
A test of the preference–performance hypothesis with stream insects: selective oviposition affects the hatching success of caddisfly eggs
Summary
- Under the preference–performance hypothesis (PPH), oviparous females select oviposition sites that optimise the fitness of their offspring (eggs or larvae). The resulting distribution and fitness of offspring may have knock-on effects for population distribution patterns and dynamics during larval and adult stages. We tested the PPH for Australian caddisflies from two genera (family: Hydrobiosidae) that oviposit in different flow conditions. Apsilochorema spp. oviposit in slow flowing water, whereas Ulmerochorema sp. favour fast flows. We expected hatching success to be higher in velocities favoured by ovipositing females.
- In a field experiment, newly laid egg masses of each species were exposed to experimental ‘fast’ and ‘slow’ flow treatments throughout development and monitored until they hatched or died. In a second field experiment, we placed egg masses in a range of velocities (0.0–1.5 ms−1) to determine the threshold beyond which eggs were damaged by shear forces.
- The results supported the PPH for one species. Apsilochorema egg masses were sheared from the substratum in fast flows, but hatched with 100% success in favoured slow flows. The threshold velocity for Apsilochorema was 0.6–0.7 ms−1, well beyond the natural oviposition range of up to 0.3 ms−1. Ulmerochorema eggs hatched in all flows, suggesting that flow-related mortality of the egg stage is unimportant for this species. Oviposition in fast flows might enhance the fitness of Ulmerochorema larvae or ovipositing females instead.
- Vulnerability to shear forces appears to explain why Apsilochorema lay eggs exclusively in slow flows. Shear may be a common cause of mortality for lotic insect eggs, and unseasonal spates or regulated flows may significantly affect recruitment of larvae. Selective oviposition affects the spatial distribution and survival of eggs and thus affects larval supply, and these supply dynamics are under-studied in stream ecology.
Introduction
A central goal in ecology is to explain why population size fluctuates through time and space. Processes occurring at multiple life stages may impact population size, and it can be difficult to disentangle which of these processes is most influential to population structure, especially for species with complex life histories such as stream insects. It is often assumed that aquatic insect populations are mediated by impacts on larvae (water quality, physical disturbance, competition, predation, etc), but events shaping reproduction may also be important (Lancaster & Downes, 2010; Lancaster, Downes & Arnold, 2011; Encalada & Peckarsky, 2012). In oviparous (egg-laying) taxa, maternal selection of oviposition sites can have strong effects on the survival and fitness of offspring, with potential consequences for population dynamics (e.g. population size, spatial distribution) and life-history evolution (Resetarits, 1996; Gripenberg et al., 2010; Refsnider & Janzen, 2010).
The preference–performance hypothesis (PPH) describes a form of maternal investment whereby females oviposit preferentially in places that enhance the performance (fitness) of their offspring. In this context, ‘preference’ describes the hierarchical ordering of different types of oviposition sites by ovipositing females, with ‘specificity’ describing the number of types of oviposition sites that a species will accept (Thompson & Pellmyr, 1991). The PPH, also known as the optimal oviposition theory, naive adaptationist theory and ‘mother knows best’ principle (see Clark, Hartley & Johnson (2011) for references), was first proposed by Jaenike (1978) and has been tested most often in the context of phytophagous (plant-eating) terrestrial insects. The simplest PPH for phytophagous insects relates preference and performance to the nutritional quality of host plants as food for developing larvae, but other environmental factors and ecological interactions (e.g. predation, parasitism, competition, mutualism) have led to the adaptation of a variety of preference–performance relationships (Thompson, 1988; Craig & Itami, 2008; Gripenberg et al., 2010; Refsnider & Janzen, 2010). For example, ovipositing females of the butterfly Ogyris amaryllis favour the presence of mutualist ants over the nutritional quality of host plants (Atsatt, 1981).
Life-history traits can influence the strength of preference–performance relationships. Selection of high-quality sites may be more critical if juveniles have low mobility and are restricted to natal sites, at least during early instars (Craig & Itami, 2008; Gripenberg et al., 2010). Likewise, selection of favourable habitat may be more crucial for species that produce few offspring or aggregate their eggs in clutches rather than distributing them widely in a bet-hedging strategy (Hopper, 1999; Gripenberg et al., 2010).
