Selection on the timing of migration and breeding: A neglected aspect of fishing-induced evolution and trait change
Ghoti papers
Ghoti aims to serve as a forum for stimulating and pertinent ideas. Ghoti publishes succinct commentary and opinion that addresses important areas in fish and fisheries science. Ghoti contributions will be innovative and have a perspective that may lead to fresh and productive insight of concepts, issues and research agendas. All Ghoti contributions will be selected by the editors and peer reviewed.
Etymology of Ghoti
George Bernard Shaw (1856–1950), polymath, playwright, Nobel prize winner, and the most prolific letter writer in history, was an advocate of English spelling reform. He was reportedly fond of pointing out its absurdities by proving that “fish” could be spelt “ghoti”. That is: “gh” as in “rough”, “o” as in “women” and “ti” as in palatial.
Abstract
Fishing can drive changes in important phenotypic traits through plastic and evolutionary pathways. Size-selective harvest is a primary driver of such trait change, has received much attention in the literature and is now commonly considered in fisheries management. The potential for selection on behavioural traits has received less study, but mounting evidence suggests that aggression, foraging behaviour and linked traits can also be affected by fishing. An important phenomenon that has received much less attention is selection on reproductive phenology (i.e., the timing of breeding). The potential for this type of “temporal selection” is widespread because there is often substantial variability in reproductive phenology within fish populations, and fisheries management strategies or fishermen's behaviours can cause fishing effort to vary greatly over time. For example, seasonal closures may expose only early or late breeding individuals to harvest as observed in a range of marine and freshwater fisheries. Such selection may induce evolutionary responses in phenological traits, but can also have demographic impacts such as shortened breeding seasons and reduced phenotypic diversity. These changes can in turn influence productivity, reduce the efficacy of management, exacerbate ongoing climate-driven changes in phenology and reduce resilience to environmental change. In this essay, we describe how fisheries management can cause temporal variability in harvest, and describe the types of selection on temporal traits that can result. We then summarize the likely biological consequences of temporally selective fishing on populations and population complexes and conclude by identifying areas for future research.
1 INTRODUCTION
The management and conservation of animal populations has traditionally focused on abundance and demographics as indicators of sustainability (Hilborn, 1985b; Ricker, 1946). More recently, the effects of human activities including harvest and habitat alteration on phenotypic traits have become broadly recognized (Allendorf & Hard, 2009; Darimont et al., 2009; Hendry, Farrugia, & Kinnison, 2008). Through phenotypic plasticity and microevolution, traits such as growth rate, behaviour, morphology and age at maturity have responded to anthropogenic influence in various taxa (Palkovacs, Kinnison, Correa, Dalton, & Hendry, 2012). Notable examples of human-induced trait change include reduced age and size at maturity in many commercial fish stocks (Sharpe & Hendry, 2009), altered travel patterns in migratory birds resulting from habitat alteration (Berthold, Helbig, Mohr, & Querner, 1992), declines in body weight and horn size in wild sheep (Ovis canadensis; Ovis dalli stonei, Bovidae) subjected to trophy hunting (Coltman et al., 2003; Douhard, Festa-Bianchet, Pelletier, Gaillard, & Bonenfant, 2016), and changes in feeding and reproductive behaviour of recreationally harvested largemouth bass populations (Micropterus salmoides, Centrarchidae; Cooke, Suski, Ostrand, Wahl, & Philipp, 2007). Such changes can profoundly affect the ecology, productivity and viability of animal populations (Palkovacs et al., 2012), and understanding the drivers of trait change is therefore critical to the sustainability of affected species (Stockwell, Hendry, & Kinnison, 2003).
