
Role of environmental factors in the spread of domestic trout in Mediterranean streams
Summary
- Overharvesting, habitat alteration and pollution have contributed to the decline of many wild populations of brown trout in Europe. Since the mid-19th century, stocking with domestic strains has added a further threat to their genetic integrity. In this study, we adopted a landscape genetic approach to evaluate the role of environmental factors in promoting or hindering invasion by an alien genome.
- Two classes of molecular markers (LDH-C1* locus and mtDNA) were used to evaluate the level of introgression between native and domestic strains of brown trout in Central Italy. A multivariate method (redundancy analysis, RDA) was employed to find relationships between genetic diversity and 19 environmental variables that describe lithology, geomorphology, fish community and human activities.
- The RDA indicated that streams with more stable ecological conditions sustain almost pure native populations, whereas streams characterised by unpredictable hydrological conditions harbour populations with high levels of introgression or pure for foreign genotypes.
- Our study revealed that the outcome of supplementary stocking with hatchery-reared brown trout is strongly linked to the environmental features of different drainage basins.
Introduction
Human-mediated secondary contacts between conspecific organisms is one of the major threats to the preservation of native biodiversity. This practice may harm native plants and animals through predation, competition, disease and parasite diffusion, and hybridisation (Rhymer & Simberloff, 1996; Allendorf et al., 2001). Loss of native gene pools through hybridisation is widespread in populations characterised by scarce density and inhabiting unstable habitat and which have come into contact with non-native conspecific taxa. This is especially common in freshwater fish because they show a natural hybridisation rate higher than in other vertebrates, and non-native fish have been introduced extensively worldwide (Campton, 1987).
In this context, European populations of brown trout (Salmo trutta) provide an exceptional opportunity to study the influence of supplementary stocking (sensu Ferguson, 2007) on native biodiversity. This salmonid represents an important resource for fishing (nowadays, mainly as a game species), and decades of overharvesting, habitat fragmentation and pollution have contributed to the decline of many wild populations. As a countermeasure, stock enhancement plans based on releases of domestic fish have been implemented for several decades. Nevertheless, these practices introduced a further threat to the maintenance of native genetic diversity because of the problems connected with gene flow from domestic to wild populations. In fact, these hatchery stocks represent only a part of the native genetic variability of the species, being hatchery strains of brown trout from Central and Northern Europe (García-Marín, Sanz & Pla, 1999; Berrebi et al., 2000; Madeira, Gómez-Moliner & Barbé, 2005). In addition, some of the current stocks have undergone more than 30 generations of captive breeding, with artificial selection, founder effects, genetic drift and a different selection regime in farm conditions that may have generated substantial differences in genetic and phenotypic traits resulting in a lowered fitness in the wild (see review by Ferguson, 2007).
In wild brown trout populations, the genetic alteration due to introgression with domestic stocks has been studied in many European countries using different classes of molecular markers (e.g. Berrebi et al., 2000; Hansen et al., 2000; Caputo et al., 2004; Almodóvar et al., 2006). These studies showed highly discordant and sometimes paradoxical results between locations. Although results from some locations indicated that introgression caused by massive stocking activity had a limited extent, others had suffered more dramatic outcomes with high levels of introgression and partial or total displacement of the native genetic diversity. Levels of farm gene introgression appear to be higher in Mediterranean drainage basins of Spain, France and Italy than in those of the Atlantic region. This is surprising given that the vast majority of brown trout used for stocking are derived from the Central-North Atlantic area and thus are genetically more similar to the other Atlantic populations than to Mediterranean ones (Ferguson, 2007). Madeira et al. (2005) proposed that different environmental conditions could explain different introgression rates between Mediterranean and Atlantic drainage basins in Spain. Mediterranean rivers are usually characterised by a highly irregular flow regime with severe droughts and floods. In this context, more adverse environmental conditions could determine a reduction in density of the Mediterranean native trout populations and therefore more susceptibility to introgression and hybridisation by repeated stocking activities.
Although the influence of hydrological processes on brown trout population dynamics has been highlighted in several studies (Campbell, 1961; Lanka, Hubert & Wesche, 1987; Cattaneo et al., 2002), there are only few examples in which the role of environmental characteristics in determining the pattern of introgression observed has been evaluated (Madeira et al., 2005; Almodóvar et al., 2006). This is despite a growing body of evidence concerning the importance of environmental factors in evaluating the dynamics of hybridisation in salmonid populations (Fausch et al., 2009; Muhlfeld et al., 2009; Marie, Bernatchez & Garant, 2012).
The aim of the present study was to evaluate the status of native brown trout genetic diversity in Central Italy from a perspective of genetic conservation and to improve the management of these populations. This was achieved (i) with a better characterisation of the level of introgression on native populations from Central Italy by revisiting and extending existing previous genetic survey based on mtDNA and LDH-C1* locus (Caputo et al., 2004) to a greater number of samples (36 wild and three domestics) and (ii) by using a landscape genetic approach to assess whether environmental factors might have had a significant role in promoting and/or constraining introgression between native and domestic strains.
