Intradermal testing of iodinated contrast media: Should we test up to pure or with diluted compounds only?
Abstract
Background
Intradermal testing (IDT) with iodinated contrast media (ICMs) is an established diagnostic tool in patients with ICM hypersensitivity. Currently, it is unclear which test concentration is the more useful one, up to pure or up to 1:10 diluted ICMs.
Methods
We searched the literature database PubMed for eligible papers dealing with ICM allergy and their IDT results. We analyzed the data presented by the papers and compared the pooled groups tested with diluted and undiluted ICMs.
Results
We identified 29 eligible original papers, and extracted data of 1137 patients that formed the study population. Although in the cohort tested with diluted ICMs the number of tested ICMs was greater, the percentage of positive tests was significantly less (9.0% vs. 24.7%; P < 0.0001; OR 0.30 [0.26–0.34]). The frequency of positive tested culprit ICMs was also lesser in the group tested with diluted ICMs (31.0% vs. 72.5%; P < 0.0001; OR 0.17 [0.12–0.23]). The number of drug provocation tests (DPTs) was greater in patients with diluted IDTs (374 vs. 89; P < 0.0001; OR 2.54 [1.93–3.36]). We detected an increased sensitivity in patients with undiluted tests (0.774 vs. 0.282) and a nearly identical specificity in both groups (1 vs. 0.983).
Conclusions
For the first time, we show that IDT up to pure ICM concentrations is superior to using diluted ICMs only. Possibly, we can reduce the number of DPTs when performing IDTs with pure ICMs. In the undiluted group, there were no hints for skin irritations or unspecific test reactions.
ABBREVIATIONS
-
- ADR
-
- adverse drug reaction
-
- CI
-
- confidence interval
-
- DPT
-
- drug provocation test
-
- ICM
-
- iodinated contrast medium
-
- IDT
-
- intradermal testing
-
- IR
-
- immediate reaction
-
- NIR
-
- non-immediate reaction
-
- NPV
-
- negative predictive value
-
- OR
-
- odds ratio
-
- PPV
-
- positive predictive value
-
- SD
-
- standard deviation
1 INTRODUCTION
Although the exact number is unclear, several hundred million doses of iodinated contrast media (ICMs) are given worldwide per year [1]. During the last decades, the number of applied ICM doses in clinical radiological routine was growing. Now, this trend changed, possibly due to the shortage of ICMs [2]. Most patients tolerate the injection of the ICMs very well, but a small percentage acquires adverse drug reactions (ADRs) [1-3]. To improve their safety, patients with a history of previous adverse ICM events should undergo a special prophylactic management. Currently, the omission of the patients' culprit ICM and the application of another non-culprit ICM is the preferred prophylaxis in such patients [1, 4, 5]. The decision-making process can be done without or with help of a contrast medium allergy tests. Especially in cases with a history of a severe hypersensitivity reaction, intradermal testing (IDT) of ICMs [6, 7] is the method of choice to make the exact diagnosis and to find a safe ICM compound.
Unfortunately, currently, the maximal test concentration for an IDT is not clear yet. While some allergists use diluted contrast agents up to 1:10 only [8-10], others test with concentrations up to undiluted (pure) contrast media [11-13]. Therefore, the aim of the present study is to find out the optimal test concentration.
2 METHODS
2.1 Literature search criteria
Two investigators independently searched the online database PubMed from January 1999 up to December 2022. We used the keywords “iodinated contrast media,” “allergy,” and “intradermal test,” in various combinations to find eligible articles. In addition, we carefully checked reference lists of all articles for other eligible papers. Inclusion criteria were articles presenting human studies and individual skin test results of ICMs that could be obtained from the paper. We included online available full-text research articles. Exclusion criteria were review articles, case series and case reports, in vitro studies, papers dealing with gadolinium-based contrast agents, and animal studies. We limited our search to articles in English or German.