A range of human activities may drive rapid trait change in wild populations such as habitat alteration (Berthold et al., 1992; Franssen, 2011), modification of thermal regimes (Bradshaw & Holzapfel, 2006), pollution (Mooney & Cleland, 2001) and harvest (Hendry et al., 2008; Palkovacs et al., 2012). Exploited populations are especially susceptible to human-induced trait change—both plastic and genetic—because hunting and fishing methods are commonly selective with regard to size and behaviour, and because reduced densities often increase growth rates via compensatory density dependence (Allendorf & Hard, 2009; Kuparinen & Festa-Bianchet, 2017; Sharpe & Hendry, 2009). Furthermore, artificial selection from harvest is often strong relative to forces of natural selection, and so rapid phenotypic change is possible (Allendorf & Hard, 2009; Edeline et al., 2007). Because harvest can impose particularly strong selection (Edeline et al., 2007), and because fishes and aquatic invertebrates make up the majority of harvested wild taxa, fisheries-induced trait change is particularly widespread and well documented (Fenberg & Roy, 2008; Palkovacs et al., 2012). Indeed, it has long been recognized that fishing can be selective with regard to morphological and life-history characteristics (Hamley, 1975; Handford, Bell, & Reimchen, 1977; Miller, 1957). Large fish are often preferentially harvested through some combination of regulations on fish size or gear, or from harvester preference, exerting selection on phenotypic traits including size, growth rate and age at maturity (Birkeland & Dayton, 2005; Edeline et al., 2007). Interest in the consequences of size-selective harvest on fisheries ecology and sustainability has grown rapidly in recent decades (Fenberg & Roy, 2008; Policansky, 1993). By 2008, size-selective harvest had been documented for over 120 species of fish and marine invertebrates (Fenberg & Roy, 2008) and many papers warned of the dangers of fisheries-induced evolution for fisheries sustainability (Conover & Munch, 2002; Fenberg & Roy, 2008; Kuparinen & Hutchings, 2012; Kuparinen & Merilä, 2007; Law, 2000). However, selective fishing is not limited to size-related traits. Recent research has documented selection on various behavioural traits (Heino & Godo, 2002) including removal of aggressive individuals by angling (Cooke et al., 2007) and individuals with bold foraging behaviour by gillnets (Biro & Post, 2008).
While the impacts of size-selective fisheries have been widely examined, the potential for reproductive phenology to be altered through anthropogenic selection as been largely ignored, even though variation in the timing of breeding and associated physiological and behavioural changes plays a critical role in the productivity of fish populations. Constraints on fishing effort imposed by management, behaviour of fishermen or environmental conditions can cause fishing intensity to vary over time, and fisheries mortality can therefore be selective with regard to important temporal traits such as spawning or migration date (Loher, 2011; Peer & Miller, 2014; Quinn, Hodgson, Flynn, Hilborn, & Rogers, 2007). In this essay, we call attention to the phenomenon of temporally selective fishing and review relevant literature from related areas of research. We argue that it is likely to be very common, can reduce the diversity, productivity and sustainability of fish populations and stock complexes and therefore merits further research. We first describe how management and harvester behaviour can cause temporal variability in mortality rates within exploited populations, and use a hypothetical population to illustrate the types of selection on temporal traits that can result. We then summarize the likely biological consequences of temporally selective fishing on single populations and population complexes composed of multiple stocks with unique timing traits, and conclude by suggesting important avenues for future research.
2 THE PREVALENCE OF TEMPORAL SELECTION IN FISHERIES
In most fishes and macroinvertebrates, breeding and migration occur predictably in association with seasonal, semilunar or other environmental cycles (Asch, 2015; Ims, 1990; Yamahira, 2004). The typical timing of these events is thought to reflect evolutionary optima given the selective pressures on adults and offspring, and reproductive phenology therefore plays a central role in the biology of many species (Lowerre-Barbieri, Ganias, Saborido-Rey, Murua, & Hunter, 2011; Quinn, McGinnity, & Reed, 2016). A series of interrelated behavioural and physiological processes commonly precede reproduction in fishes, including gametogenesis, migration, altered feeding behaviour, aggregation and courtship (Figure 1). Variation in any of these traits can influence susceptibility to fishing within or between populations, and fisheries can therefore selectively remove fish based on various aspects of their reproductive phenology. Furthermore, fishing practices and regulations are commonly organized around these events, either to exploit or protect the migrants or breeders (van Overzee & Rijnsdorp, 2014; Sadovy de Mitcheson, 2016). As a result, there is significant potential for fishing to interact with biologically important phenological traits (Hard et al., 2008), which may result in what we hereafter refer to as temporal selection.