Methods
Study area
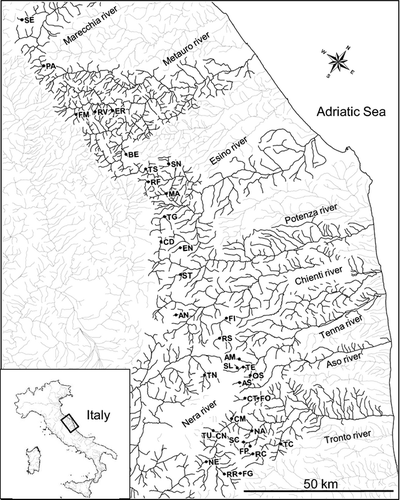
The studied area extends between 42°31′ and 43°47′N and encompasses the upper part of eight Central Apennine river systems (Marecchia, Metauro, Esino, Potenza, Chienti, Tenna, Aso e Tronto, draining into the Adriatic Sea, and Tiber River that drains into the Tyrrhenian Sea; Fig. 1).
Sample collection and genetic analyses
A total of 712 individuals were collected by electrofishing from 28 sites. Adipose fin biopsies were conserved in 95% ethanol until DNA extraction. Total genomic DNA was extracted following a phenol–chloroform method (e.g. Taggart et al., 1992). The subsequent data analyses were performed on both these 712 samples and 193 individuals (eight wild and three domestic samples) previously collected and analysed by Caputo et al. (2004). A total of 36 sampling locations were investigated (Fig. 1; for additional information, see Table 1).
| Stream name | River basin | Site code | Longitude (E) | Latitude (N) | Year | Sample size (mtDNA; LDH-C1* locus) |
|---|---|---|---|---|---|---|
| Torrente Senatello | Marecchia | SE | 12.113 | 43.768 | 1999 | 14, 14 |
| Torrente Auro | Metauro | PA | 12.241 | 43.652 | 1999 | 14, 14 |
| Rio Vitoschio | RV | 12.489 | 43.578 | 1999 | 18, 18 | |
| Fosso dell'Eremo | ER | 12.491 | 43.578 | 1999 | 9, 10 | |
| Fosso Menatoio | FM | 12.394 | 43.547 | 1999 | 6, 6 | |
| Torrente Bevano | BE | 12.631 | 43.471 | 2005 | 21, 21 | |
| Torrente Sanguirone | Esino | SN | 12.820 | 43.462 | 2010 | 20, 20 |
| Torrente sentino | TS | 12.736 | 43.431 | 2010 | 48, 48 | |
| Rio Freddo | RF | 12.759 | 43.396 | 2008 | 10, 14 | |
| Torrente Marena | MA | 12.835 | 43.378 | 2010 | 22, 22 | |
| Torrente Giano | TG | 12.853 | 43.309 | 2000 | 19, 25 | |
| Fiume Esino | EN | 12.918 | 43.230 | 2010 | 18, 21 | |
| Fosso di Campodonico | Potenza | CD | 12.860 | 43.241 | 2010 | 38, 52 |
| Torrente Scarsito | ST | 12.933 | 43.185 | 2010 | 8, 21 | |
| Torrente Fiastrone | Chienti | FI | 13.185 | 43.062 | 1999 | 18, 18 |
| Torrente Santangelo | AN | 12.988 | 43.038 | 2009 | 15, 15 | |
| Rio Sacro | RS | 13.177 | 43.001 | 2000 | 12, 12 | |
| Torrente Ambro | Tenna | AM | 13.288 | 42.951 | 2006 | 24, 41 |
| Fiume Tenna | TE | 13.285 | 42.930 | 2006 | 24, 43 | |
| Torrente il Rio | SL | 13.275 | 42.923 | 2010 | 50, 50 | |
| Torrente Cossudro | OS | 13.329 | 42.920 | 2006 | 16, 22 | |
| Fiume Aso | Aso | AS | 13.305 | 42.892 | 2006 | 17, 36 |
| Fiume Nera | Tevere | TN | 13.152 | 42.887 | 2006 | 38, 46 |
| Torrente Fluvione 1 | Tronto | FO | 13.376 | 42.857 | 2005 | 26, 26 |
| Torrente Fluvione 2 | CT | 13.328 | 42.848 | 2005 | 27, 24 | |
| Fosso della Camartina | CM | 13.290 | 42.776 | 2005 | 4, 4 | |
| Rio Noce Andreana | NA | 13.384 | 42.760 | 2005 | 15, 15 | |
| Torrente Castellano | TC | 13.503 | 42.743 | 2005 | 5, 5 | |
| Fosso Tufo | TU | 13.254 | 42.735 | 2005 | 48, 48 | |
| Torrente Chiarino | CN | 13.309 | 42.729 | 2005 | 8, 8 | |
| Torrente Scalandro | SC | 13.363 | 42.727 | 2005 | 14, 24 | |
| Fosso della Prata | FP | 13.390 | 42.726 | 2005 | 6, 6 | |
| Rio Valle Castellana | RC | 13.418 | 42.701 | 2005 | 20, 20 | |
| Torrente Neia | NE | 13.236 | 42.642 | 2005 | 22, 22 | |
| Fosso di Selva Grande | FG | 13.371 | 42.632 | 2005 | 19, 19 | |
| Fiume Tronto | RR | 13.317 | 42.620 | 2005 | 19, 19 | |
| Cantiano | Hatchery | H1 | 12.631 | 43.471 | 1999 | 22, 36 |
| Visso | Hatchery | H2 | 13.086 | 42.931 | 1999 | 20, 20 |
| Visso | Hatchery | H3 | 13.088 | 42.931 | 1999 | 17, 20 |
| Total | 771, 905 |
Mitochondrial haplotypes were characterised by PCR-RFLP analysis of a segment containing the subunits 5 and 6 of the NADH dehydrogenase gene (ND 5/6; c. 2.5 kb) using five restriction enzymes: AvaII, HinfI, TaqI, AluI and HpaII. These enzymes were selected based on the results reported in Caputo et al. (2004) and Splendiani et al. (2006), which showed the occurrence of several polymorphic diagnostic restriction sites for four main brown trout evolutionary lineages: Adriatic (AD), Mediterranean (ME), marmoratus (MA) and Atlantic (AT) (sensu Bernatchez, 2001). PCR conditions and electrophoresis procedures were as described in Hansen & Loeschcke (1996).