2.2 Data extraction and study design
We searched eligible publications according to criteria of meta-analyses, but we did not perform a meta-analysis. Two investigators independently extracted relevant data from each article of single patients and documented age, gender, culprit ICM, tested ICMs and test results, the kind of hypersensitivity reaction (immediate reaction [IR] or non-immediate reaction [NIR]), and the used ICM concentration for IDTs (1:10 diluted or undiluted/pure). We solved possible discrepancy by consensus.
In addition, we carefully analyzed articles that describe IDTs with pure contrast media for hints of irritant or unspecific test reactions. We also searched in the PubMed online database for articles that provide insight into the history of allergy skin testing by using contrast media.
In papers dealing with IDTs up to 1:10 diluted ICMs, we searched the reason for the procedure and analyzed cited articles, if mentioned.
2.3 Statistical analysis
Demographic data, clinical characteristics, and test results were analyzed by descriptive statistic tests. For categorical data, we used the Chi-squared test or Fisher's exact test. The continuous variables were expressed by mean and standard deviations (mean ± SD) and compared by Mann–Whitney U test or the Student's T test. Odds ratios (ORs) and their 95% confidence intervals (95% CI) were calculated, and comparisons between different groups were made.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were analyzed through the comparison of patients and controls.
We considered P values of less than 0.05 to be statistically significant. We used the STATA software version 12.1, Inc., Chicago, IL, USA, for the statistical analysis.
3 RESULTS
3.1 Study overview
We found 173 papers and excluded 144 of them (Figure 1). The remaining 29 original articles [8-36] met the above-mentioned inclusion criteria and were analyzed.
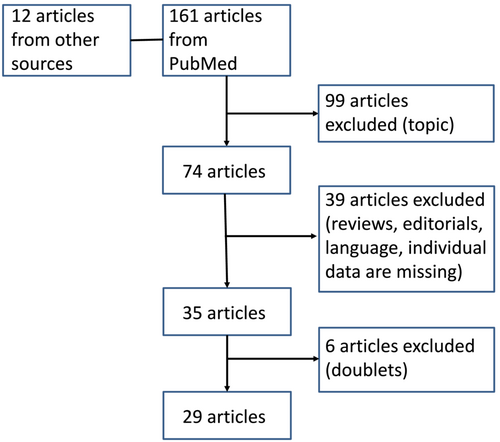
In 12 papers, the authors reported on test concentrations up to pure ICMs [11-13, 17, 19, 21, 22, 28, 29, 32, 33, 35]. Prieto-Garcia et al. [19] tested iopamidol, ioversol, and iobitridol pure, and Vernassiere et al. [29] used undiluted test concentrations but not when testing iodixanol or ioxaglate.
In 16 papers, the authors used diluted ICM test concentrations only [9, 10, 14-16, 18, 20, 23-27, 30, 31, 34, 36]. In one paper [8], the authors used both pure (in patients with NIRs) and diluted ICM test concentrations (in patients with IRs).
3.2 Extracted data
For the further detailed analysis, we used extracted data from individual patients. We identified 1137 patients (men n = 312, women n = 563, unknown gender n = 262) with a median age of 54.9 ± 15.2 years who formed the pooled study population. Most patients (n = 1121) had one culprit ICM, and the remaining patients had two culprit ICMs. Table 1 shows additional details.
| Parameter | Result |
|---|---|
| Age in years, mean ± SD | 54.9 ± 15.2 |
| Men | 312 (27.4%) |
| Women | 563 (49.5%) |
| Unknown | 262 (23.0%) |
| Immediate reactions | 741 (65.2%) |
| Non-immediate reactions | 393 (34.6%) |
| Biphasic reactions | 1 (0.09%) |
| Unknown | 2 (0.18%) |
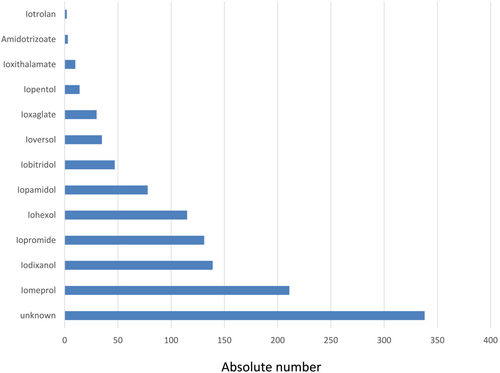
In the pooled cohort, we found that 7994 tested ICMs induced 1027 (12.8%) positive reactions. The culprit ICM was positive in at least 387 instances. Further test results are presented in Table 2. The name of the culprit ICM was mentioned in 799 (77.8%) cases. Figure 2 shows both the names of the culprit ICMs and their frequencies.