Stream insects use a range of oviposition strategies (Hinton, 1981; Encalada & Peckarsky, 2007; Lancaster & Downes, 2013), but, to our knowledge, the performance of offspring has not been compared between species with different strategies. It is widely suggested, however, that selective oviposition behaviour should benefit the fitness of eggs more than larvae, because the larvae of most aquatic insects are mobile and able to relocate from unfavourable habitat (Siva-Jothy, Gibbons & Pain, 1995; Peckarsky, Taylor & Caudill, 2000; Hoffmann & Resh, 2003; Reich & Downes, 2003a,b), and because oviposition sites are not necessarily associated with larval food resources. Preference–performance relationships seem unlikely to develop in species that scatter their eggs at the water surface except, for example, where selection of particular waterbodies (e.g. with or without detectable conspecifics, predators, food) may benefit larvae (Lancaster & Downes, 2013). In contrast, strong preference–performance relationships may be likely to develop among species that attach a single egg mass per female (entire clutch of eggs) to an actively selected oviposition substratum, thus putting all of their eggs into one basket. Several such freshwater insects lay their egg masses exclusively on ‘emergent rocks’ (rocks that protrude from the water), including the caddisflies Neophylax rickeri (North America), Rhyacophila dorsalis (Europe), and Hydrobiosidae (Australia), and the mayflies Baetis bicaudatus (North America) and B. rhodani (Europe) (Peckarsky et al., 2000; Reich, 2002; Hoffmann & Resh, 2003; Lancaster, Downes & Arnold, 2010a,b). Unlike the host plants of phytophagous insects, emergent rocks do not offer a direct source of nutrition for hatching larvae, but differ in other characteristics that may influence the survival of offspring. The size of emergent rocks and surrounding water velocity, in particular, are characters that factor highly in oviposition site selection (Hoffmann & Resh, 2003; Reich & Downes, 2003a,b; Encalada & Peckarsky, 2006; Lancaster et al., 2010b). Most species prefer large rocks, which may benefit developing eggs as they are less likely to roll and typically occur in deeper water, reducing the risk of desiccation (Downes et al., 1998; Peckarsky et al., 2000). In selecting oviposition sites with particular water velocities, however, females may have to trade off different components of fitness. Ovipositing in fast flows may facilitate embryo development and survival, as demonstrated for the damselfly Calopteryx splendens xanthostoma (Siva-Jothy et al., 1995), but could also increase the risk that eggs or ovipositing females will be dislodged by swift currents. Female mortality during oviposition can influence oviposition site selection in insects, with some females sacrificing the fitness of individual offspring to enhance their own survival (a parent-offspring conflict) (Clark et al., 2011). Freshwater insects typically die shortly after oviposition, but parent–offspring conflicts may still arise if larvae fare best in environments that carry a high mortality risk for ovipositing females.
Our aim was to test the PPH for stream insects with selective oviposition behaviours, to determine whether the survival and hatching success of eggs in preferred and non-preferred flow environments is consistent with a preference–performance relationship. We compared oviposition site preference with the performance (hatching success) of egg masses for lotic caddisflies of two genera (family: Hydrobiosidae) that oviposit in different flow conditions. Ulmerochorema egg masses are most common on emergent rocks in fast flows (≥0.4 ms−1), whereas Apsilochorema prefer to oviposit in slow flows (≤0.3 ms−1) (Reich & Downes, 2003b). We tested the PPH that hatch rates are higher when eggs develop in favoured flow conditions. Unlike the flattened egg masses of Ulmerochorema, Apsilochorema egg masses are bulbous and project from the substratum, which may make the latter vulnerable to dislodgement by flow forces if laid in high velocities. We therefore tested a second hypothesis that Apsilochorema egg masses would shear from the substratum in fast flows.
Methods
Study taxa
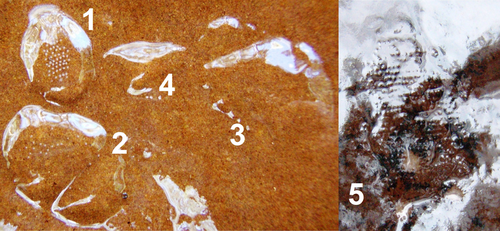
Caddisflies of the genera Apsilochorema and Ulmerochorema predominantly occur in the Australasian and Neotropical regions (Neboiss, 1986). Reich (2004) described the egg masses of these genera by hatching and identifying neonates from egg masses collected in our study system. Apsilochorema eggs are attached to the substratum and covered with soft spumaline (jellylike material) that spans 20–40 mm in diameter, and projects 8–10 mm from the substratum (Reich, 2004; Bovill, 2013) (Fig. 1). Ulmerochorema egg masses are smaller (8–12 mm diameter) and more flattened, projecting 3–5 mm from the substratum. The spumaline of Ulmerochorema masses is also firmer than that of Apsilochorema and more difficult to remove physically. Ulmerochorema egg masses appear better adapted, morphologically, to withstand fast flows and are most common on rocks in fast velocities (≥0.4 ms−1), throughout the stream channel. Apsilochorema egg masses appear more fragile and are more common on emergent rocks in slower velocities (≤0.3 ms−1), which typically occur along stream margins (Lancaster, Downes & Reich, 2003; Reich & Downes, 2003b).

