity, Reynolds stress, turbulent kinetic energy and volumetric flow)
Mean water velocity averaged over the vertical water column and the patches (Ū) was similar for P. natans and R. fluitans, Ū ranging from 0.02 m s−1 to 0.19 m s−1 for P. natans and from 0.02 m s−1 to 0.21 m s−1 for R. fluitans. The minor differences were due to slight changes in the external forcing. The patch was deflected by increasing water velocity in both species. Initially, patches of P. natans and R. fluitans reached the water surface. As expected, the bending of the plants increased with velocity, until the patch height at 0.3 m s−1 was about 0.04 and 0.12 m for P. natans and R. fluitans, respectively (data not shown).
For P. natans at 0.1 m s−1, high positive Reynolds stress values (τxz > 1000 cm² s−2) were observed at 2 m from the leading edge of the patch in the fully filled flume configuration and close to the downstream edge of the patch in the half-filled flume configuration, indicating a downward flow into the patch and the presence of a downstream wake (Fig. 2). In addition, negative τxz was observed close to the downstream edge of the patch in the fully filled flume configuration. At the higher velocity, the Reynolds stress reached a maximum for both configurations near the 2.5 m point.
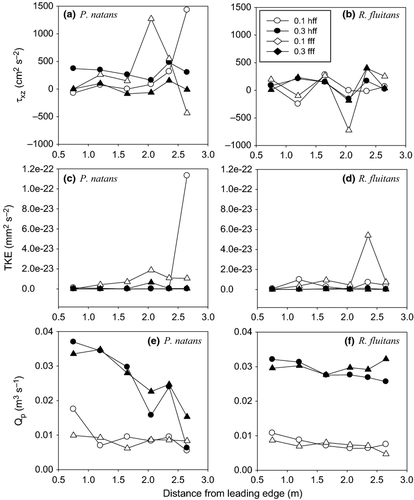
For both flume configurations at 0.1 m s−1, alternation of positive and negative τxz was at the leading edge of the R. fluitans patches, with a minimum observed 2 m from the leading edge in the fully filled flume configuration. In contrast to high velocity, τxz was positive, except for minima also located at 2.5 m from the leading edge (Fig. 2).
Turbulence within the patches, represented by TKE, was related to flow velocity and was comparable between the two species for all flume configurations. Nevertheless, the position of TKE maxima differed slightly. For P. natans, maximum TKE was observed at 2.5 m downstream from the leading edge at 0.1 m s−1 in the half-filled flume. For R. fluitans, this maximum value was observed between 2 and 2.5 m from the leading edge at a velocity of 0.1 m s−1 in the fully filled configuration (Fig. 2c,d).
The flow volume passing through the patches (Qp) was similar for P. natans (range, 0.0055–0.0369 m3 s−1) and R. fluitans (range, 0.0047–0.0322 m3 s−1). Generally, maximum Qp was observed close to the leading edge of the P. natans patches; water flow then decreased towards the downstream edge of the patch, except for a small peak observed at 2 m from the leading edge. For R. fluitans, maximum Qp was also observed close to the leading edge, except for the experiment performed at 0.3 m s−1 in the fully filled flume configuration, where maximum Qp was observed close to the backward edge of the patch (Fig. 2).
Influence of species, velocity and patch properties on ammonium uptake
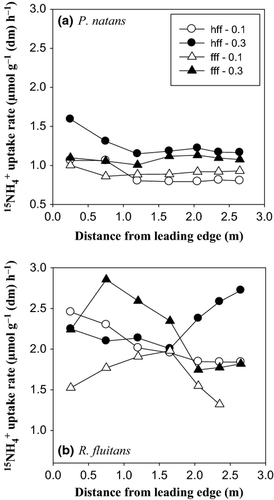
Ammonium uptake rates measured in the flume at velocities of 0.1 and 0.3 m s−1 ranged between 0.79 and 1.59 μmol g−1 dry mass h−1 for P. natans and between 1.32 and 2.86 μmol g−1 dry mass h−1 for R. fluitans. Ammonium uptake rate was significantly higher for R. fluitans than for P. natans at both water velocity treatments (Fig. 3; Mann–Whitney rank-sum test: P < 0.001) with a mean (±SD) of 2.07 μmol g−1 dry mass h−1 ± 0.75 for R. fluitans, almost double that for P. natans (1.06 μmol g−1 dry mass h−1 ± 0.46). The specific surface of R. fluitans was two times higher than for P. natans (550 and 260 cm² g−1, respectively), probably helping to explain the observed difference in uptake rates for both species.