The occurrence of allochthonous genotypes was also estimated through the analysis of the nuclear locus LDH-C1*, which discriminates hatchery stocks that in Europe are fixed for, or show a very high frequency for, the *90 allele, from the native Mediterranean populations, characterised by the *100 allele (McMeel, Hoey & Ferguson, 2001). Protocols for the LDH-C1* locus genotyping were as described in McMeel et al. (2001).
Statistical treatment of the data
Mitochondrial DNA
Assessment of genetic introgression at mtDNA level was based directly from the frequency of the Atlantic haplotypes observed in the wild samples.
LDH-C1* locus
The RFLP data from LDH-C1* were used to estimate the spread of the allochthonous *90 allele in the area. Correlation between the frequency distribution of this allele and AT mitochondrial haplotypes was determined using a nonparametric test (Pearson's rs). Conformity to Hardy–Weinberg expectations was evaluated by estimating FIS using GENEPOP 4.0 (Raymond & Rousset, 1995). The exact FIS P-values were obtained implementing a Markov chain method based on 1000 dememorisations, 1000 batches and 10 000 iterations per batch.
Relationship between environmental factors and genetic introgression
Several environmental factors detected at subcatchment scale were evaluated to determine the extent to which they have influenced the diffusion of Atlantic hatchery genes in the study area. A total of 19 variables, classified into five different categories (geology, geomorphology, land use, fishing management and fish community), were estimated for each sampling location and pooled into a single environmental matrix (see Table 2 for further details). Due to the lack or scarce collection of hydrological attribute records for the study area (i.e. flow data, water temperature, pH, dissolved oxygen, degree of sedimentation), the geological setting at subcatchment level was used as a substitute for these parameters (cf. Magalhães, Batalha & Collares-Pereira, 2002; Miller, Burnett & Benda, 2008). To evaluate the influence of human activities expected to produce habitat disturbance, land-use data (% of agricultural, urban and forested territories), total road density and total road crossing were included. The role of fishing pressure acting as a selective force against Atlantic trout and their hybrids (García-Marín et al., 1999) was defined as a dummy variable, 0 for samples collected in protected areas (i.e. National Parks) and 1 otherwise. The occurrence of impassable barriers (natural and/or artificial) acting as obstacles to hybridisation due to up-stream invasions was taken into account. Finally, the number of other fish species from sampled streams was included. For each sampling location, the subcatchment boundaries and the hydrographic network were delineated from a 10-m resolution digital elevation model (DEM) using the plug-in watershed delineation implemented in MAPWINDOW (a geographic information system GIS, downloadable at http://www.mapwindow.org/). Before analysing data, the environmental variables were tested for normality and appropriate transformations (logarithmic for continuous data or square-root-arcsine for proportional data) were applied.