| Characteristics | Result |
|---|---|
| Total number of tested ICMs (IDT) | 7994 |
| Number of positive tested IDTs | 1027 (12.8%) |
| Patients with diluted IDTs | 797 |
| Patients with undiluted IDTs | 340 |
| Positive tested culprit ICM | 387 |
| Drug provocation tests | 463 (40.7%) |
3.3 Evaluation of the ICM test concentration
The central purpose of this paper is the comparison of patient groups tested with different ICM test concentrations (pure versus diluted) in IDT.
Papers with diluted ICMs [8-10, 14-16, 18, 20, 23-27, 30, 31, 34, 36] report on a cohort of 4192 patients. In 336 patients, the authors found positive test reactions. The cohort of patients tested with pure ICMs comprises of 766 patients, and 335 individuals had a positive test result [8, 11-13, 17, 19, 21, 22, 28, 29, 32, 33, 35]. When testing up to pure CM concentrations, the number of patients with a positive test result was five times higher than the number of patients with positive test result following testing with diluted CMs only (43.7% vs. 8.0%), the result was statistically significant (P < 0.0001; OR 8.92 [7.44–10.69]).
In the next step, we compared subgroups with diluted and pure ICM concentrations for IDTs (Table 3). Although in patients tested with diluted compounds, the number of tested ICMs was significantly greater, the number of positive tested contrast agents was significantly less than in the pure group (Table 3).
| Diluted tests (n = 797) | Undiluted tests (n = 340) | Significance (P values) | OR (95% CI) | |
|---|---|---|---|---|
| Mean age (±SD) | 56.5 (±14.4) | 53.4 (±15.9) | ns | |
| Men:women | 1:1.7 | 1:2.15 | ns | |
| ICMs tested | 6030 | 1964 | <0.0001 | 0.30 (0.26–0.34) |
| Positive tested ICMs | 542 (9.0%) | 485 (24.7%) | ||
| 1–6 ICMs tested | 259 | 225 | <0.0001 | 4.06 (3.11–5.32) |
| 7–12 ICMs tested | 538 (67.5%) | 115 (33.8%) | ||
| Culprit positive | 152 (31.0%) | 235 (72.5%) | <0.0001 | 0.17 (0.12–0.23) |
| Culprit negative | 338 | 89 | ||
| Patients | <0.0001 | 0.1152 (0.086–0.155) | ||
| Correct positive | 225 | 263 | ||
| False negative | 572 | 77 | ||
| Controls | ns | |||
| False positive | 5 | 0 | ||
| Correct negative | 290 | 148 | ||
| Patients with drug provocation tests | 374 (46.9%) | 89 (26.2%) | <0.0001 | 2.54 (1.93–3.36) |
| Number of DPTs | 400 | 111 | <0.0001 | 2.08 (1.59–2.71) |
- Abbreviation: ns, not significant.
We calculated a sensitivity of 0.282 in the diluted group and of 0.774 in the undiluted cohort. The specificity is 0.983 in the diluted and 1 in the pure group.
3.4 Drug provocation tests (DPTs)
In the diluted group 374 and in the undiluted group 89 patients underwent DPTs (Table 3).
In the diluted group, DPTs induced 57 adverse reactions (in seven cases with positive IDT). The remaining 343 DPTs were negative (174 DPTs performed with unknown ICMs). Of 169 negative DPTs tested with known ICMs, in 159 cases, IDTs were negative, in eight cases positive, and in two cases, the ICMs used for DPT were not skin tested. Consequently, we calculated a sensitivity of 0.467, a specificity of 0.761, a PPV of 0.123, and a NPV of 0.925.