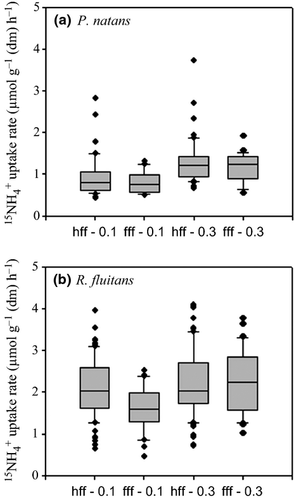
Additionally, the uptake rates for both species doubled at high velocity (0.3 m s−1) over that of low velocity (0.1 m s−1; P. natans: t-test, P < 0.0001; R. fluitans: t-test, P = 0.012). There was no significant difference in uptake rate between the two flume configurations involving P. natans shoots (at 0.3 m s−1, t-test: P = 0.673 and at 0.1 m s−1, t-test: P = 0.486). Similar results are observed for R. fluitans incubated at 0.3 m s−1 (t-test: P = 0.990).
At both velocities and flume configurations, uptake was greater at the leading edge of the P. natans patches. Uptake generally declined downstream of the leading edge up to 1 m and then remained constant (Fig. 4a). Similarly, at both velocities but only for the half-filled flume configuration, uptake rate was enhanced in R. fluitans at the leading edge of the patch. In contrast, uptake was greater at 1.5 m from the leading edge at low velocity and at 0.5 m at high velocity for R. fluitans in the fully filled flume configuration. A general decrease in R. fluitans uptake was observed downstream of the leading edge, except for the incubation performed at high velocity in a half-filled flume. In this experiment, uptake increased 1.5 m downstream of the leading edge (Fig. 4b). These results showed that 15NH4+ uptake exhibited species-specific and spatially explicit patterns. Contrary to P. natans, a higher sensitivity of uptake rate to the flume configuration was found in R. fluitans.
Combination of hydraulics and species on ammonium uptake
The existence of a spatial pattern for both nutrient uptake and several hydrodynamic parameters (Figs 2 & 4) raises the question of how these are related. When all patches were considered together, as expected, turbulent kinetic energy (TKE) was significantly associated with Reynolds stress (τxz) and to a lesser extent with mean water velocity (Ū) and canopy water flow (Qp; Table 2). Patch properties (height and bending angle) were strongly correlated with Ū and Qp and, to a lesser extent, with TKE. Nevertheless, despite species-specific differences in patch density, Qp was strongly correlated with Ū, illustrating homogeneity in height of the patch and patch compression among the two species.
When each species was examined individually, 15NH4+ uptake rate by P. natans was significantly correlated with bending angle, Ū, TKE and Qp. Ammonium uptake rate by R. fluitans was significantly correlated with nearly all patch properties and hydrodynamic parameters, although to a lesser extent than in P. natans (Table 3). For both species, the correlation coefficient between uptake and Qp was one of the highest, at 0.71 and 0.51 for P. natans and R. fluitans, respectively. For both species, uptake was not correlated with Reynolds stress, which indicates the weak effect of turbulent mixing in determining ammonium uptake in these plants.
When the combined data sets for both macrophyte species were considered, 15N-ammonium uptake was significantly correlated with all patch properties and with Ū and Qp, although to a lesser extent (Table 3). Closer examination of the data shows that while both patch and hydrodynamic variables show weak correlations with uptake rates independently of the species studied, Ū and Qp seem to be the only variables that could explain variation in the uptake of the P. natans species.
Discussion
Results from this study demonstrate the effect of both increasing water flow velocity and spatial position on 15N-ammonium uptake rates of the shoots of two macrophyte species with different patch properties (i.e. plant density, patch length, leaf morphology; Fig. 4). Our results are consistent with previous studies of seagrass species highlighting spatial patterns in NH4+ uptake within patches (e.g. Morris et al., 2008).
For both species, the correlation coefficient between ammonium uptake and Qp was one of the highest, at 0.71 and 0.51 for P. natans and R. fluitans, respectively, showing that patch flow (Qp) was the most important variable that could explain both the spatial- and water velocity-related variations in ammonium uptake for both species. The strong effect of water velocity on Qp emphasises the importance of patch compression (Thomas & Zande, 2000; Abdelrhman, 2007), indicating that flexibility may be an important trait that can simultaneously determine both the local phylloclimate and the ecosystem engineering capacity of submerged macrophytes (Bouma et al., 2005; Brun et al., 2006; Peralta et al., 2006, 2008; Hendriks et al., 2010). This canopy opening and closing locally changed water velocity, which is consistent with our finding that patch flow was the most important parameter. In addition, the Qp values of P. natans (range, 0.0055–0.0369 m3 s−1) and R. fluitans (range, 0.0047–0.0322 m3 s−1) were similar, although plant density was lower in the P. natans patches (Table 1).