| Environmental variables | Unit/expression | Description | Source of data |
|---|---|---|---|
| Geology | |||
| Permeable rocks | (%) | Area of permeable rocks in the subcatchment/subcatchment area | 1 : 250 000 Hydrogeological complexes map of Italya |
| Geomorphology | |||
| Subcatchment area | (km2) | MAPWINDOW [a geographic information system (GIS) software] was used to determine the subcatchment boundaries | 10-m resolution digital elevation model (DEM) |
| Catchment area | (km2) | (GIS) | (DEM) |
| Total stream length | (km) | (GIS); total length of the main stream channel | (DEM) |
| Mean elevation | (m. a. s. l.) | Calculated as the average of three stream elevation measurement | (DEM) |
| Distance to mouth | (km) | (GIS); length from the sampling site to main ramification point | (DEM) |
| Drainage density | (km km−2) | (GIS); length of all stream channels in the subcatchment/subcatchment area Horton (1945) | (DEM) |
| Sinuosity | (km km−1) | (GIS); along-channel length/the straight line Brice (1984) | (DEM) |
| Stream order | Classified 1–5 | (GIS), sensu Strahler (1957) | (DEM) |
| Slope | (%) | (GIS) | (DEM) |
| Knickpoints | Count | Gradient steps > 10% | (DEM) |
| Land use | |||
| Forested territories | (%) | Area of forested territories/subcatchment area | CORINE Land Cover 2006 databaseb |
| Agricultural territories | (%) | Area of agricultural territories/subcatchment area | CORINE Land Cover 2006 databaseb |
| Urban territories | (%) | Area of urban territories/subcatchment area | CORINE Land Cover 2006 databaseb |
| Road density | (km km−2) | Length of all road segments in the subcatchment/subcatchment area Baxter, Frissell & Hauer (1999) | OpenStreetMapc |
| Road crossing | Count | Number of road intersections | OpenStreetMapc |
| Barriers | Count | Main waterfalls and/or dams | Field and/or topographic reconnaissance |
| Fishing management | |||
| National Parks | Dummy variable | Streams localised in a protected area = 1, otherwise = 0 | Site boundariesd |
| Biotic | |||
| Other species | Count | Number of other fish species | Carta Ittica della Regione Marche Lorenzoni & Esposito (2012) |
- a Complessi idrogeologici, Agenzia per la Protezione dell'Ambiente e per i Servizi Tecnici, APAT, (http://www.sinanet.isprambiente.it).
- b CORINE Land Cover 2006 database, CLC2006; European Environmental Agency, (http://www.eea.europa.eu).
- c Map data © OpenStreetMap contributors, CC BY-SA; (http://www.openstreetmap.org/).
- d Aree protette ufficiali, Agenzia per la Protezione dell'Ambiente e per i Servizi Tecnici, APAT, (http://www.sinanet.isprambiente.it).
Influence of the spatial data distribution was addressed from Cartesian geographical coordinates of each sampling location adopting the algebraic calculation (x, y, xy, x2, y2, x2y, xy2, x3, y3) described in Legendre (1990).
In this analysis, genetic data were used as dependent variables. A matrix was constructed using LDH-C1* allele frequencies and mtDNA haplotypes frequencies. The frequencies of the native haplotypes were treated as a single variable (AD-MA-ME). A square-root-arcsine transformation was applied before successive analyses.
An ordination approach was adopted to assess the relationship between genetic introgression and environmental characteristics. Before starting, the pattern extracted from all variations was first interpreted by an indirect gradient analysis (detrended correspondence analysis, DCA). Because DCA showed turnovers along the first axes close to two SD units, a linear model of ordination (redundancy analysis, RDA) was chosen, as recommended by ter Braak (1995). This analysis and the following RDAs were carried out using the program CANOCO version 4.5 (ter Braak & Smilauer, 1998). Both environmental and spatial matrices were related independently to the genetic data, and in both cases, the variables that were more likely to account for the different distribution of the genetic native and alien variants were extracted using the forward selection procedure available in CANOCO. Variables were selected on the basis of their statistical significance (P < 0.05) and on the proportion of the variance in dependent variables that they explained, both individually and in linear combination with other such variables based on 1000 Monte Carlo permutations. The selected variables were then used to perform a RDA whose contribution to explaining the genetic pattern of introgression observed was determined from the sum of all canonical eigenvalues. A permutation procedure (Monte Carlo test) was used to assess significance.
Finally, the total variation in genetic data was partitioned into four components: (i) pure environmental; (ii) pure spatial; (iii) shared by spatial and environmental variables and (iv) unexplained variation following the method of Borcard, Legendre & Drapeau (1992).
Results
Mitochondrial DNA
A total of 12 haplotypes were detected (Table 3). According to Caputo et al. (2004) and Splendiani et al. (2006), the haplotypes 1–8 are mtDNA variants of hatchery-reared trout of Atlantic origin (AT lineage). In fact, the RFLP pattern D (produced by the endonuclease Alu I) is a diagnostic character of the AT lineage. The haplotypes 9–12 represent variants of native origin. Haplotype 9 with the RFLP pattern BCCAA showed a combination of diagnostic restriction sites for the Adriatic lineage (AD). In the same way, the haplotype 10 (pattern DCCAB) can be ascribed to the marmoratus lineage (MA) and the haplotypes 11 and 12 (patterns ADCCC and AECCC, respectively) belong to the Mediterranean lineage (ME).