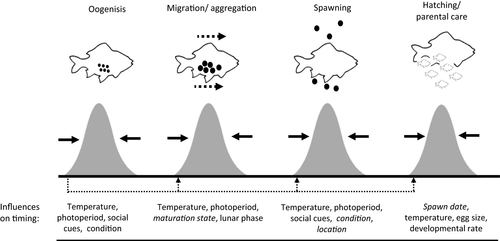
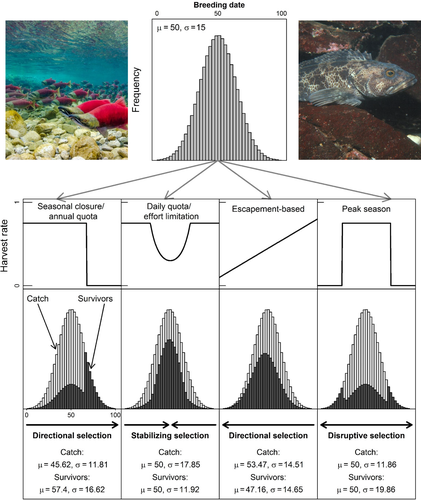
Temporal selection occurs when (i) individuals within a population vary in the timing of migration, spawning or other important life-history events; and (ii) vulnerability to capture in a fishery depends on the timing of those same events. Temporally selective fishing is therefore any pattern of harvest that results in variable mortality rates across individuals as a result of their reproductive phenology. Figure 2 illustrates how several common forms of selection may act on a phenological trait. For example, directional temporal selection can occur when either early or late migrating or spawning individuals are more susceptible to harvest than other parts of the population (Figure 2a,c), resulting in a directional change of the mean phenotype. When both early and late migrants are harvested at high rates, the resulting stabilizing selection can reduce variability in timing (Figure 2b). Lastly, disruptive selection results when early and late individuals are harvested at lower rates than the central portion of the population. This can increase variability in timing, although it may also result in fewer individuals spawning at the optimum time (Figure 2d).
2.1 Direct temporal management
Many fishes undertake spawning migrations or form large groups during reproduction (Harden Jones, 1981; Neuenfeldt et al., 2013; van Overzee & Rijnsdorp, 2014; Sadovy de Mitcheson, 2016), aggregating a fisheries resource in time and space. Indeed, the majority of global fisheries catch is comprised of species that display seasonal movement patterns (Harden Jones, 1981). Fishermen have long exploited these movements and aggregations, and management measures such as spatial and seasonal closures—restricting fishing in certain areas, during certain times of the year or a combination of the two—are common for protecting fish during these vulnerable periods (Erisman et al., 2012; Loher, 2011; Sadovy de Mitcheson, 2016). Because they are explicitly temporal in nature, typically consistent across multiple years and commonly have some basis in the reproductive biology of the targeted species, seasonal closures are a likely cause of temporal selection in fisheries; almost inevitably some fish migrating or breeding outside the closed period are vulnerable to harvest. When migratory or reproductive behaviour is distributed across several weeks or months, it is often technically or politically difficult to enforce a seasonal closure that protects all breeders equally. This is the case in many river fisheries for Pacific salmon (Oncorhynchus spp., Salmonidae), where efforts to balance the conservation of depleted stocks with full exploitation of more abundant stocks have produced a complex system of seasonal closures that can lead to differential exploitation between early and late migrating fish (Larson & Ward, 1955; Washington Department of Fish and Wildlife, 2016). A similar type of selection occurs in the Canadian Pacific halibut (Hippoglossus stenolepis, Pleuronectidae) fishery, which utilizes a winter closure (typically mid-November through mid-March) to protect fish during their offshore migration and spawning. However, telemetry studies have revealed that the traditional closure does not completely protect migrants in either the spring or fall, and the fishery can therefore exert either directional or stabilizing selection (Loher & Seitz, 2008).