We are confident that we studied only one species of Ulmerochorema, as the egg masses of this genus are readily divided into different morphotypes, which almost certainly represent different species, and are impossible to confuse (W. Bovill personal observations). We studied the Ulmerochorema morphotype used in all studies of Ulmerochorema oviposition to date and suggested to be U. rubiconum (references given above). Two species of Apsilochorema, A. gisbum and A. obliquum inhabit the study region. A recent analysis of clutch size (Bovill, 2013) suggests that there may also be two cryptic groups of Apsilochorema egg masses and that each may be laid by one of the two different species (but this is yet to be established). Clutch size varies enormously both within and between species, but mean (range in parentheses) clutch size is 210 (52–384) and 394 (153–636) eggs per mass, respectively, for the two cryptic groups of Apsilochorema egg masses, and 186 (37–406) eggs per mass for Ulmerochorema. Unfortunately, splitting the cryptic groups of Apsilochorema egg masses can be difficult in the field and was not possible in this study, but oviposition site selection (preference for slow flows) and egg mass morphology are consistent for both groups, which appear to differ only in clutch size. Any effects of water velocity on hatching success are therefore likely to be similar for all Apsilochorema egg masses.
Study sites
Field experiments were conducted at two sites on the Little River in Cathedral Range State Park, Victoria, Australia (145° 48′ 510″ S, 37° 23′ 345″ E). The Little River has been used extensively as an arena for studies of hydrobiosid oviposition (Lancaster et al., 2003; Reich & Downes, 2003a; Reich, 2004; Reich et al., 2011). Experiments were run at separate sites for each species, but sites were selected to be similar in gradient, substratum geology and surrounding land use (dry upland forest) and were separated by <400 m stream length.
Experiment 1: Testing the preference–performance hypothesis
Eggs hatch synchronously from hydrobiosid egg masses and, excepting occasional unfertilised eggs that fail to hatch, typically the hatching success or failure of egg masses is complete (Bovill, 2013). The performance (hatched versus failed to hatch) of egg masses in favoured and unfavoured flow conditions was tested in field experiments conducted simultaneously for both species during February – March 2011. Emergent rocks with recently laid egg masses were allocated at random among three velocity treatments: (i) control (‘C’, preferred velocity) – the egg-laden rock remained in its original fast (Ulmerochorema) or slow velocity (Apsilochorema) location. Hatching success in the control should reflect the hatching success of egg masses under favoured conditions; (ii) procedural control (PC) – the egg-laden rock was moved to a different location within the same velocity category. There should be no difference in hatching success between the control and procedural control unless the act of moving rocks affects hatching success; (iii) treatment (T) – the egg-laden rock was moved from one flow category to the other (Apsilochorema, slow to fast; Ulmerochorema, fast to slow). If velocity affects hatching success, hatch rates should differ between the treatment and the procedural control.
To acquire replicate emergent rocks with freshly laid egg masses, we positioned 200 rocks at each site in conditions suitable for oviposition, based on the preferences outlined for each species by Reich & Downes (2003b). Emergent rocks ≥15 cm b-axis, with the bottom surface not embedded in the stream bed, were positioned in velocities ≥0.4 ms−1 for Ulmerochorema, and ≤0.3 ms−1 for Apsilochorema. The b-axis (measured as the shortest axis of the largest surface plane of a particle) determines the minimum mesh size through which a particle may pass and generally offers a suitable estimate of mean particle diameter (Gordon et al., 2004). If available, in situ emergent rocks were used, otherwise submerged rocks were sourced from within the stream. All experimental rocks were positioned within the range of depths exploited naturally during oviposition. Any egg masses already present were removed so that all rocks commenced the oviposition period bare. A long stretch of stream (length: 137 m, area: 1137 m2) was required to accommodate 200 emergent rocks in places suitable for Apsilochorema, because slow flows were most common along the margins. Rocks at the Ulmerochorema site could be placed throughout the width of the channel, so the site was shorter (length: 73 m, site area: 637 m2). Site dimensions were unimportant, as our hypotheses related to processes that play out at rock scales.
An oviposition period of 4 days resulted in 35 rocks with Apsilochorema egg masses and 37 rocks with Ulmerochorema egg masses. These were the replicates to be spread randomly among the three experimental treatments. Rocks allocated to the procedural control and treatment were carried by hand to the nearest suitable fast or slow flow location, taking care not to damage eggs in the process. Rocks were rarely moved further than 2 m from their original position. The original orientation of each rock (upstream/downstream surface, top/bottom surface) was maintained, and rocks were never fully submerged. Physicochemical attributes of egg-laden rocks (rock size, velocity, water depth, dissolved oxygen, temperature) were measured immediately before (C, PC, TC) and after (PC, TC) manipulation to test for confounding environmental variables between treatments. Dissolved oxygen and temperature were measured with the pH/Oxi 340i probe of a ‘Multi 340i’ Multi-Parameter Instrument (Wissenschaftlich-Technische Werkstätten, Weilheim, Germany). Velocity was recorded using a MiniAir2 velocity meter with a ‘mini snap head’ impeller (22 mm diameter) (Schiltknecht™, Gossau, Switzerland). Measures were taken just beneath the surface, and immediately upstream of experimental rocks so as to be comparable with Reich & Downes (2003b).
