
Synergistic and species-specific effects of climate change and water colour on cyanobacterial toxicity and bloom formation
Summary
- Cyanobacterial blooms are a worldwide phenomenon in both marine and freshwater ecosystems and are predicted to occur more frequently due to global climate change. However, our future water resources may also simultaneously suffer from other environmental threats such as elevated amounts of humic content and consequent increased water colour, a phenomenon called ‘brownification’.
- In order to investigate the effects of temperature and water colour in combination, we performed a mesocosm experiment combining a 3 °C increase in temperature and a doubling in water colour. With this, we created a projected future scenario for our water resources, and we specifically focused on how these changes would affect cyanobacterial bloom formation and toxicity.
- We showed that despite total cyanobacterial biomass remaining unaffected, the abundance of one individual cyanobacterial species, Microcystis botrys, increased in response to the combination of elevated temperature and increased water colour. Furthermore, population fluctuations in M. botrys explained the majority of the variations in microcystin concentrations, suggesting that this species was responsible for the more than 300% higher microcystin concentrations in the future scenario treatment compared to the ambient scenario. Hence, it was not a change in cyanobacterial biomass, but rather a species-specific response that had the most profound impact on bloom toxicity.
- We argue that understanding such species-specific responses to multiple stressors is crucial for proper management decisions because toxic blooms can significantly affect both biodiversity and ecosystem functioning, as well as ecosystem services such as drinking water supply and recreation.
Introduction
The occurrence of harmful algal blooms, and specifically cyanobacterial blooms, is a worldwide phenomenon, and much attention has been focussed on this subject in the light of eutrophication and the formation of toxic blooms (Paerl et al., 2011; Paerl & Paul, 2012). However, besides eutrophication, water bodies around the globe are now faced with additional challenges, such as elevated temperatures resulting from climate change (Christensen et al., 2007), and “brownification” (Granéli, 2012) due to increased leakage of terrestrial humic substances into the water resulting from, for example, reduced acid deposition (Hongve, Riise & Kristiansen, 2004; Evans, Monteith & Cooper, 2005; Monteith et al., 2007; Ekström et al., 2011). In the next 100 years, global mean temperatures are projected to increase by between 2 and 5 °C in the Northern Hemisphere (Christensen et al., 2007). Furthermore, if the rate of increase in water colour continues at current rates, a doubling in water colour can be expected during the same period (Hansson et al., 2013).
Cyanobacteria are generally well adapted to grow at high temperatures, with higher species-specific growth rates than some common classes of phytoplankton (Paerl & Paul, 2012), but not all (Lürling et al., 2013). Temperature alone is probably the single most important variable for explaining the proportion of cyanobacteria found in shallow lakes from South America to northern Europe (Kosten et al., 2012). Furthermore, Kosten and co-workers also found synergistic effects between temperature and nutrient loading, which further enhanced the proportion of cyanobacteria. This is consistent with the findings of Jeppesen et al. (2011) who also observed these synergistic patterns in cyanobacterial biomass in Danish lakes.
In contrast to studies of temperature effects, studies on cyanobacterial responses to increasing water colour are scarce (but see e.g. Kirkwood, Nalewajko & Fulthorpe, 2003), and focus has mainly been on cyanobacterial tolerance of low light conditions in general and their ability to change vertical position (Scheffer et al., 1997; Huisman et al., 2004; Jöhnk et al., 2008).
One of the main concerns associated with cyanobacterial blooms is that many species are able to produce a variety of toxins (Codd, Bell & Brooks, 1989; Codd, 1995). If cyanobacterial blooms increase due to projected global change, this could considerably increase toxicity in waterbodies. Increased toxicity may have strong effects on both biota (Hansson et al., 2007) and humans using the water resource (Carmichael, 2001; Falconer, 2005), and already much research focuses on ways to manage and reduce cyanobacterial blooms due to eutrophication (Hansson et al., 1998; Lürling & Faassen, 2012; Reitzel et al., 2013).
Since increases in temperature and water colour are expected to co-occur in lakes, we performed a mesocosm experiment to address potential interactive effects between the two factors. Based on a study by Hansson et al. (2013), who found increased Microcystis spp. abundance as a response to elevated temperature, we hypothesised that elevated temperature would promote cyanobacterial proliferation and would thereby lead to higher toxicity in the waterbody. Increased water colour was expected to reduce overall total algal growth due to light limitation, thereby reducing cyanobacterial biomass in relation to less coloured water. Furthermore, we wanted to determine whether there are synergistic effects between temperature and water colour, as found between temperature and nutrients, and if so, how this would affect overall toxicity in the water.