In the pure group, DPTs induced adverse reactions in two cases with previous positive IDT and in 35 patients with negative IDT. All patients with negative DPTs (n = 74) were previously negative skin tested by IDTs. Therefore, the sensitivity is 1, the specificity 0.679, the PPV 0.054, and the NPV 1.
3.5 Patients with IR versus NIR
The comparison of patients with IR and NIR shows that the first group is greater than the latter (Table 4). In both groups, more tests with diluted contrast materials were done. The number of patients with IRs was significantly greater in the group tested with diluted than with pure contrast agents (Table 4). In other words, of 742 IRs, 576 were tested with diluted and 166 with undiluted ICMs. In the cohort with NIRs (n = 394), 220 were tested with diluted and 174 with undiluted contrast media (Table 4). NIR patients have a significant greater proportion of both positive tested culprit and positive tested ICMs. The percentage of patients who underwent a DPT is greater in IR patients (Table 4).
| NIR (n = 394) | IR (n = 742) | Significance (P values) | OR (95% CI) | |
|---|---|---|---|---|
| Mean age (±SD) | 54.5 (±11.9) | 54.4 (±15.7) | ns | |
| Men:women | 1:1.66 | 1:1.89 | ns | |
| Diluted IDT | 220 | 576 | <0.0001 | 2.74 (2.11–3.57) |
| Undiluted IDT | 174 | 166 | ||
| ICMs tested | 2854 | 5140 | <0.0001 | 2.09 (1.83–2.39) |
| Positive tested ICMs | 526 (18.4%) | 501 (9.7%) | ||
| Culprit positive | 188 (54.5%) | 199 (42.4%) | <0.0009 | 1.62 (1.23–2.15) |
| Culprit negative | 157 | 270 | ||
| Patients with drug provocation tests | 126 (32.0%) | 337 (45.4%) | <0.0001 | 0.57 (0.44–0.73) |
- Abbreviation: ns, not significant.
3.6 Analyses to detect irritant or unspecific skin reactions
We carefully screened all papers using IDTs up to undiluted ICM concentrations [8, 11-13, 17, 19, 21, 22, 28, 29, 32, 33, 35] for hints to unspecific and/or irritant skin reactions, but we did not find them.
In addition, we analyzed the number of controls who were tested with undiluted or diluted ICMs. Most authors did not test the ICMs in healthy volunteers or patients without CM hypersensitivity [8-18, 22, 24, 26, 27, 29-34, 36]. Only few papers dealing with pure IDTs included controls in their study group [19, 21, 28, 35]. We revealed a pooled cohort of 154 subjects who served as of controls. None of them had a positive test reaction. In papers with diluted ICMs, we found only four papers that included 295 controls in their studies with five (1.7%) individuals who showed a positive test reaction [21, 24, 26, 37].
3.7 Reasons for using diluted ICMs for IDTs
Next, we qualitatively analyzed the related papers for reasons why IDTs were performed with diluted ICMs [8-10, 14-16, 18, 20, 23-27, 30, 31, 34, 36, 38]. Figure 3 summarizes the results. One paper [34] mentions that 1:10 is the known non-irritant concentration but does not cite this statement. Another paper [31] states that they tested according to ENDA/EAACI guidelines without citation of an article.

4 DISCUSSION
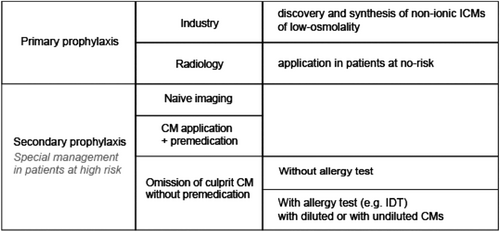
In order to find the optimal test concentration, we for the first time compared IDT results obtained with pure and diluted ICM concentrations. We reveal that testing up to pure ICM has several advantages such as increased percentage of positive test reactions, decreased number of tested ICMs, and decreased number of DPTs, for example (Table 3). The optimization of the test concentration is one step in a complex scenario to improve the safety of ICMs (Figure 4). Since eight decades [39], allergy workups (e.g., IDTs) are established methods to verify an allergy to the culprit CM and to find out individually tolerated alternate compounds [7]. Therefore, the finding of the optimal test concentration for IDTs is also an important step.