Although we assessed the hatching success of egg masses, egg-laden rocks were the true replicates in this experimental design. To ensure independence, when rocks contained more than one egg mass, a single mass was selected at random and monitored as the replicate. Replicate egg masses were monitored every 2 days until they either hatched or failed to hatch. Egg masses were deemed fully hatched once all neonates had emerged from the chorion, regardless of whether the neonates had emerged from the spumaline matrix of the egg mass (e.g. Fig. 1). Egg masses typically hatched after about 10–12 days, but sheared egg masses usually disappeared completely within the first 48 h, so it was simple to distinguish between hatched and sheared egg masses. Further, Apsilochorema neonates remained within the spumaline for about 2 days before dispersing, confirming successful hatching (Fig. 1). Ulmerochorema neonates dispersed immediately upon hatching, but the empty chorions (eggshells) remained visible for several days, and these were used to confirm successful hatching. Egg masses were deemed to have failed to hatch if they were physically removed from the substratum (Fig. 2), infested with fungi, or if eggs turned opaque indicating that they were dead or unfertilised. The fate of all egg masses (including egg masses not counted as replicates in Experiment 1) was monitored to establish the hatch rates and dominant modes of egg mortality for each species in favoured flow conditions (C, PC).
Experiment 2: Velocity threshold of egg masses
In this experiment, egg masses were exposed to a range of water velocities to identify the velocity threshold beyond which egg masses were sheared from the rock surface. We predicted that mortality of Apsilochorema egg masses would be low in the flow range favoured by ovipositing females (≤0.3 ms−1) and that a velocity threshold would occur beyond the maximum velocity at which eggs of this species have been observed in previous surveys (0.51 ms−1) (Reich & Downes, 2003a). We were unable to calculate the shear stress exerted on individual egg masses, but because there is a strong positive correlation between velocity and shear stress (Gordon et al., 2004), we used velocity as a suitable surrogate measure of hydraulic forces.
A total of 79 rocks containing 250 fresh Apsilochorema egg masses were obtained in the manner described for Experiment 1 and allocated at random among 15 velocity categories. Categories were defined at intervals of 0.1 ms−1 (i.e. 0.00–0.09, 0.10–0.19… 1.40–1.49 ms−1) and encompassed the full range of water velocities available at the site under summer flow conditions. Each category received between four and nine rocks, providing a total of 10–35 egg masses per category. Rocks were positioned with the bottom surface not embedded in the streambed and eggs on the underside, and the original orientation of top, bottom, upstream, downstream surfaces was maintained. In a departure from Experiment 1, velocity was recorded immediately beneath rocks, centrally, to more accurately represent the flow environment experienced by eggs, and the fate of all egg masses was recorded. A pilot experiment indicated that Apsilochorema egg masses may remain intact for some time when exposed to fast flows, but partial damage was apparent after 24 h for all egg masses that were subsequently removed by fast flows (e.g. Fig. 2). Velocities that did not damage egg masses within 24 h did not subsequently damage egg masses. We evaluated egg masses after 24 h of exposure as either damaged or undamaged, with ‘damage’ indicating a loss of some eggs, spumaline or complete removal of the egg mass from the rock surface. The same method was used to test flow tolerance of 254 Ulmerochorema egg masses (spread over 12 rocks). As Ulmerochorema egg masses were not expected to shear at low or medium velocities, they were subjected only to the fastest flows available at the site (0.79–1.38 ms−1).
Statistical analysis
The preference–performance hypothesis was tested for each species with separate 3 × 2 contingency tables analysed with G-tests of independence (with the Williams correction) (Sokal & Rohlf, 1995). The G-test tests the null hypothesis that hatching success is independent of flow treatment. We expected higher mortality in the treatment than in the procedural control if the PPH was to be supported. A difference in hatching success between the control and procedural control would indicate harmful effects of rock movement on egg masses. Although G-tests are generally robust to small sample sizes and low values in some cells (Bradley et al., 1979), we also conducted Fisher's exact tests (which are robust to small sample sizes and low cell values) on 2 × 2 tables comparing treatments with controls where there was no difference between controls and procedural controls.
Physicochemical properties of rocks in Experiment 1 were compared between treatments, before and after rock manipulation, with one-way analysis of variance. We used planned comparisons (Sokal & Rohlf, 1995) to test for physicochemical differences between (i) control versus procedural control, to ensure environmental variables did not differ between these treatments; and (ii) procedural control versus treatment, to ensure that velocity was the only environmental variable to differ between these treatments.
The relationship between water velocity and egg mass damage (Experiment 2) was analysed with non-linear regression. Within each velocity category, the percentage of egg masses damaged by shear forces after 24 h was calculated as the response variable. A logistic model (see Neter et al., 1996) was fit using SPSS Statistics version 19 (IBM, NY, U.S.A.) to identify the velocity threshold of Apsilochorema egg masses.