Methods
Experimental set-up
We performed an experiment between April and September 2011 consisting of 24 insulated 400-L mesocosms (diameter = 0.7 m, height = 1 m) divided into four treatments, each replicated six times. In addition to the control (C), which mimics the present environmental and climatic conditions of northern temperate lakes, one treatment had water temperature elevated by 3 °C compared to ambient temperature (T), a temperature projected to occur around 2100 (Christensen et al., 2007). In another treatment, we approximately doubled water colour compared to the control (B), to mirror projected ‘brownification’ during the coming decades (Hansson et al., 2013). Finally, we combined the above-mentioned factors in the fourth treatment (TB), which represented the projected conditions with respect to both temperature and water colour.
The top centimetres of sediment were collected at 1-m depth from the shallow (mean depth = 1.5 m) and eutrophic Lake Krankesjön (N55° 42′ 27′′, E13° 27′ 58′′) on 28 March 2011 using hand nets. The sediment was put into several dark plastic boxes and transported to the experimental facility within two hours of sampling. Each mesocosm received equal amount of sediment from each sediment box until a total sediment thickness of 60 mm was reached. This was done to avoid differences in sediment quality, particularly with respect to the amount of resting cysts present that might have arisen from patchiness in cyst distribution in the lake. After the sediment was added, each mesocosm received 320 L of unfiltered Lake Krankesjön water (80% of the final volume). The final 20% of water differed between clear water (C and T) and humic water treatments (B and TB). The clear water treatment received 80 L of 20 μm filtered Lake Krankesjön water, and the humic treatments received 80 L of 20 μm filtered lake water from the more humic Lake Liasjön (N56° 26′ 47′', E13° 59′ 34′'), resulting in an 81% increase in absorbance at 420 nm in the humic water treatments. Everything caught on the filters were rinsed into a bucket with filtered lake water and pooled into a slurry of which 2 L was added to each mesocosm to avoid differences in biotic communities due to differences in lake origin.
The 3 °C increase in temperature was achieved by using a PC-controlled temperature system that regulated the elevated temperature in the treatments in relation to the control mesocosms. Temperature was measured every ten seconds using temperature sensors (National Semiconductor, LM335AZ, precision temperature sensor) in the mesocosms, and if the temperature in any of the elevated mesocosms differed more than 0.2 °C from the desired temperature, the aquarium heater (Jäger 150W) of that specific mesocosm was turned on or off until the desired difference was re-established. In this way, each temperature-elevated mesocosm (T and TB) always followed the temperature patterns of the ambient-temperature mesocosms (C and B), but on a 3 °C higher level. Hence, organisms experienced both the diel and seasonal variations in temperature in all our mesocosms. Mean daily water temperatures in the ambient temperature treatment went from 10.0 °C in April to 14.0 °C at the end of the experiment in September, with a maximum temperature of 23.5 °C in June. To avoid temperature differences in the mesocosms, a gentle airflow was induced inside a small Plexiglas tube in which the heater was mounted at one side of the mesocosm. For more information regarding the temperature system, see Nicolle et al. (2012) and Ekvall & Hansson (2012).
Maintaining the mesocosms
To ensure a constant water level, and to maintain differences in water colour (humic content), weekly additions of either distilled water or 20 μm filtered humic water mixed with distilled water were added to the mesocosms. To avoid nutrients being bound up in periphyton, the walls of the mesocosms were gently scrubbed each week. Along with the scrubbing of the walls, filamentous algae and macrophytes were removed from the mesocosms each week. Furthermore, weekly nutrient additions of commercial plant nutrients were made (Blomstra växtnäring, Cederroth, Upplands Väsby, Sweden). Total phosphorus concentration, used as a proxy for nutrient additions in the mesocosms, did not differ among the treatments during the experiment (anova: F3,116 = 0.50, P = 0.69), with an overall mean concentration of 40.6 μg L−1 during the course of the experiment.