| Code | Haplotype | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (AT) | 2 (AT) | 3 (AT) | 4 (AT) | 5 (AT) | 6 (AT) | 7 (AT) | 8 (AT) | 9 (AD) | 10 (MA) | 11 (ME) | 12 (ME) LDH-C1* | |||
| BAADB | CAADB | AAADB | BBBDB | CABDB | BABDB | BABDB | BBADB | BCCAA | DCCAA | ADCCC | AECCC | *90 | *100 | |
| SE | 0.50 | 0.29 | 0.21 | 1.00 | 0.00 | |||||||||
| PA | 0.14 | 0.21 | 0.64 | 1.00 | 0.00 | |||||||||
| RV | 0.06 | 0.50 | 0.44 | 0.94 | 0.06 | |||||||||
| ER | 0.22 | 0.78 | 0.85 | 0.15 | ||||||||||
| FM | 0.17 | 0.83 | 1.00 | 0.00 | ||||||||||
| BE | 0.14 | 0.05 | 0.43 | 0.38 | 0.36 | 0.64 | ||||||||
| SN | 0.20 | 0.05 | 0.75 | 0.98 | 0.03 | |||||||||
| TS | 0.09 | 0.38 | 0.29 | 0.02 | 0.22 | 0.77 | 0.23 | |||||||
| RF | 0.20 | 0.20 | 0.60 | 1.00 | 0.00 | |||||||||
| MA | 0.73 | 0.23 | 0.05 | 0.76 | 0.24 | |||||||||
| TG | 0.11 | 0.11 | 0.68 | 0.11 | 0.98 | 0.02 | ||||||||
| EN | 0.05 | 0.95 | 0.33 | 0.67 | ||||||||||
| CD | 0.05 | 0.11 | 0.29 | 0.39 | 0.16 | 0.34 | 0.66 | |||||||
| ST | 0.88 | 0.13 | 0.53 | 0.48 | ||||||||||
| FI | 0.06 | 0.17 | 0.22 | 0.33 | 0.06 | 0.06 | 0.11 | 0.59 | 0.41 | |||||
| AN | 1.00 | 0.89 | 0.11 | |||||||||||
| RS | 0.33 | 0.17 | 0.25 | 0.25 | 0.41 | 0.59 | ||||||||
| AM | 1.00 | 0.12 | 0.88 | |||||||||||
| TE | 0.04 | 0.96 | 0.14 | 0.86 | ||||||||||
| SL | 0.72 | 0.28 | 0.00 | 1.00 | ||||||||||
| OS | 0.06 | 0.94 | 1.00 | 0.00 | ||||||||||
| AS | 0.18 | 0.12 | 0.71 | 0.46 | 0.54 | |||||||||
| TN | 0.03 | 0.05 | 0.11 | 0.82 | 0.24 | 0.76 | ||||||||
| FO | 0.08 | 0.42 | 0.50 | 0.98 | 0.02 | |||||||||
| CT | 0.04 | 0.74 | 0.22 | 1.00 | 0.00 | |||||||||
| CM | 0.50 | 0.25 | 0.25 | 0.88 | 0.13 | |||||||||
| NA | 0.53 | 0.20 | 0.20 | 0.07 | 0.97 | 0.03 | ||||||||
| TC | 0.20 | 0.20 | 0.20 | 0.40 | 1.00 | 0.00 | ||||||||
| TU | 0.04 | 0.02 | 0.06 | 0.35 | 0.50 | 0.02 | 0.50 | 0.50 | ||||||
| CN | 1.00 | 0.81 | 0.19 | |||||||||||
| SC | 0.21 | 0.79 | 1.00 | 0.00 | ||||||||||
| FP | 0.50 | 0.17 | 0.33 | 1.00 | 0.00 | |||||||||
| RC | 0.20 | 0.70 | 0.05 | 0.05 | 0.83 | 0.18 | ||||||||
| NE | 0.14 | 0.09 | 0.45 | 0.32 | 0.77 | 0.23 | ||||||||
| FG | 0.21 | 0.26 | 0.53 | 1.00 | 0.00 | |||||||||
| RR | 0.11 | 0.05 | 0.84 | 0.84 | 0.16 | |||||||||
| H1 | 0.59 | 0.27 | 0.14 | 0.88 | 0.13 | |||||||||
| H2 | 0.20 | 0.20 | 0.05 | 0.55 | 1.00 | 0.00 | ||||||||
| H3 | 0.35 | 0.00 | 0.65 | 1.00 | 0.00 | |||||||||
- AD, Adriatic; AT, Atlantic; MA, marmoratus; ME, Mediterranean.
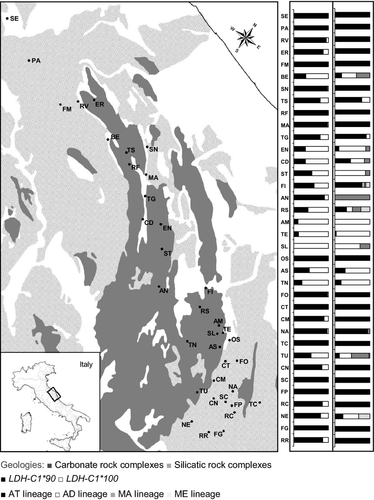
Overall, the Atlantic haplotypes were observed in 410 (53.2%) of 771 individuals analysed. The remaining individuals bore native haplotypes (lineages AD, MA and ME) with frequencies of 30.3, 9.77 and 1.8%, respectively (Table 3, Fig. 2). At single population level, the exclusive presence of AT haplotypes was observed in 19 of 36 wild populations. Conversely, only four local samples (ST, AN, AM and SL) were lacking in these alien variants. In the rest of the wild samples, the percentage of Atlantic haplotypes ranged from 4.17 in TE to 93.33% in NA. The three hatchery samples (H1, H2 and H3) were characterised by individuals bearing only AT haplotypes.