Results
Experiment 1: The preference–performance hypothesis
Moving rocks had no observable effect on egg survival for either species. All egg masses in C and PC treatments hatched successfully (Table 1). The hypothesis that oviposition preference benefits egg performance was supported for Apsilochorema egg masses, which hatched with 100% success in slow flows (C, PC) but suffered significant mortality (64%) in fast flows (Table 1). Physical damage of egg masses from shear forces was the only source of mortality observed for Apsilochorema. All replicate Ulmerochorema egg masses hatched successfully within about 12–14 days, irrespective of flow conditions. A single failure in the Ulmerochorema treatment (Table 1) was caused by accidental removal during rock manipulation and may be disregarded.
| Treatment | Hatched | Failed | G | d.f. | P |
|---|---|---|---|---|---|
| Apsilochorema | |||||
| C | 8 | 0 | 19.93 | 2 | <0.001 |
| PC | 13 | 0 | |||
| T | 5 | 9 | |||
| Ulmerochorema | |||||
| C | 10 | 0 | 1.274 | 2 | >0.5 |
| PC | 14 | 0 | |||
| T | 12 | 1 | |||
Other than velocity, physicochemical parameters did not appear to influence the outcome of Experiment 1. Prior to manipulation, there were no systematic differences in the size or physicochemical surroundings of emergent rocks containing egg masses of either species (Table 2). Following manipulation, velocity in ‘fast’ and ‘slow’ treatments differed statistically, as expected. Velocities differed between the control and procedural control for Ulmerochorema (C = 0.47 ± 0.06 ms−1, PC = 0.64 ± 0.04 ms−1, Table 2), but both treatments satisfied the a priori definition of ‘fast’ flowing water (≥0.4 ms−1). For Apsilochorema, water depth varied significantly in both planned comparisons. Water depth affects the hydrostatic pressure, which can influence the shear force exerted on an object, but a difference in mean depth of 3.6 cm between treatments would be negligible. Dissolved oxygen and temperature did not differ significantly between fast and slow environments.
| Apsilochorema | Ulmerochorema | |||||
|---|---|---|---|---|---|---|
| F | d.f. | P | F | d.f. | P | |
| Pre-move | ||||||
| Rock size (b-axis) | 0.196 | 2,31 | 0.823 | 0.661 | 2,35 | 0.523 |
| Velocity | 2.046 | 2,31 | 0.146 | 0.818 | 2,35 | 0.449 |
| Water depth | 0.400 | 2,31 | 0.674 | 0.027 | 2,35 | 0.973 |
| Dissolved oxygen | 0.268 | 2,31 | 0.767 | 0.470 | 2,35 | 0.629 |
| Temperature | 0.483 | 2,31 | 0.622 | 0.051 | 2,35 | 0.950 |
| Post-move | ||||||
| Velocity | 94.178 | 2,32 | <0.001 | 51.603 | 2,35 | <0.001 |
| C versus PC | t = 0.077 | 32 | 0.939 | t = 3.006 | 35 | 0.005 |
| PC versus T | t = 12.260 | 32 | <0.001 | t = −10.006 | 35 | <0.001 |
| Water depth | 8.127 | 2,32 | 0.001 | 0.575 | 2,35 | 0.568 |
| C versus PC | t = 2.156 | 32 | 0.039 | t = 0.619 | 35 | 0.540 |
| PC versus T | t = 2.094 | 32 | 0.044 | t = −1.062 | 35 | 0.296 |
| Dissolved oxygen | 1.220 | 1,25 | 0.280 | 0.382 | 1,26 | 0.542 |
| Temperature | 0.010 | 1,25 | 0.921 | 1.173 | 1,26 | 0.289 |
When the fate of all egg masses (replicate and non-replicate) is considered, hatching success was high for both species under favoured flow conditions. All 46 Apsilochorema egg masses developing in slow flows hatched successfully, and just eight of the 435 Ulmerochorema egg masses developing in fast flows failed to hatch (1.8% mortality). Fungus was observed on two Ulmerochorema egg masses and was the only biotic enemy observed for eggs of either species. A further six Ulmerochorema egg masses contained opaque, non-viable eggs that either died of an unknown cause or else were unfertilised and failed to develop.
Experiment 2: Velocity threshold of egg masses
As with Experiment 1, emergent rocks were the true replicates in this experiment. Preliminary analyses indicated that the fate of eggs did not differ appreciably between rocks in each category, so we pooled the fate of all eggs on all rocks per category to calculate a % survival rate for each flow category.