Sampling and analysis
Mesocosms were sampled every second week using a Plexiglas tube (length: 1 m, diameter: 70 mm) that was carefully lowered into the mesocosm, reaching from the surface to c. 10 cm above the sediment surface. Before taking the tube up from the mesocosm, it was sealed with stoppers at each end to get water from the whole water column. Three tube samples were taken along the diameter of each mesocosm and were pooled into an integrated sample from which subsamples were taken for colour, nutrients (total-P), phytoplankton and cyanobacterial toxins (microcystins, anatoxins and cylindrospermopsins). Phytoplankton samples were preserved with Lugol's solution and stored in a cold room at 4 °C until later enumeration. Colour was measured as absorbance at 420 nm using a spectrophotometer (Beckman DU800 Coulter), after filtration through a GF/C filter (WhatmanTF).
Cyanotoxins
Microcystin samples (20 mL) were stored at −20 °C until analysis. Prior to analysis, the samples were thawed and frozen three times and ultrasonicated for two minutes in an ice bath to release the intracellular toxins into the water (Hansson et al., 2007). Following ultrasconication, samples were centrifuged at 4590 g for 20 min, and the supernatant was analysed for microcystins (microcystin-LR equivalents) according to enzyme-linked immunosorbent assay (ELISA) standard procedure (Microcystins-DM ELISA Microtiter Plate, Abraxis LLC, Warminster, PA, U.S.A.). Absorbance was measured at 450 nm on a Biochrom Asys Expert 96 Microplate Reader (Biochrom Ltd., Cambridge, U.K.).
Samples for analysis of anatoxins and cylindrospermopsins (20 mL) were immediately acidified with formic acid (FA) to 0.1% (v:v) before being stored in the same way as the microcystin samples. The samples were concentrated, and cells were destructed by lyophilisation and subsequently reconstituted in 5 mL of water with 0.1% FA. Samples were extracted for ten minutes at 95 °C and dried in a SpeedVac (SPD121P, Thermo Scientific Savant, Asheville, U.S.A.). Dried samples were then reconstituted in 50 μL water with 0.1% FA and 950 μL acetonitrile with 0.1% FA and cleaned up by solid-phase extraction (SPE) conforming to Faassen et al. (2012). Samples were analysed for anatoxin-a, homoanatoxin-a, (homo)anatoxin-a degradation products, cylindrospermopsin and deoxy-cylindrospermopsin by LC-MS/MS according to Faassen et al. (2012). All solvents used were at least of analytical grade, and water was purified by a Q-Pod (Millipore, Billerica, U.S.A.).
Cyanobacterial community composition
Cyanobacteria were identified and counted in sedimentation chambers (2, 5, 10 or 25 mL) at 200× and 400× magnification according to Utermöhl (1958) using a Nikon TMD inverted phase contrast microscope. Sedimentation time varied between 4 and 24 h depending on the size of the chambers. Cyanobacteria were counted in the entire chamber, per diagonal of the chamber or field of view. For the counting of cyanobacteria growing in filaments and colonies, a special eyepiece graticule, where a grid divided in equal-sized squares covered the whole field of view, was used. Microcystis species were counted with the square grid and each square multiplied with estimated cells per square. The estimation was based on a study by Cronberg (1982) where colonies of different colonial cyanobacterial species were separated by ultrasonic treatment and thereafter counted, giving an estimation of number of cells per square for the different species.
Identification of cyanobacteria was based on Komarkova-Legnerova & Cronberg (1994), Komárek & Anagnostidis (1999) and Komárek & Zapomelova (2007, 2008 (and references therein)). Different Microcystis species were distinguished based on colony size and shape as well as cell size. All species observed in the experiment occur frequently in Scanian lakes. Other cyanobacterial species present in the samples were counted at the same time as the Microcystis sp. and Anabaena sp., which, however, constituted the majority of the algae in the samples. Cyanobacterial biomass was calculated from cell size measurements using stereometric equations. The experiment started in April, although cyanobacterial biomasses were too low (total cyanobacterial biomass <0.01 mg L−1) before late May to allow for accurate biomass estimations of individual species.