Non-migratory species can also be subjected to temporal selection because spawning date often varies by weeks or months within populations (Martell, Walters, & Wallace, 2000). For example, lingcod (Ophiodon elongatus, Hexagrammidae) typically spawn between January and April, and fisheries in Washington, British Columbia and Alaska all observe seasonal closures intended to protect nest-guarding males. Guarding males are sedentary, aggressive and highly susceptible to both hook and line and spearfishing mortality (Martell et al., 2000; Stahl, Green, & Vaughn, 2014; Withler et al., 2004). However, in each of these regions at least some males have been observed guarding after the spring opening dates (Smith, McFarlane, & Cass, 1990). Nest-guarding species in fresh water including important recreational species such as largemouth bass could also be susceptible to similar temporal selection (Gwinn & Allen, 2010). This type of incomplete spawning closure selects strongly against late reproduction because such guardian males would be vulnerable, and their death also exposes the clutch to egg predators (Withler et al., 2004).
2.2 Indirect temporal management
In addition to the fixed-date seasonal closures described above, flexible closures also occur (Melnychuk, Banobi, & Hilborn, 2014). In such cases, fishery openings vary from year to year, depending on in-season monitoring of abundance, environmental conditions, physiological state (e.g., proportion ripe, spawned or moulted) or presence of non-target species. For example, in some Pacific herring (Clupea pallasii, Clupeinae) fisheries, harvest is delayed until the majority of females have fully developed (and therefore marketable) roe (Thynes, Gordon, Harris, & Walker, 2016). This effectively reduces the harvest rate on early spawning fish and may impose directional selection on spawning date (Ware & Tanasichuk, 1989). Fisheries that target anadromous species often are managed to allow a fixed number of individuals—“the escapement”—to pass upstream for spawning (Walters, 1981). Even when managers endeavour to distribute the escapement evenly throughout a salmon run, several temporally selective mortality patterns can result from limited harvest capacity or management uncertainty (Sethi, Costello, Fisher, Hanemann, & Karp, 2005). If the capacity of a fishing fleet is limited, then a “predator swamping” effect may occur during the period of peak abundance. This results in stabilizing selection by lowering harvest rates during the middle of the migration relative to the beginning and end (Figure 2b; Ims, 1990). Alternatively, precautionary management can limit fishing during the early portion of the migration because of uncertainty in overall abundance, shifting to higher exploitation rates once the management targets have been met (i.e., directional selection as in Figure 2c). Quinn et al. (2007) demonstrated both patterns of selective mortality in the Egegik fishing district of the Bristol Bay sockeye salmon (Oncorhynchus nerka, Salmonidae) fishery. During the early 1970s, harvest rates were lower during peak abundance (as in the generalized example in Figure 2b), but in recent decades the managers have been reluctant to allow fishing early in the season, resulting in strongly directional selection on migration date (as in Figure 2c with data shown in Figure 3).

The case of Bristol Bay sockeye salmon demonstrates that even when management is “conservation minded” and not explicitly temporal in nature, selective harvest may nevertheless occur. Efforts to control overfishing have led to the introduction of strict harvest quotas in many fisheries, and in some cases, there is a “race for fish” as fishermen strive to fulfil these quotas over very short time periods (Chu, 2009). Annual quotas that lead to early closure of fisheries result in the same temporal pattern of fisheries mortality as seasonal closures (Figure 2a), whereas daily quotas or trip limits impose stabilizing selection by reducing harvest rates during periods of peak abundance (Figure 2b). While comprehensive statistics on the prevalence of fisheries management strategies are not available, fixed or variable temporal closures are very common management practices (Melnychuk, Banobi, & Hilborn, 2013). The increasing focus on preventing overfishing around the world is likely to bring more fisheries under seasonal or quota management systems capable of producing temporally selective fishing mortality (Anderson et al., 2012).
2.3 Fishermen's behaviour
In addition to management actions, the behaviour of fishermen is likely to result in temporally selective fishing effort because people tend to fish when it is most economical, safe or productive. In many areas of the world, seasonally bad weather or other environmental conditions (e.g., ice, high river flows) limit fishing at certain times of the year (Smith & Wilen, 2005). Because migration and breeding commonly coincide with seasonal changes in environmental conditions (ice, storms, high river flows, etc.), selection can occur if part of the population has already reproduced or migrated by the time fishing becomes safe or practical (Figure 2a). Fishermen are also sensitive to economic forces, and fishing effort commonly changes throughout the year in response to seasonal variability in the absolute or relative profitability of targeting different species or stocks (Katsukawa & Matsuda, 2003). Fishing that targets periods of abundance (such as spawning aggregations or migrations) can increase profitability, but might also impose disruptive selection if less abundant early and late migrants or breeders experience lower fishing mortality (Figure 2d). Thus, various forms of temporal selection can result from intentional management actions, or as unintended consequences of management and the behaviour of fishermen. Furthermore, the phenomenon is likely widespread in both commercial and recreational fisheries, freshwater and marine environments, and across a range of taxa.