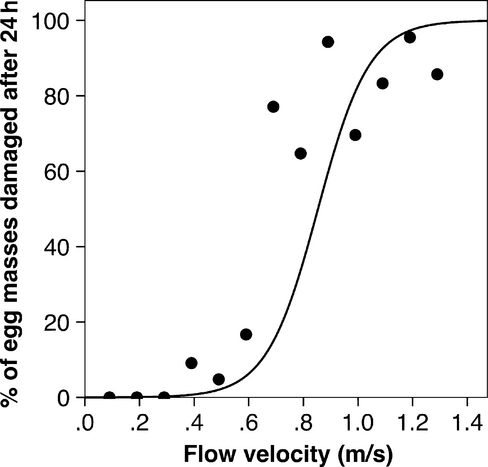
The relation between velocity and shear of Apsilochorema egg masses was described by a logistic model, and we defined the velocity threshold of Apsilochorema egg masses as the point at which the slope of the curve underwent maximum acceleration (where x approximates 0.6–0.7 ms−1, Fig. 3). This point describes the velocity beyond which survival rapidly decreases and is a more relevant threshold than the inflection of the curve (at which point 50% of egg masses are damaged by flow). The precise value of the velocity threshold could be calculated analytically (as the third derivative of the logistic function), but this seems unnecessarily precise given that we were unable to measure hydraulic forces at the scale of individual egg masses. Mortality of Apsilochorema egg masses was 0% in the flow range favoured by ovipositing females (≤0.3 ms−1), but on average 81.5% of Apsilochorema egg masses were damaged at velocities ≥0.7 ms−1. The damage to Apsilochorema egg masses by water forces suggests that, if there are indeed two cryptic species represented, the outcomes for both species are the same. The first sign of shear was usually that eggs were missing from apparently random positions throughout the mass. How this occurs is unclear, but individual eggs appear to be shaken free of the egg mass, perhaps by parallel flow vibrating the spumaline and, in doing so, weakening the integrity of the spumaline. Once the spumaline was damaged, egg masses degraded rapidly. No Ulmerochorema egg masses were damaged in the fastest summer flows attainable in the experimental stream reach. In fact, some hatched Ulmerochorema egg masses (spumaline only) survived 24 h exposure to flows of 1.38 ms−1.
Discussion
Our results support the preference–performance hypothesis for the caddisfly Apsilochorema, with oviposition site selection positively affecting egg survival. Hatching success of Apsilochorema egg masses was higher in slow flows (the preferred environment) as the large, soft egg masses could not withstand the physical stresses (shear) associated with high water velocities. This vulnerability of Apsilochorema egg masses to shear is a plausible explanation for the high selectivity of ovipositing females for emergent rocks in slow flows, but we acknowledge that other unmeasured factors may influence the observed patterns, as discussed below. Flow-related egg mortality may be a widespread phenomenon in streams, because a variety of taxa lay bulbous egg masses like those of Apsilochorema (also discussed below). In contrast, oviposition preferences were not associated with the hatching success of Ulmerochorema egg masses. Ulmerochorema egg masses hatched equally well in fast and slow flows, even though the egg masses of this species are rarely found in slow flow environments. It is unclear why Ulmerochorema prefers to oviposit in fast flows (which are, presumably, more hazardous to ovipositing females), but if there is an adaptive explanation for selective oviposition by this species, it may be that fast flows benefit the fitness of adults or, more likely, larvae.
Identifying life stages at which significant mortality occurs, and the knock-on implications for subsequent life stages, is fundamental to understanding the ecology of species with complex life cycles. Most studies in freshwater ecology consider only the larval stage (Downes & Reich, 2008), and the dynamics of eggs, pupae and adults remain largely unknown. As a starting point, the PPH may offer a useful framework for studies attempting to build a more holistic view of stream insect life cycles, because testing the PPH encourages a consideration of factors affecting the fitness of adults, eggs and larvae. The theoretical context of the PPH is well established and may be relevant to many freshwater species, but empirical tests to date are restricted to terrestrial taxa. The different responses of our study species to the experiment (Apsilochorema supports the PPH, Ulmerochorema does not) suggest different implications for the population dynamics of each species. Apsilochorema seems likely to suffer significant mortality during the egg stage if flows suddenly accelerate (e.g. during spates that raise water velocity above the shear threshold), with potential knock-on implications for the abundance and distribution (spatial, temporal) of larvae, pupae and adults. In contrast, very little mortality was observed during the egg stage for Ulmerochorema under natural (control) and manipulated conditions, suggesting that mortality during other life stages probably has a greater influence on population dynamics.
We first discuss several potential mechanisms that may underlie the selective oviposition behaviours of each species and then consider the potential population-level implications of oviposition behaviour, particularly for taxa with egg masses that are vulnerable to shear.
Possible mechanisms for the flow selectivity of ovipositing Hydrobiosidae
We have delivered strong evidence that oviposition in preferred slow flows enhances the survival of Apsilochorema egg masses. This is consistent with the PPH, but does not provide definitive proof that oviposition preference has evolved to enhance egg survival. We must therefore caution against an interpretation of our results that fits the ‘adaptationist paradigm’ (Gould & Lewontin, 1979). Low survivorship of egg masses in fast flows may have led to the adaptation of selective oviposition behaviour, but a correlation between oviposition behaviours and egg survival may merely be a secondary effect of adaptations that favour (for example) adult or larval fitness. Alternatively, oviposition in slow flows may not be adaptive at all.