Finding potential microcystin producers
A total of 12 cyanobacterial species were found in the experiment (Table 1). In order to assess which of the species were most likely to be microcystin producers in the experiment, we applied a two-step procedure. The first step was to assess how common each species was in the mesocosms regardless of treatment and sampling occasion. Species that occurred in fewer than 5% of all the samples (n = 216) were not included in further analysis (Table 1). In step two, the biomasses of the remaining species, as well as the microcystin concentrations, were put into a stepwise multiple regression to select the most likely microcystin producers.
| Cyanobacterial species |
|---|
| Anabaena |
| A. cf. crassa |
| A. lemmermannii* |
| A. macrospora* (a.k.a. Dolichospermum macrosporum) |
| Microcystis |
| M. aeruginosa |
| M. botrys* |
| M. flos-aquae |
| M. wesenbergii |
| M. sp. * (unidentified Microcystis sp. colonies) |
| Other cyanobacteria |
| Snowella spp. |
| Picocyanobacteria spp.* |
| Planktolynbya spp. |
| Pseudoanabaena spp. |
Statistics
All data were square-root-transformed prior to analysis to achieve normality. A stepwise multiple regression was used to select the species most likely to produce microcystins. In the stepwise multiple regression analysis, species-specific biomasses were added in a sequence to evaluate whether they contributed significantly to explaining the variation in microcystin concentrations. If an added species contributed to the success of the model, it was retained, otherwise it was removed. The model was subsequently retested by adding and removing species until only significant contributors remained. The total cyanobacterial biomass and the total biomass of the two dominant genera, Anabaena spp. and Microcystis spp., as well as the biomass for species selected by the multiple regression analysis as potential microcystin producers, were analysed with repeated-measures anovas. This was done using temperature and water colour as fixed factors and biomasses as dependent variables. The same procedure was used for analysing microcystin concentrations. Greenhouse–Geisser corrected values were used for all the repeated-measures anovas in order to compensate for any lack in sphericity. All statistical analyses were made using SPSS 21 for Macintosh.
Results
Cyanobacterial community composition
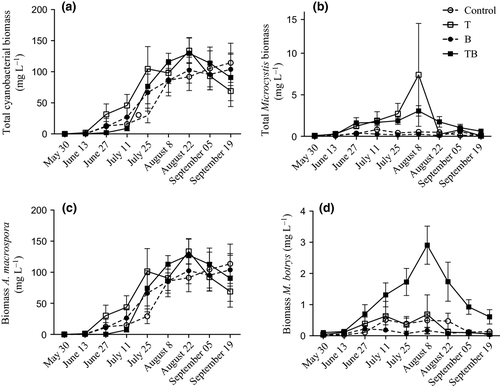
Total cyanobacterial biomass, dominated by Anabaena spp., was similar among treatments (Fig. 1a) and increased significantly during the experiment (Table 2). However, this increase was neither affected by temperature, nor water colour nor by the interaction between the two factors. Average biomasses of total Anabaena spp. also increased significantly with time (Fig. 1c), but again, the increase did not differ between treatments (Table 2). Total Microcystis spp. biomass increased significantly with time and had a faster increase in temperature-elevated treatments (T and TB) (Fig. 1b). This faster development in total Microcystis spp. biomass also led to higher average biomass of Microcystis spp. in temperature-elevated treatments (T and TB) (Table 2). However, neither water colour nor the interaction between temperature and water colour had any effect on total Microcystis spp. biomass (Table 2).
| Factor | Total cynobacterial biomass (mg L−1) | Total Anabaena spp. biomass (mg L−1) | Total Microcystis spp. biomass (mg L−1) | M. botrys biomass (mg L−1) | A. macrospora biomass (mg L−1) | Microcystin concentrations (μg L−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F2.81,56.18 | P | F2.78,21.09 | P | F2.64,52.73 | P | F3.31,66.15 | P | F2.77,55.45 | P | F2.70,53.93 | P | |
| (a) Within-subject effects | ||||||||||||
| Time | 56.57 | <0.001 | 54.53 | <0.001 | 8.74 | <0.001 | 10.23 | <0.001 | 54.78 | <0.001 | 26.80 | <0.001 |
| Time × Temperature | 1.96 | 0.134 | 1.70 | 0.180 | 3.10 | 0.040 | 2.20 | 0.091 | 1.72 | 0.178 | 2.66 | 0.063 |
| Time × Colour | 0.43 | 0.720 | 0.56 | 0.633 | 0.53 | 0.643 | 1.62 | 0.188 | 0.56 | 0.629 | 1.28 | 0.290 |
| Time × Temperature × Colour | 1.24 | 0.303 | 1.34 | 0.271 | 0.85 | 0.461 | 5.42 | 0.002 | 1.33 | 0.275 | 4.98 | 0.005 |
| F1,20 | P | F1,20 | P | F1,20 | P | F1,20 | P | F1,20 | P | F1,20 | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (b) Between-subject effects | ||||||||||||
| Temperature | 0.71 | 0.408 | 0.42 | 0.523 | 18.62 | <0.001 | 9.87 | 0.005 | 0.42 | 0.525 | 13.44 | 0.002 |
| Colour | 0.02 | 0.888 | 0.04 | 0.849 | 0.15 | 0.699 | 4.99 | 0.037 | 0.03 | 0.859 | 3.74 | 0.067 |
| Temperature × Colour | 0.40 | 0.535 | 0.39 | 0.541 | 1.46 | 0.241 | 12.00 | 0.002 | 0.36 | 0.557 | 9.43 | 0.006 |
- Significant results (<0.05) are displayed in bold.