The positive values of Reynolds stress (τxz) and slightly enhanced Qp observed 2 m from the leading edge of the P. natans patches in the experiments with the fully filled flume at 0.1 m s−1 indicated that the ‘wake’ resulting from the flow separation initiated at the leading edge (Morris et al., 2008) was responsible for enhanced ammonium uptake. Nevertheless, despite this individual example of the effect of τxz on ammonium uptake of P. natans, Reynolds stress was not correlated with uptake rate for both species. In contrast to the results of Morris et al. (2008), our results suggest a weak relationship between turbulent mixing and ammonium uptake in freshwater plant species. As turbulent mixing depends on vertical vortices penetrating the plant canopy, this highly periodic process (Ghisalberti & Nepf, 2005) could be reduced by the number of stems floating on or near the surface as result of reduced flow above the vegetation patches at low velocities. The fact that TKE was significantly associated with ammonium uptake in R. fluitans highlights the importance of turbulent transport and mixing of the rapid overflow into the patch in determining uptake in the dense R. fluitans patches. The weak correlation between TKE and ammonium uptake for P. natans indicates that turbulence may not be as important in transporting nutrients to the plants in species with sparse foliage.
Because the uptake affinity (i.e. physiological assimilation efficiency in static conditions) for each species was not determined, the observed differences in ammonium uptake could be species specific, due to the patch properties, or accounted for by how these properties interacted under the experimental conditions. The estimated specific surface area of R. fluitans, being double that of P. natans, may explain the differences between the uptake rates. The positive relationship between velocity and the ammonium uptake capacity of the two species appears to be a function of hydrodynamics and the response of the plant patches to the unidirectional flow. Mean ammonium uptake rate of R. fluitans (on average 2 μmol g−1 dry mass h−1) was double that of P. natans (on average 1 μmol g−1 dry mass h−1). To compare our 15N-ammonium uptake rate to values reported in literature as ammonium uptake, our results must be treated taking into account that the approximate abundance of 15N in the label water was 30%. Our range of ammonium uptake (6.3–53 μg N g−1 dry mass h−1) is comparable with the uptake rates of seagrass ranging between 15 and 66.6 μg N g dry mass−1 h−1 (Morris et al., 2008).
Short-term uptake rates (a few hours) may not necessarily represent growth rates, but N uptake can be converted to growth rates by multiplying the ammonium uptake by the N content to obtain plant growth rates of 0.04 ± 0.02 day−1 (mean ± SD) for P. natans (n = 198) and 0.08 ± 0.04 day−1 for R. fluitans (n = 239). These values are in the range of optimal growth rates for similar freshwater species (Nielsen & Sand-Jensen, 1991).
Other studies have highlighted the fact that factors other than species identity, spatial position of individuals within the vegetation patch and water current are also important in explaining ammonium uptake. For instance, the importance of turbulent mixing in the architecture-specific N canopy uptake (patch in our case; Morris et al., 2008) supports the hypothesis that spatial patterns in N uptake dependent on the patch properties of different species (Peterson et al., 2004). Results from this study show that species, flow velocity and spatial position also have an effect on 15N-ammonium uptake rates for freshwater species. The observed existence of spatial patterns for both nutrient uptake and some of the hydrodynamic parameters raises the question of how these are related.
Our results showed the importance of patch properties on 15N-NH4+ uptake rate of P. natans and R. fluitans. Height and bending angle were significantly correlated with uptake rate when the combined data sets for both macrophyte species were considered, while angle was significantly correlated with 15N-NH4+ uptake rate of P. natans and to a lesser extent, with that of R. fluitans when each species was examined individually. This discrepancy is the result of differences in individual bending angles in the front or at the end of the patch. Plant individuals at the end of the patch had a more upright stature than those located at the leading edge. This non-rigid state of submerged species enables these species to persist under higher velocity conditions than emerged species (Schutten & Davy, 2000). These differences in bending angles have been shown to have an impact on the performance (drag generation) of submerged plants (Bal et al., 2011a). Concomitant reduction in drag and thus flow conditions within the patch is the result of differences in stem flexibility. Investment in components like lignin and cellulose regulates plant stiffness. With increased lignin concentration, stems have greater stiffness (Kaufman et al., 1999). From Schoelynck et al. (2010), it can be seen that aquatic vegetation has variable cellulose (between 104 and 387 mg g−1 dry mass) and lignin concentrations (between 3 and 192 mg g−1 dry mass). This flexibility determines the typical waving movement (monami) of submerged aquatic vegetation. This ‘opening’ of the vegetation canopy with increased turbulence has been shown to enhance ammonium uptake by the plants (Thomas & Cornelisen, 2003), as seen in our study.
Acknowledgments
K.D.B. would like to thank BELSPO for funding Manudyn II, the research project in which this experiment was performed. K.D.B. would also like to thank NRF, VLIR and the Dean of the Faculty of Science and Agriculture at the University of Limpopo for post-doctoral funding. We would also like to thank S. Marr and the editor (Alan Hildrew) for their constructive comments and suggestions that significantly improved the final manuscript.