There are three potential explanations for the observation that Apsilochorema egg masses only occur in slow flows: (i) females are incapable of ovipositing in fast flows and may perish in the attempt; (ii) females oviposit indiscriminately in all flow conditions, but only those egg masses in slow flows survive to be surveyed; or (iii) females oviposit preferentially on emergent rocks in slow flows. The second explanation seems unlikely because egg masses typically required 24–48 h to shear completely, yet in no study of Apsilochorema oviposition (including this one) have partially sheared egg masses been observed in fast flows (Reich, 2002, 2004; Lancaster et al., 2003; Reich & Downes, 2003a,b, 2004; Reich et al., 2011; Bovill, 2013). Unfortunately, we cannot disentangle whether Apsilochorema oviposits in slow flows via choice (explanation iii) or necessity (explanation i). If Apsilochorema oviposit in slow flows by necessity, higher egg survival in these flows may be a secondary effect of oviposition in slow flows. If so, a hypothesis that oviposition preference benefits eggs is not supported, and adult survival might instead drive selective oviposition behaviour. However, there is ample evidence that hydrobiosids and other stream insects can actively choose oviposition sites in particular flows (explanation iii). Ulmerochorema females land more frequently on emergent rocks in the fast flows favoured for oviposition, suggesting that they may assess flow velocity on the wing (Reich & Downes, 2003b). Female Baetis bicaudatus can also asses flow conditions during flight, using visual cues such as splash and polarised light to locate emergent rocks in suitable flows (Encalada & Peckarsky, 2006). Although we cannot define the mechanisms driving selective oviposition, what our study delivers beyond doubt is that Apsilochorema eggs benefit from oviposition in slow flows. Whether this arises due to adult choice or necessity is less important for Apsilochorema (and other taxa with bulbous egg masses) than the fitness and population implications of being vulnerable to mortality in fast flows.
The strong preference of Ulmerochorema for oviposition sites in fast flows remains to be explained. Indeed, there may not be an adaptive cause for this behaviour, but it seems unlikely that females would preferentially oviposit in fast flows unless some benefit was incurred by the eggs, larvae or the adults themselves. Oviposition in fast flows had no observed benefit for Ulmerochorema eggs, but Siva-Jothy et al. (1995) reported higher hatch rates for the damselfly C. s. xanthostoma, when eggs were inserted into macrophyte stems in fast velocities (c. 0.4 ms−1). Plant stems in slow velocities (<0.04 ms−1) became encrusted with algae, and the damselfly neonates were unable to break through after hatching. In a similar way, oviposition by Ulmerochorema in fast flows may reduce the number of egg masses that are infected by fungal hyphae. Fungal infection appears to be the most common source of mortality for Ulmerochorema egg masses (Reich, 2004; W. Bovill personal observations), but accounts for a very small proportion of eggs on the Little River (about 1%, Reich et al., 2011).
Oviposition in fast flows may benefit larvae by facilitating dispersal in the drift. Ulmerochorema often deposit hundreds of egg masses on a single rock, and drift dispersal may alleviate competition with kin (or other species) in early life. Fonseca & Hart (1996) observed density-dependent drift dispersal from natal sites by blackfly neonates (Simuliidae), which voluntarily entered the drift to escape competition with conspecifics. Oviposition by Ulmerochorema in fast flows might also reduce competition with Apsilochorema neonates at natal sites via resource partitioning, but this has not been tested. Alternatively, oviposition in fast flows may improve survival rates of adult females, but this seems counterintuitive because females presumably stand a greater chance of being dislodged from rocks in fast flows. It may be that rapid or turbulent flow offers protection from predators such as water spiders (Pisauridae), which were commonly observed on emergent rocks in the Little River, but predominantly along the stream margins in slow flows. Oviposition behaviours may also affect the vulnerability of gravid females to predation by fish. For example, females that oviposit on emergent rocks (such as hydrobiosids) appear less prone to fish predation than species that occupy the surface during oviposition (Encalada & Peckarsky, 2007), or swim in the water column to locate oviposition substratum (e.g. see: Duffield, Flint & Nelson, 1994).
Some oviposition behaviours benefit the fitness of adults at a cost to individual offspring, a ‘parent-offspring conflict’ (Mayhew, 2001; Clark et al., 2011). For example, oviposition on food plants favoured by adults, but not by larvae, enhances the longevity and fecundity of adults of the leaf-mining fly Chromatomyia nigra, at a cost to the fitness of larvae (Scheirs, De Bruyn & Verhagen, 2000). Parent–offspring conflict may be more common when adult and juvenile stages occupy different environments (Clark et al., 2011), and so may be prevalent among the freshwater insects but, to our knowledge, this idea is yet to be tested in a stream setting.