4.1 Advantages and disadvantages of diluted and undiluted ICM concentrations
- The number of patients with diluted IDTs is twice of that with undiluted tests.
- IRs dominate in the diluted group, while IR and NIR are nearly identical in the pure group.
- Increased number of tested ICMs in the dilution group: the number of tested ICMs per patient is greater and the percentage of patients receiving tests with 7–12 ICMs as well.
- Significant increased number of positive tested ICMs and of positive tested culprit ICMs in the pure group.
- Increased sensitivity and decreased specificity in the pure group.
- Increased PPV and NPV in the pure group.
- Significant increased number of DPTs in the dilution group.
As expected, the ratio of positive test reactions was significantly greater in the group tested with undiluted ICMs (24.7% vs. 9.0%; P < 0.0001). Consequently, we found in the pure group an increased significant frequency of positive tested culprit ICMs (72.5% vs. 31.0%; P < 0.0001), and the percentage of correct positive reactions was greater (Table 3). Interestingly, in the diluted group, the number of tested ICMs and the number of patients tested with a great panel of ICMs (7–12) were higher than in the undiluted group. This fact possibly reflects the uncertainty when testing with diluted ICMs only. In other words, when testing with diluted ICMs, one should be aware that the result shows only some of the patients' ICM allergies. In summary, the test results in the pure group seem to be exacter than these obtained in the diluted group.
In addition to diluted tests, several authors suggest to perform undiluted IDTs in cases of a non-immediate type hypersensitivity reaction for optimal sensitivity [8, 40, 41]. Therefore, one may ask, why do they recommend undiluted IDTs for patients with delayed reactions only?
Although most authors prefer IDTs with diluted contrast materials [9, 10, 14-16, 18, 20, 23-27, 30, 31, 34, 36, 42], citations/explanations are missing or incorrect (Figure 3).
4.2 Criticism and uncertainty of skin testing
Some authors presenting data on IDTs with CMs mention that this procedure has a low sensitivity or low predictive value [9, 10, 29, 34]. As possible explanation, the authors mention the low number of IgE-mediated reactions due to ICMs [9, 15]. However, other yet unknown factors were also taken into account [9].
The major criticism for tests with undiluted ICMs seems to be unspecific or irritant test results [43]. Therefore, we carefully screened all papers dealing with undiluted ICM test concentration. Two independent researchers did not find hints for irritant or unspecific skin reactions. Therefore, the argument of authors preferring diluted ICMs for IDTs can be refuted. Moreover, the percentage of false positive reactions (Table 3) was greater in the diluted than in the undiluted group. In controls tested with undiluted contrast materials, we did not find positive reactions (unpublished data).
The percentage of positive tested ICMs is significantly greater in the group tested with pure compounds. Therefore, it could be possible that besides correct tested ICMs, false positive tested ICMs may account for the increased number of positive test results. With respect to clinical safety aspects, these false positive tests do not matter. On the other hand, false negative tested compounds in the group tested with diluted ICMs only can be harmful for the patient. These considerations also support IDTs with pure ICMs.
Since the classic paper of Katayama et al. [3], it is clear that the incidence of hypersensitivity reactions to ICM has decreased with the change from ionic, high-osmolality to non-ionic, low-osmolality ICMs and is currently estimated to be between 0.15% and 0.69% [44-46].