Nuclear DNA: LDH-C1* locus
The comparison of the introgression patterns detected with the LDH-C1* locus and the mtDNA haplotypes showed a high and significant correlation (Spearman's rs = 0.83, P < 0.0001). However, discrepancy was detected between the presence of the allele *90 and AT haplotypes in some of the populations studied (Table 3). In nine wild samples (BE, ST, AN, RS, AM, TE, TN, TU and NE), characterised by the prevalence of native haplotypes (mean frequency of AT haplotypes = 12.60%), the LDH-C1* locus showed a higher value of introgression (mean frequency of allele *90 = 40.69%). Although in some cases discrepancies in introgression estimates might have been affected by small sample size, like in ST and RS samples (8 and 12 individuals, respectively), discrepancies were also clear in collections with large sample size, like TU, TN AM and TE (Table 1). In contrast, in the wild samples characterised exclusively by AT haplotypes, LDH-C1* locus showed a residual of native genetic variability (mean frequency of allele *100 = 4.8%). In fact, while mtDNA analysis revealed 19 wild samples where the native variants were displaced by AT variants, LDH-C1* showed full introgression in 10 wild samples. However, the possibility that the low frequency of the allele *100 detected in these latter samples is not a residual of the original genetic diversity cannot be rule out. Indeed, it could be related to the presence of this allele in the hatchery sample H1 as a consequence of farm's naive practice of inseminating hatchery females with wild males (see Caputo et al., 2004). The only population in which, consistent with mtDNA data, the domestic allele *90 was not observed is the sample SL. The observed genotype frequencies at this locus showed no significant departure from HW expectations after correcting for multiple tests (data not shown).
Relationship between environmental factors and genetic introgression
The RDA revealed a strong relationship between environmental variables (see Table S1 for values) and the spreading of domestic and native genetic variants in the 36 wild samples of brown trout from Central Italy. The forward selection procedure retained two of 19 environmental variables: percentage of permeable rock complexes and drainage density. The pairwise correlation between the environmental variables selected was not more than 0.49. Combination of these variables accounted for 62.5% of the total variance in LDH-C1* locus and haplotypes mtDNA frequencies distribution. This relationship was statistically significant (P < 0.001) as evidenced by a Monte Carlo permutations test (1000 permutations).
The sample distribution on the ordination plots (Fig. 3) revealed a strong association with the first axis (62.2% of the variance), mainly related to the geological characteristics of the streams. Indeed, the brown trout collections characterised by the elevated presence of genetic variants of native origin were progressively located at the right end of the plots, along a gradient of increasing rock permeability. In contrast, samples showing the dominance of genetic variants of domestic origin were located at the left end. The second axis explained only a small portion (0.3%), although significant (P < 0.001), of the total variance.
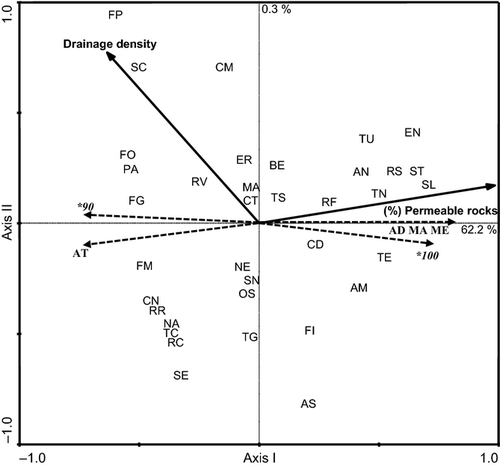
A significant relationship between spatial coordinates and the level of introgression observed was also detected. A single spatial term was forward selected: Y2. This variable accounted for 25.5% of the total variance. The relationships between genetic variables and explanatory variables were also statistically significant in this case (Monte Carlo test, 1000 permutations).
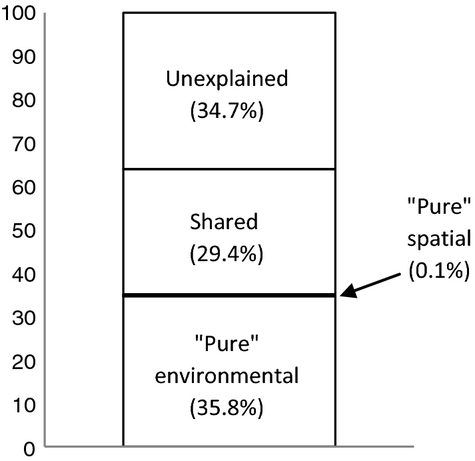
Variance partitioning procedure (pRDA) indicated that the environmental component, compared to the spatial one, was the prevalent descriptor of the genetic variation (Fig. 4). Pure environmental component explained 35.8% (P < 0.001 after 1000 permutations of a Monte Carlo test) of the observed genetic variance. In contrast, pure spatial component explained only 0.1 of the total variance and, in both cases, was not significant (P > 0.05). The variance shared by the environmental and spatial variables accounted for 29.4% of the total variance.