Potential implications of flow-selective oviposition on populations
Although the egg stage is seldom considered in freshwater ecology, it is apparent from the few studies conducted that oviposition behaviours can influence the reproductive success of lotic insects in a way that might impact the spatial and temporal distribution of populations. At the simplest level, selective oviposition behaviours and hatching success may influence the spatial distribution of larvae between sites or streams that differ in the availability of particular velocity regimes and emergent rocks (i.e. oviposition sites). However, fluctuating water levels (and velocities) may also influence reproduction over temporal scales, by affecting (i) the spatial and temporal abundance of oviposition sites (e.g. emergent rocks may become submerged and vice versa) and; (ii) the survival of shear intolerant egg masses. This in turn may affect the number and spatiotemporal distribution of larvae entering the stream.
For example, the availability of emergent rocks in streams of the East River catchment (Rocky Mountains, Colorado) is dependent on the timing of snowmelt and runoff, and female Baetis bicaudatus emerging early in the season may be unable to oviposit at some sites (Peckarsky et al., 2000). Such a scenario has obvious implications for individual females, which may be forced to trade off the costs associated with dispersing to locate suitable oviposition substratum, ovipositing on sub-optimal substratum or waiting for water levels to drop and potentially failing to oviposit at all. The population-level implications of oviposition patterns are, of course, dependent on whether larvae persist in natal sites or disperse elsewhere. Compellingly, by simply manipulating the abundance of emergent rocks in 45 m-long stretches of river (East River catchment, Colorado), Encalada & Peckarsky (2012) affected not only the supply of B. bicaudatus egg masses, but also the subsequent abundance of late instar larvae at those sites. Corroborating this result, Lancaster et al. (2011) also reported persistent effects of egg supply on the distribution of early and mid instar B. rhodani larvae in south-east Scotland. With such strong links between egg supply and larval abundance through to the late instars, raised water levels affecting the abundance of oviposition substratum may be more important to the distribution and dynamics of some benthic larval populations than other impacts of flow disturbance, such as displacement of mobile larvae. For species with shear intolerant egg masses, such as Apsilochorema, mortality of egg masses in high velocities may complement the impact of high water levels on substratum availability, delivering a ‘one-two knockout punch’ to reproduction rates (i.e. removing egg masses and preventing females from laying new ones). Many aquatic invertebrates lay soft, bulbous egg masses similar to those of Apsilochorema (e.g. several Trichoptera, Diptera, Mollusca), and these taxa may be similarly affected by shear forces in elevated flows. Conceivably, species with shear resistant eggs, which attach their eggs to plants (e.g. several Odonates, Diptera, Hemiptera), may also be affected if those plants are damaged or dislodged by swift currents. Consequently, egg mortality could be significant for a variety of aquatic invertebrates and yet is highly under-studied.
While elevated flows could preclude freshwater insects from colonising particular habitats, the relative effects of reduced oviposition versus egg mortality are likely to differ depending on the flow regime. Seasonal fluctuations in water level dictate when and where oviposition can take place, but in many systems, these may be gradual, ramping fluctuations (sensu Lake, 2000), taking place over timescales that allow egg masses to complete a 10- to 14-day development period in a relatively constant flow. In contrast, flashy spates that rapidly raise velocities may be catastrophic for egg masses that are vulnerable to shear, but probably have little impact on oviposition rates if adults are able to postpone egg-laying until the water level recedes. Perhaps the greatest impact on aquatic insect reproduction could occur in regulated streams. Inverted flow regimes (high summer flows, low winter flows, when the reverse is usual) may submerge oviposition substrata throughout the peak breeding period, prohibiting the supply of eggs and thus restricting the supply of larval colonists to crawling or drifting individuals. Sudden releases (e.g. for irrigation or hydroelectricity, and environmental flows) are also common in these systems and could be detrimental to existing egg masses. Specific research is warranted to determine the impact of regulated flows on oviposition and egg survival, and whether this might influence the composition and dynamics of communities downstream of dams and weirs.
The spatial distribution of populations holds implications for ecological interactions at individual, species and community levels, and although hydraulics have clear implications for reproduction in some aquatic insects, the knock-on effects for larval and adult populations remain all but unexplored (but see Lancaster et al., 2011; Encalada & Peckarsky, 2012), representing a huge gap in our knowledge of most freshwater species. Whether egg mortality significantly affects populations requires an understanding of relative mortality rates at all life stages which, for stream taxa, may be difficult to achieve (but see Willis & Hendricks, 1992; Hildrew et al., 2004). Such studies are possible, however, and enhancing our understanding of oviposition and hatching success in aquatic insects will provide crucial insights into the ecology of stream insects, providing the critical links needed to connect adult and larval dynamics.
Acknowledgments
This study was funded by an Australian Postgraduate Award (scholarship) and grants from the Holsworth Wildlife Research Endowment awarded to W. Bovill. Special thanks to Beatrice Choong and Julia Y. White for field assistance and to Dylan and Julia H. White for field accommodation. Research in Cathedral Range State Park was conducted under a permit from the Department of Sustainability and Environment, State Government Victoria. We are thankful for the constructive comments of two anonymous referees.