3 BIOLOGICAL CONSEQUENCES OF TEMPORAL SELECTION
Although little research has directly focused on the issue of temporal selection, and indeed relatively few empirical estimates of fishing selection on any trait exist (Kendall & Quinn, 2011, 2012; Quinn et al., 2007), a sizeable body of literature suggests that adverse effects are probable in populations with artificially altered phenology. While biological responses will no doubt be species, fishery and context-specific, three main categories of impacts are likely to result from temporal selection. First, evolutionary responses within populations are possible if the phenological traits experiencing selection are heritable. Second, temporal selection can drive plastic and demographic trait change by altering the spawning period or by selective removal of individuals with other traits that covary with phenology (Wright & Trippel, 2009). For example, early breeders often differ in size or age from later breeders (Horrall, 1981; Hutchings & Myers, 1994; Quinn et al., 2016). Finally, when fisheries target stock complexes, temporal selection can lead to over-exploitation of stocks with specific timing patterns, altering the overall timing of the complex and reducing intraspecific variability (Collie, Peterman, & Walters, 1990). Regardless of the pathway, the biological impacts of temporally selective fishing are likely to interact with ongoing changes in reproductive phenology driven by climate change (Parmesan, 2006), and this interaction may dampen or enhance the effect of temporal selection on fisheries productivity and sustainability.
3.1 Evolutionary impacts
Following evolutionary theory, spawning should be timed to maximize reproductive success by optimizing the balance between survival of parents and their progeny; reproductive phenology should reflect the prevailing environmental and ecological conditions experienced throughout the period of maturation, spawning and hatching (Cushing, 1969; Sinclair & Tremblay, 1984; Wright & Trippel, 2009). In some cases, reproductive phenology can be very plastic, responding to environmental changes such as water temperature (e.g., American shad [Alosa sapidissima, Clupeinae] Leggett & Whitney, 1972; striped bass [Morone saxatilis, Moronidae] Peer & Miller, 2014). However, in other cases, especially in salmonids, the genetic control over timing of upstream migration and reproduction is much stronger, as inferred from (i) population-specific differences in timing (Hodgson & Quinn, 2002; Webb & McLay, 1996), (ii) selective breeding experiments (Neira et al., 2006; Siitonen & Gall, 1989), (iii) evolution of timing in hatchery populations (Hard, 2004; Quinn, Peterson, Gallucci, Hershberger, & Brannon, 2002; Tipping & Busack, 2004), (iv) estimates of heritability in wild populations (Dickerson, Willson, Bentzen, & Quinn, 2005; Lin et al., 2016), (v) changes in response to fishery selection (Quinn et al., 2007), and (vi) the rapid evolution of timing outside the native range of the species (Quinn, Unwin, & Kinnison, 2000). Indeed, there is often substantial variation in reproductive phenology—both migration and spawning timing—among and within salmon populations (Quinn et al., 2016), and heritability in these traits appears quite high (Carlson & Seamons, 2008; Dickerson et al., 2005; Lin et al., 2016). In some populations, more than half the variability of spawn timing in progeny can be predicted by spawn timing in adults (Quinn et al., 2000; Thériault, Garant, Bernatchez, & Dodson, 2007). Thus, at least in salmonids the key ingredients for adaptive evolution of reproductive phenology are both present; variability on which selection can act and heritability of timing traits.