Discussion
The results presented in this study confirm the previously reported alarming prevalence of allochthonous genetic variability in populations of S. trutta from several Apennine streams of Central Italy (Caputo et al., 2004; Splendiani et al., 2006). However, the addition of new samples and the genetic data treatment using a landscape genetics approach demonstrated that: (i) in a few streams, the original genetic diversity of brown trout is still dominant and (ii) the main geological characteristics of the Central Apennines could have played an important role in shaping the levels of introgression detected in the wild brown trout populations of this area.
The study highlights the relevance of including multiple types of genetic markers in investigations of population structure and introgressive hybridisation as already recommended in several studies (Poteaux, Bonhomme & Berrebi, 1998; Hansen et al., 2000; Sanz et al., 2009). Although an elevated and significant correlation between the LDH-C1*90 allele and AT haplotypes was revealed in the whole sample, discrepancies were detected at some locations, suggesting asymmetric gene flow. According to Hansen et al. (2000), a higher tendency of hatchery females to become anadromous and a stronger selection acting against them could explain the lowest contribution of hatchery females to interbreeding at some locations. This hypothesis seems to be supported by the observations of smolting trout of hatchery origin that were characterised by the predominance of females in the coastal waters of the Adriatic Sea (Splendiani et al., in preparation). A further explanation for the lower contribution of female domestic trout to interbreeding is related to the differences in spawning times between Mediterranean and northern brown trout populations. According to Gortázar et al. (2007), the hydrological unpredictability of southern streams, characterised by a strong variation in water flow, may be a reason for the longer spawning periods observed in southern populations. In this context, it was shown that in heavily stocked Mediterranean streams, gonad maturation in females presented a pattern similar to that observed in populations from Central Europe (i.e. November–January) (Caputo, Giovannotti & Splendiani, 2010). Therefore, the role of a genetic component in determining a more definite maturation time span in female trout of Atlantic origin could explain the pattern of male-mediated introgression observed in several streams. The deposition of eggs in a short time period could therefore result in an uncoupling with adequate habitat conditions (Bernardo et al., 2003). In the case of males, a longer spawning time (see Burton, 1998), in conjunction with the possible presence of mature male parr (sneaker individuals), would guarantee a higher probability of fitting the reproductive effort with suitable environmental conditions. The detection of mature male parr of domestic origin in the Metauro River (e.g. Caputo et al., 2010) supports this hypothesis. Genetic drift is another important mechanism that may produce sex bias in the direction of hybridisation. In fact, taking into consideration the maternal inheritance of the mitochondrial genome and therefore its smaller effective population size than that of nuclear loci, genetic drift is expected to remove introduced mitochondrial haplotypes faster than nuclear alleles, thus resulting in an apparently lower introgression measured by mtDNA.
Whatever the mechanisms that may have produced the phenomena of asymmetric introgression observed in some populations, they could not completely explain the pattern of introgression observed in other samples. In fact, mtDNA and LDH-C1* locus indicated the almost total absence of the original genetic diversity in 20 of 36 wild samples. According to Hansen (2002), introgression due to stocking can be regarded as immigration (m)/selection (s) balance. If the immigration rate of hatchery trout into wild populations is high enough to overcome the rate at which stocked trout are removed by selection (m > s), introgression will persist over time and finally replace wild trout. The different patterns of introgression detected in this study seem to indicate that the simultaneous action of these two opposing forces resulted in different outcomes. The most direct explanation could be related to a non-homogeneous stocking effort; unfortunately, as mentioned above, historical stocking records are lacking, and therefore, a direct comparison between the extent of these practices and the degree of introgression detected was not possible. Nevertheless, it is difficult to say whether the different levels of introgression detected can merely be attributed to different stocking histories between streams, especially when the limited size of the study area (c. 160 km from north to south) and the long tradition of massive stocking activities are considered (Sommani, 1950; Bianco, 1994; Caputo et al., 2004). Although stocking records are scarce, historical documents could be helpful to roughly predict the different fate of stocking activities. For example, documents, dating back to the 15th century, suggest the occurrence of native brown trout populations in the area of the Nera River (sampling location TN) long before the start of stocking (see Cecchi, 1966). On the contrary, in similar documents dating back to 16th century and regarding the area of the Metauro River, only Cottus gobio is mentioned (Caputo, 2003). The fact that S. trutta was not cited in the above documents might suggest that in this part of the study area trout was very rare and localised. Similar conclusions can be proposed for the area of the Tronto River on the basis of a publication dating back to the 19th century (Cappello, 1843). These historical reports offer useful information to roughly depict the original brown trout distribution in the study area before the advent of modern stocking activities. The presumed rarity of this fish in the Metauro and Tronto rivers could explain the more severe introgression levels exhibited by the samples from these rivers. The low density of trout inhabiting these two river basins could have lowered the strength of selective pressure and promoted extensive introgression or even total replacement of original genetic diversity owing to stocking. In these two main river basins, traces of the original brown trout genetic diversity are detectable only at three localities (BE for the Metauro River and TU and NE for the Tronto River), which correspond to the parts of the rivers more suitable for salmonids (see below). On the contrary, stocking does not seem to have promoted severe introgression in streams harbouring healthy self-sustaining native trout populations. Similar considerations have been already suggested for S. trutta (Berrebi et al., 2000; Heggenes et al., 2002; Madeira et al., 2005; Van Houdt et al., 2005; Hansen et al., 2009) as well as for other salmonids like Salmo salar (Tessier & Bernatchez, 1999), Salvelinus fontinalis (Marie et al., 2012) and Thymallus thymallus (Koskinen, Piironen & Primmer, 2002).