Moreover, non-ionic ICMs do not induce irritant skin reactions, because we found no corresponding evidence in the literature analyzed (see Section 3.6). Even in patients with contrast extravasation, an irritant phenomenon did not occur (unpublished data). Therefore, in papers dealing with test concentrations up to pure ICMs [11-13, 17, 19, 21, 22, 28, 29, 32, 33, 35], we did not find hints for irritant or unspecific test results. Moreover, there is a body of evidence that non-ionic ICMs are no obligate histamine liberators [47]. Consequently, even pure ICMs are suitable for intradermal tests.
In the EAACI guideline [48], ICM concentrations of 300–320 mgI/mL are recommended for testing, while in radiology practice, many institutions use ICM with higher concentrations of 350–370–400 mgI/mL. Currently, it is unknown how this will influence the results of IDT with ICM, because to the best of our knowledge, there are no studies available dealing with this topic.
4.3 DPT
DPTs verify a positive or a negative IDT result [7]. DPTs are necessary in the diagnosis of drug hypersensitivity reactions, when a decreased sensitivity of skin testing requires it [49].
In the herein analyzed patient group, 463 DPTs are reported [8-11, 13-21, 24-26, 29, 33, 34, 36]. Patients tested with diluted ICMs underwent significantly more often a DPT than those tested with pure ICMs (Table 3). This is a hint for the assumption that IDTs with diluted ICMs are less reliable and cause greater uncertainty.
Since DPTs are much more time-consuming than IDTs and bear greater risks to harm the patient [50], IDTs with concentrations up to pure ICMs is the safer alternate test procedure. Moreover, IDTs up to undiluted compounds seem to fill the gap between IDTs with diluted ICMs and DPTs. The herein presented data provide evidence for the assumption that it is useful to test up to undiluted ICMs if necessary.
Most importantly, an allergy tests should be done in patients suspected for an ICM hypersensitivity reaction. Allergy testing without hint in the medical history for a hypersensitivity reaction is not recommended. It has been shown that IDTs in such patients are of low sensitivity and of low PPV [34].
4.4 IR and NIR
Interestingly, some authors suggest the use of diluted skin tests for patient with a history of immediate ICM hypersensitivity and undiluted IDT for patients with history of non-severe NIRs [51]. We found in NIR patients a significant greater percentage of undiluted IDTs than in patients with IR (Table 4). Consequently, the proportion of positive tests (culprit and total number) was significantly greater in patients with NIR than with IR. Therefore, the statement that allergic reactions are more common in non-immediate than in IR seems to be due to the use of different test concentrations. Future studies must show whether other factors also play a role. For example, complement activation-related pseudo-allergy (CARPA) could have a meaning, too [52].
4.5 Historical overview
In initial intradermal test procedures, physicians used undiluted contrast materials [39, 53]. The origin to use diluted contrast materials only is unclear, so far. To the best of our knowledge, a study to establish the optimal ICM test concentration does not exist. In a case report of 1999, the authors wrote that they performed patch and intracutaneous tests with a series of ionic and non-ionic radiocontrast media according to international guidelines [54]. Unfortunately, at that time, there did not exist guidelines for testing contrast agents. Consequently, it is reasonable that the authors meant general guidelines without special regard to contrast media. Although unknown, it is tempting to speculate that in vitro experiments done more than 40 years ago and their results [55] might probably have influenced some clinicians to use diluted contrast agents for IDTs only. The major finding of this paper is histamine release from peripheral mononuclear blood cells obtained from controls and patients following in vitro stimulation with ionic ICMs. In parallel to increasing concentrations of the ICMs, the histamine release increased, too [55]. Since these were (1) in vitro and not in vivo experiments and (2) done with ionic ICMs of high osmolality [55], we should neither overestimate such results nor should use them as basis for in vivo tests. We should realize that we no longer use ionic ICMs in clinical radiology routine for intravenous injections.
4.6 Limitations/data extraction
Our study has the following limitations. Since we extracted data from the literature, we were not able to re-evaluate all patients published. In particular, patients with completely negative test results were rarely mentioned in the publications [6-9, 12, 14, 20]. The general problem of an insufficient documentation [56] hinders not only radiological routine but also scientific analyses. Therefore, we recommend documenting exactly patients suspected for CM hypersensitivity reactions.