Quinn and Adams (1996) hypothesized that because the environments experienced by anadromous salmon during migration and spawning are temporally distant from the conditions experienced by their progeny, the optimal strategy is to track long-term mean conditions rather than short-term variation. In contrast, for other anadromous fish such as American shad and striped bass that have shorter incubation periods, environmental conditions at the time of migration or spawning better predict conditions at hatching, thus favouring plasticity in spawning date (Peer & Miller, 2014). The strength and rate of evolutionary responses to temporal selection is therefore expected to vary between taxa and salmonids may represent a somewhat extreme case. Nevertheless, even in species with more plastic reproductive phenology, evolution of timing traits is possible because trait variability commonly reflects an underlying, genetically controlled norm of reaction to external cues (Crozier & Hutchings, 2014). Reaction norms such as size at maturity evolve in response to size-selective fishing (Kendall, Dieckmann, Heino, Punt, & Quinn, 2014), and similar responses in both the mean and variability of timing traits seem probable responses to temporal selection. Fisheries-induced evolution of timing traits could have significant and long-lasting impacts on fisheries sustainability by disrupting the relationship between phenology and the ecological conditions that optimize reproductive success (Beaugrand, Brander, Alistair Lindley, Souissi, & Reid, 2003; Cushing, 1990; Sinclair & Tremblay, 1984). However, even in the absence of a genetic response, temporal selection can drive trait changes that influence the productivity of populations and stock complexes (Lowerre-Barbieri et al., 2011; Wright & Trippel, 2009).
3.2 Ecological and demographic impacts
Although reproduction follows predictable cycles in most fishes, there is often significant variation in timing between individuals from the same population (Scott, Marteinsdottir, Begg, Wright, & Kjesbu, 2006). This variation can buffer the population against an uncertain environment by ensuring that at least some offspring are likely to encounter favourable conditions. This variation in larval survival, commonly known as the match-mismatch hypothesis, is foundational in fisheries oceanography (Cushing, 1990) and has often been demonstrated in marine systems where fishes rely on secondary productivity of zooplankton and other small pelagic prey (Beaugrand et al., 2003; Cushing, 1990; Mertz & Myers, 1994; Sinclair & Tremblay, 1984). These fundamental principles apply to freshwater systems as well, so alteration of the normal phenology is likely to affect recruitment in lakes (e.g., Ludsin, Devanna, & Smith, 2014). Consistent with the match-mismatch hypothesis, empirical (Mertz & Myers, 1994) and simulation (James, Pitchford, & Brindley, 2003) studies have found that longer spawning seasons increase a population's productivity and reduce recruitment variability in uncertain environments.
Because mortality from fishing reduces the probability of fish growing old and large, exploited populations—even in the absence of any size or temporal selectivity—can nevertheless experience reductions in the duration and shifts in the peak of spawning activity as a result of altered demographics (Hixon, Johnson, & Sogard, 2014). Size and reproductive timing are commonly correlated; early migrating and spawning salmonids are commonly (though not universally) larger or older than those later in the run (Anderson & Beer, 2009; Bracis & Anderson, 2013; Doctor & Quinn, 2009; Quinn et al., 2016). Indeed, this pattern occurs in many fishes, although in some cases the reverse pattern is seen (Horrall, 1981). For example, in Atlantic cod the larger and older individuals tend to spawn later and over a longer period compared to younger ones (Hutchings & Myers, 1994). Thus, the loss of large, old individuals in heavily exploited populations is likely to reduce overall reproductive output, but also shorten the spawning season, potentially reducing the likelihood of a “match” of juveniles with abundant prey (Cushing, 1990; Mertz & Myers, 1994). Indeed, Wright and Trippel (2009) reviewed the potential impact of demographic-driven changes in spawn timing and duration on reproductive success in fishes and argued that this is likely a major component of reduced productivity in age-truncated populations. In similar fashion to this process of demography-driven contraction of spawning seasons in exploited populations, directional and stabilizing temporal selection both serve to reduce the number individuals spawning at the extremes of the temporal distribution. As such, temporal selection may result in the same negative outcomes for impacted populations including increased variability and reduced productivity.
Size selectivity, especially the common pattern of increased exploitation of large individuals, only serves to exacerbate the issue of spawning season contraction as it further reduces the abundance of large individuals with particularly early or late spawn timing (Hixon et al., 2014). Additionally, because timing often covaries with size and age, there is the potential for complex interactions between multiple forms of selection and demographic impacts of fishing (Anderson et al., 2008; Wright & Trippel, 2009); just as size-selective fishing may influence timing, temporally selective fishing may in turn influence size. Depending on the temporal pattern of fisheries mortality, the direction of size selectivity and the relationship between size and timing, various outcomes seem plausible in the case of dual selectivity including counteracting and reinforcing effects on correlated traits. To further complicate matters, size and phenology may also be associated with other behavioural traits such as boldness or aggression (Biro & Post, 2008; McPhee & Quinn, 1998). These interactions highlight the fact that both the process of, and biological responses to, all forms of fisheries selection do not happen in a vacuum and trait changes in exploited fish populations may result from selection on ostensibly unrelated traits.