Cyanobacterial toxins
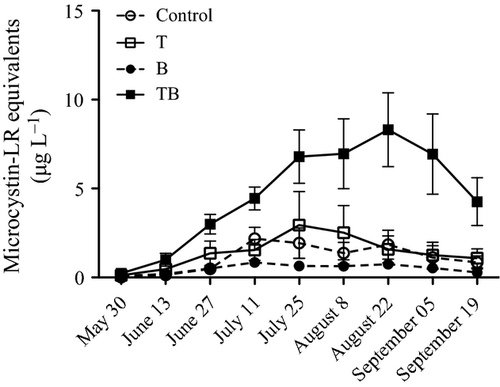
Average microcystin concentration was affected by both temperature and the interaction between temperature and water colour (Table 2) and increased significantly over time (Table 2). The increase in microcystins over time was neither affected by temperature nor water colour acting alone. However, there was an interactive effect between elevated temperature and water colour over time yielding a higher microcystin concentration in the TB treatment (Table 2; Fig. 2). No anatoxins, cylindrospermopsins or any of their degradation products were detected in any samples.
Potential microcystin producers
Of the five species entered into the stepwise multiple regression (Table 1), abundance of two species, Microcystis botrys and Anabaena macrospora (also known as Dolichospermum macrosporum), explained 73% of the total variation, and these were considered as the most likely microcystin producers (adjusted R square = 0.73, F2,213 = 285.9, P < 0.001; M. botrys: beta = 0.83, t = 23.1, P < 0.001, A. macrospora: beta = 0.13, t = 3.7, P < 0.001). Furthermore, of these two species, M. botrys was the only one differing among treatments (Table 2). The average biomass of M. botrys during the experiment was significantly affected by both temperature and water colour separately, as well as by the interaction between the two factors (Table 2), showing the highest biomass in the TB treatment (Fig. 1d). Furthermore, as with the pattern in microcystin concentrations, the increase in M. botrys biomass over time was highest in the TB-treatment (Fig. 2).
Discussion
Our future water resources are expected to suffer from both increasing temperatures (Christensen et al., 2007) and increasing water colour (Monteith et al., 2007; Hansson et al., 2013). However, these are but two of several potential stressors affecting aquatic ecosystems. This emphasises the need for multiple stressor research in order to make more realistic predictions about how different species will respond to environmental changes. This is especially the case since, as shown here, subtle interactions between stressors can lead to quite different degrees of impact when combined, compared to those shown by each in isolation. When increased temperature was combined with increasing water colour (TB-treatment), microcystin concentrations increased by more than 300% compared to the control scenario. This level of response was not seen in any of the single-factor treatments. Furthermore, we also show that the pathway leading to changing microcystin concentrations in the water might be through alterations in species composition rather than through changes in total cyanobacterial biomass, which remained unaffected by our treatments. Hence, our results highlight the importance of performing studies at different taxonomic levels. Rather than focusing only on changes among genera or higher taxonomic levels, it is important to disentangle alterations at the species level as this can reveal completely different patterns of response (Hansson et al., 2004).
Although previous studies have shown positive correlations between cyanobacterial abundance and water temperature (e.g. Park et al., 2004; Kosten et al., 2012), our analysis of total cyanobacterial biomass showed no response to elevated temperature. Furthermore, we did not observe any effect of neither changing water colour nor any interaction between temperature and water colour on total cyanobacterial biomass. Microcystis was the only genus that responded to the temperature increase in our experiment, producing higher biomasses in both the temperature-elevated treatment (T) and in the future scenario treatment (TB). These patterns are consistent with the findings of Hansson et al. (2013), who also found the genus to increase in response to elevated temperatures. Increased biomass of Microcystis, and other cyanobacterial species, being less nutritional food and also toxic, could pose a severe threat to zooplankton growth and survival (Claska & Gilbert, 1998; Lürling & Beekman, 2006; Sarnelle, Gustafsson & Hansson, 2010). Initially, we hypothesised a decreased cyanobacterial biomass in the brownification treatments in relation to the control scenario due to the reduced light climate (i.e. decreasing overall algal production). The only recorded effect of water colour separately in this study was on M. botrys biomass with a small decrease in abundance in the B-treatment compared to the control. However, the fact that there was a significant interaction between water colour and temperature, with a much higher biomass of M. botrys in the TB-treatment, makes it hard to draw any general conclusions regarding the effect of changing water colour on this species as the effect of water colour on M. botrys seems to be strongly coupled to temperature regime.