A possible clarification of the different patterns of introgression observed in the study area emerged when the environmental characteristics were considered. The variable that best explained the variance of both LDH-C1* alleles and ND-5/6 haplotypes frequency distributions was the percentage of permeable rocks. In fact, the samples characterised by a high percentage of native genetic variability were detected in streams arising from the hydrogeological complex of the carbonates ridge (continental formation, Meso-Cenozoic), where the water courses show stable flow conditions. On the other hand, most of the wild samples hosting almost exclusively genes of hatchery origin were detected in streams arising from siliceous sedimentary rock complexes (marine terrigenous formations, Mio-Pliocene), where the presence of impermeable rocks (sandstone and clays) renders the discharge of main water courses low and irregular (e.g. Boni et al., 2009).
According to Nicola, Almodóvar & Elvira (2009), the hydrogeological variability plays a central role in population dynamics of brown trout from lower latitudes. In fact, the high annual variability of the Mediterranean climate has a strong influence on the hydrological behaviour of the water courses; for example, the regional climate of the study area, characterised by dry and hot summers and rainfall concentrated in autumn and winter, causes irregular water conditions with phases of flood disproportionately large when compared to the phases of drought (Bisci & Dramis, 1991). Therefore, we speculate that the severe degree of introgression detected in most locations sampled in impermeable catchments represents indirect evidence that populations of native trout in these rivers were absent or very small, even before stocking began. In fact, the incidence of severe summer droughts in these streams would never have allowed the establishment of large populations of brown trout. Probably these populations were subjected to continuous phenomena of local extinction and subsequent recolonisation from neighbouring populations inhabiting the more suitable limestone catchments, at both intra- and inter-river basin level. The detection of the trout native genetic diversity relegated in BE (51.82% of permeable rocks) for the Metauro River and in TU and NE (92.04 and 22.75%, respectively) for the Tronto River would suggest that these localities served as refuges for brown trout inhabiting these two unstable river systems. As a consequence, the massive stocking with domestic strains would have quickly overwhelmed the already small original populations giving rise to the current populations of Atlantic origin. Similar considerations have been proposed for S. trutta by Madeira et al. (2005) to explain the different introgression rates observed between Cantabrian and Mediterranean rivers.
In conclusion, this study has highlighted the importance of taking into account environmental factors when assessing the underlying causes of hybridisation in wild brown trout populations. The influence of catchment geology on the distribution of native brown trout populations in Central Italy represents an important finding not only to improve conservation strategies for this salmonid at a local scale, but also for other salmonid species from similar peripheral southern areas. Moreover, the identification in a few streams of ‘pure’ or almost ‘pure’ trout represents a significant marker for the future conservation of this salmonid, especially if the rarity of non-impacted salmonid populations in Italy is taken into consideration (e.g. Meraner et al., 2013; Querci et al., 2013).
Acknowledgments
The authors thank the local angling associations Arcipesca, FIPSAS and UNPEM for their assistance with specimen collection, in particular Stefano Breccia, Alfeo Busilacchio, Luca Ercoli, Luigi Evangelisti, Lucio Santoni, Adriano Togni and Mauro Zavaldi. We are grateful to Adriano Mancini for helping with statistical methods, Mario Marconi (University of Camerino, Italy), Massimo Lorenzoni (University of Perugia, Italy), Luca Esposito, Roberto Barbaresi (provincial administration of Pesaro-Urbino), Pierfrancesco Gambelli (provincial administration of Ancona) for their valuable support during the field activities. We are also grateful to the provincial administrations of Pesaro-Urbino, Ancona, Macerata, Ascoli Piceno and Ente Parco Nazionale dei Monti Sibillini for permitting specimen collection. Finally, we are very grateful to two anonymous reviewers who greatly improved the manuscript. This research was supported by funds from Università Politecnica delle Marche (Ancona, Italy), the provincial administrations of Pesaro-Urbino and Ancona and ASTERIA Institute (Istituto per lo Sviluppo Tecnologico e la Ricerca Applicata, Monteprandone, Ascoli Piceno, Italy).