3.3 Impacts on stock complexes
Advances in population genetics have revealed that many commercially exploited fish populations once thought to be relatively homogeneous have complex stock structure (Stephenson, 1999; Waples & Naish, 2009). Subpopulations with unique morphology, phenology, population dynamics and ecological interactions commonly comingle despite reproductive isolation, and can therefore be harvested by a single fishery (Hilborn, 1985a). When populations vary in their productivity or are differentially vulnerable to harvest, management as a single stock can lead to population-specific depletion or extirpation (Hilborn, 1976). Populations exploiting even subtly different ecological niches commonly vary in productivity; thus, applying a single harvest rate to a stock complex will often deplete the less productive populations (Hilborn, 1985a; Ricker, 1958). In salmon, differences in migratory timing between populations returning to the same river system have been exploited by managers through temporal closures to protect the weaker stocks while harvesting the more abundant (Collie et al., 1990). However, in cases where populations overlap significantly in migration timing, or timing differences are not appreciated, temporally selective fishing can shift the overall run-timing by altering the relative abundances of the substocks (Figure 4). For example, the sockeye salmon run to Bear Lake, Alaska, is characteristically bimodal, with distinct early and late peaks, and management was based on the idea that there were distinct early and late populations. However, Boatright, Quinn, and Hilborn (2004) revealed a series of populations with a continuum of migration timing. The higher exploitation rate in the middle of the season had created the appearance of bimodality by suppressing the populations migrating at that time.

Long-term monitoring of stock complexes with fine-scale population structure has demonstrated that the relative productivity of individual stocks fluctuates significantly over time, and increased diversity therefore stabilizes overall variability in abundance, a phenomenon now commonly referred to in ecology as the portfolio effect (Schindler et al., 2010). Thus, reduced genetic and phenotypic diversity resulting from over-exploitation of individual stocks serve to erode the portfolio effect and can therefore increase overall variability (Hilborn, Quinn, Schindler, & Rogers, 2003) and reduce the long-term resilience of stock complexes to environmental change (Moore, McClure, Rogers, & Schindler, 2010). The importance of maintaining population structure and intraspecific diversity has been appreciated for decades in Pacific salmon management (Thompson, 1959) but has been less widely known in marine fishes until more recently (Stephenson, 1999). Reproductive timing in Pacific herring varies significantly between populations spawning in discrete locations (Haegele & Schweigert, 1985; Hay, 1985). However, many populations comingle in the winter and during pre-spawn staging periods in coastal areas where they are fished (Ware & Tanasichuk, 1989). Variability between populations in spawning date or the duration of staging is likely to influence susceptibility to fisheries, and population-specific over-exploitation might occur. Such losses of genetic and phenotypic diversity may also impede the recovery of depleted stocks. For example, Ames (2004) reported that prior to intensive fishing, the Atlantic cod stock complex in the Gulf of Maine was composed of many smaller subpopulations with unique spatial and temporal migration patterns. Many of these subpopulations were extirpated during decades of intensive harvest, and even after years of drastically reduced fishing, the historic diversity in migratory routes and spawning areas has not recovered (Ames & Lichter, 2013).
4 CONCLUSION: IMPLICATIONS OF TEMPORAL SELECTION FOR FISHERIES SUSTAINABILITY
Here, we have called attention to the prevalence of temporal selection in fisheries and briefly considered its causes and potential consequences. Many common fisheries management strategies and fishermen's behaviours can result in mortality that is selective with regard to reproductive phenology, and various evolutionary and plastic changes in temporal traits can result from such selection. Although the potential consequences of temporal selection have received little attention from researchers and managers, the phenomenon nevertheless has the potential to impact exploited fish populations, particularly in combination with rapid changes in phenology resulting from global climate change (Parmesan, 2006). In order to clarify the risks to fisheries sustainability resulting from temporally selective fishing, we encourage further research on several key topics.