The multiple regression selected both M. botrys and A. macrospora as potential microcystin producers in the mesocosms. However, based on the corresponding increase in M. botrys biomass and microcystin concentrations in the future scenario treatment (TB), it is most likely that the increase in microcystin concentrations was a result of the increase in M. botrys biomass. Hence, although cyanobacteria can change their toxin production over time, the population fluctuations in M. botrys explain most of the variations in the microcystin concentrations. A. macrospora biomass made a much smaller contribution to explaining microcystin concentrations and, unlike M. botrys biomass, did not show any response to the treatments and had similar biomasses everywhere. This conclusion is strengthened by a lake study showing that highest microcystin concentrations occurred during the period when the cyanobacterial community was dominated by M. botrys (Cronberg, Annadotter & Lawton, 1999). Hence, both our replicated experiment and studies in natural systems suggest that M. botrys has a considerable impact on the overall microcystin concentrations.
Since microcystins are harmful to both aquatic organisms (Hansson et al., 2007; Urrutia-Cordero et al., 2013) and humans (Carmichael, 2001; Falconer, 2005), the increased microcystin concentrations in the future scenario treatment (TB) may have implications both for ecosystem functioning and for ecosystem services, such as drinking water supply. The removal of toxins from raw water involves a number of methods (Falconer, 2004), but we may conclude that increased toxicity in our future waters will increase the demand for effective methods to remove these substances to ensure drinking water of good quality. Although our study only detected the cyanobacterial toxin microcystin, previous studies have demonstrated that other cyanobacterial toxins, such as anatoxin-a, are positively correlated with environmental variables such as temperature (Cerasino & Salmaso, 2012).
The reason why just M. botrys reacted synergistically to temperature and water colour, and thereby increased microcystin concentration, is not clear, and more research is needed to identify the underlying mechanisms. However, we may conclude that synergistic effects, such as the ones previously observed between elevated temperatures and nutrient availability (Jeppesen et al., 2011; Kosten et al., 2012), can also be expected to occur between elevated temperatures and increasing water colour.
Although it is important to acknowledge the limitations of extrapolating findings from experimental studies to future natural waters (Carpenter, 1996; Caceres & Schwalbach, 2001), mesocosm experiments have been suggested to be valid for upscaling to more natural aquatic systems when it comes to algal responses to nutrient enrichment (Spivak, Vanni & Mette, 2011). Also, mesocosm experiments give us the opportunity to design scenarios that have not yet occurred and allow us to make predictions about how organisms may respond to changing environments. Hence, with the precautions associated with mesocosm experiments in mind, we conclude that it is not necessarily an increase in total cyanobacterial biomass that leads to the most drastic responses when it comes to the toxicity of cyanobacterial blooms, but rather the responses by individual species, such as M. botrys. We demonstrate here that although the total cyanobacterial biomass was similar, population fluctuations in mainly one species (M. botrys) could potentially lead to several orders of magnitude more toxic water in our lakes in the future. Knowledge of these species-specific responses to multiple stressors is crucial for proper management decisions, as these single-species responses can have considerable impacts on both biodiversity and ecosystem functioning, as well as ecosystem services, such as drinking water supply and recreation (Brookes & Carey, 2011).
Acknowledgments
We would like to thank Johan Bäckman, Andreas Bäckman and Wendy Beekman-Lukassen for technical support during the experiment. Also we would like to thank P.A. Nilsson and Kim Berndt for statistical support. The project was financed by The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), The Royal Physiographic Society in Lund and the Swedish Research Council (VR) through the Centre for Animal Movement Research (CAnMove) through the Linnaeus grant 349-2007-8690. E. J. Faassen was supported by Grant 817.02.019 from the Netherlands Organization for Scientific Research.