4.7 Conclusion and recommendations
Today, contrast media may be among the safest drugs in the general population. This result is the outcome of a long process in which industry, radiologists and skin testing procedures were/are involved (Figure 4). Safety in patients at risk is still a challenge, because they may react again upon re-exposure to a CM [1, 4-7].
IDT is an established diagnostic tool in patients suspected for ICM-mediated hypersensitivity reaction, it is of simple execution, and it can identify safe alternatives for further real-life setting injection of ICM; therefore, it is considered a great help, particularly if the ICM needs to be re-administrated. Unluckily, in clinical practice, there is little clarity about the maximal test concentration, pure (undiluted) or up to 1:10 diluted ICMs.
Our analyses provide evidence for a significant lower percentage of positive test reactions with diluted IDT, whereas the usage of undiluted IDT results in a greater percentage of positive test reactions. One major criticism for pure IDT use seems to be irritant or unspecific skin reactions; however, after a careful analysis of the data of the literature, this seems an incorrect assumption. This leads to the conclusion that leading to a higher result accuracy, undiluted IDT consequently could be considered of a bigger help for clinical radiology routine. Therefore, for a higher IDT diagnose reliability and selection of a safe ICM compound, we propose considering IDT with pure contrast agent, as it is more sensitive than the usual dilution (1:10) criteria.
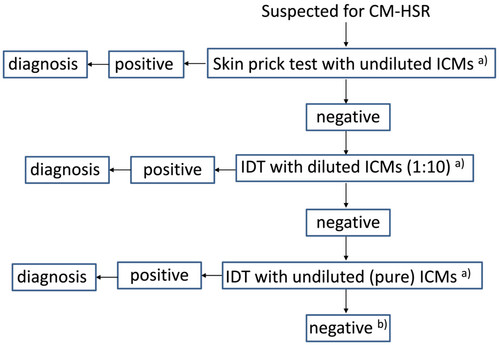
- Allergy testing should start with a skin prick test with undiluted ICMs.
- Negative tested ICMs should be followed by intradermal tests. In cases with a history of severe previous hypersensitivity reaction, the initial concentration of the ICMs should be diluted 1:1000 or 1:100, while patients with mild reactions can be tested with dilutions of 1:10.
- For negative tested ICMs, we should continue testing with the next dilution step (1:100 → 1:10).
- Undiluted intradermal tests are reserved for cases with negative tested ICM at 1:10 dilution.
Whether there will be in vitro tests [57, 58] to replace skin tests in the future remains to be elucidated.
AUTHOR CONTRIBUTIONS
Project idea: Ingrid B. Boehm. Data analyses: Adrian A. Schmid and Ingrid B. Boehm. Literature search: Adrian A. Schmid and Ingrid B. Boehm. Manuscript writing: Paolo Lombardo, Adrian A. Schmid, Martin N. Hungerbühler, and Ingrid B. Boehm. Manuscript revision: Paolo Lombardo, Adrian A. Schmid, Martin N. Hungerbühler, and Ingrid B. Boehm. Figures: Martin N. Hungerbühler and Ingrid B. Boehm. Tables: Paolo Lombardo, Adrian A. Schmid, Martin N. Hungerbühler, and Ingrid B. Boehm. Final approval: Paolo Lombardo, Adrian A. Schmid, Martin N. Hungerbühler, and Ingrid B. Boehm.
ACKNOWLEDGMENTS
The authors thank Elena Samanta Bertagna for her critical manuscript review, Professor Tokio Nakada (Tokyo, Japan) for sending us his data, Professor Gisèle Kanny (Vandoeuvre-Lès-Nancy Cedex, France) for sending us one of her papers, and Dr. Marga Tomas (Madrid, Spain) for sending us the number of tested controls. There is no funding. Open access funding provided by Inselspital Universitatsspital Bern.
CONFLICT OF INTEREST STATEMENT
None.