First, a prerequisite for understanding the potential impacts of temporal—or indeed any type—of fisheries selection is determining the stock structure of the exploited population or stock complex (Stephenson, 1999). Within- and between-population variability in phenology may indicate genotypic variability on which temporal selection can act, but may also reflect plastic responses to a variable environment. As noted previously, such intraspecific variability in traits can also arise from either differences between individuals or differences between populations that co-occur in time and space and may therefore be captured in the same fishery. As such, the interpretation and consequences of an observed trait change depend fundamentally on these two facets of variability. Both within- and between-population variability can produce a stabilizing portfolio effect (Schindler et al., 2010), and fisheries that erode diversity in traits including reproductive phenology are likely to have negative consequences for population productivity and resilience (Ames, 2004; Carlson & Satterthwaite, 2011; Smith, Francis, & McVeagh, 1991). Diversity in phenology may be particularly important as temporal traits appear to the most likely path for adaptive evolution in response to climate change (Bradshaw & Holzapfel, 2006, 2008). Thus, an improved understanding of stock structure will be critical for identifying and interpreting phenological change in response to fisheries selection and to crafting appropriate management responses.
Second, there is limited understanding of the impacts of fisheries selectivity in general (Kuparinen & Hutchings, 2012) and selection on reproductive phenology in particular will have on fish population dynamics and productivity. Although theory predicts that human-driven shifts in reproductive timing could cause a larger portion of a population to spawn during less favourable periods, the consequences of such changes are difficult to observe in the real world and may be confounded by environmental modulation of reproductive phenology (Wright & Trippel, 2009). As such, it is difficult to predict how different species or populations will respond to temporal selection. Simulation and experimental studies are needed to explore the consequences of human-induced changes in phenological traits and to differentiate plastic and genetic components of trait change. Individual-based models have been used to explore the relative importance of genetic, plastic and demographic factors in other instances of fisheries-induced trait change (e.g., Dunlop, Shuter, & Dieckmann, 2007; Kuparinen & Hutchings, 2012), and the demographic impacts of fisheries-induced evolution (Kuparinen & Hutchings, 2012), and should also be valuable for studying changes in reproductive phenology. Such studies will help to identify the circumstances under which temporally selective fishing reduces productivity and will also be useful for guiding conservation and management efforts by identifying species and life-history strategies that are particularly susceptible to temporal selection.
Third, the impacts of fisheries-induced changes in reproductive timing are likely to interact with ongoing change in environmental drivers of phenology such as temperature (Asch, 2015; Crozier & Hutchings, 2014). Depending on the form and direction of selection, these independent forces may reinforce or dampen phenological change. For example, in a case where both environmental and selective fishing impose reinforcing directional selection on spawning date, reproductive timing could change rapidly. In contrast, if environmental change and selective fishing oppose each other, it is possible that timing would remain the same but temporal selection would nevertheless be driving maladaptation to climate change and could drastically reduce productivity. This type of counteracting selection was observed in a hatchery population where unintentional artificial selection drove a rapid advance in spawn timing in Chinook salmon, despite a warming environment that favoured later spawning (Quinn et al., 2002), ultimately requiring refrigeration to avoid mortality of embryos from high water temperatures in the hatchery. In the light of the complex and potentially cryptic outcomes of simultaneous artificial and natural selection, we encourage researches and managers observing phenological change in exploited populations to consider the role of temporally selective fishing in addition to climate change.
Finally, the effectiveness of temporal fisheries managements such as seasonal closures will be impacted by changes in phenological traits, whether induced by selective fishing or climate change (Melnychuk et al., 2014). For example, as the reproductive phenology shifts away from historical conditions, a closed season may become more or less protective of spawners (e.g., Peer & Miller, 2014), or a closure that once protected, all spawners may become temporally selective if management fails to respond to altered phenology. In response to these challenges, we encourage thoughtful examination of temporal fisheries management strategies and their potential interaction with timing traits. In particular, the biological basis of management measures that target or protect reproductive periods should be critically evaluated, and their effectiveness in the light of environmental change should be considered.




