HTE 3.0: Knowledge-based systems in cascade for familial hypercholesterolemia detection and dyslipidemia treatment
Funding information: Generalitat de Catalunya, Grant/Award Number: 2017 SGR 1551; Sanofi España
Abstract
HTE 3.0 aims to support clinicians in the detection of patients with dyslipidemia, especially patients with familial hypercholesterolemia (FH), and in the recommendation of personalized lipid-lowering treatments. The core of HTE 3.0 is a clinical decision support system in which several knowledge-based systems are serialized: patient detection, therapeutic target setting, personalized treatment assessment, and treatment combination and prioritization, according to different criteria. The experimental evaluation of HTE 3.0 shows that the use of HTE 3.0 would mean increasing the capacity to detect FH by 5.7 times compared with usual clinical practice. Regarding the lipid-lowering treatment, a comparison of 18 cases among seven lipidologists shows that the differences between treatments provided by HTE 3.0 and human lipidologists are smaller than the differences between human experts.
1 INTRODUCTION
Atherosclerotic cardiovascular disease (CVD) is the leading cause of death and disability in Spain (Dégano et al., 2013), and represents a major public health burden throughout the world (de la Sierra et al., 2015). Major risk of CVD has been associated with an abnormal level of lipids and cholesterol in the blood, mainly caused by inactivity, obesity, and an unhealthy diet, but also due to genetics, namely as familial hypercholesterolemia (FH) (de la Sierra et al., 2015). FH is underdiagnosed, with a diagnosis rate of around 6%–10% (Nordestgaard et al., 2013), while it is a major public health concern because a considerable percentage of untreated patients with FH have a high risk of coronary heart disease (CHD) at early ages: men at 50 years old (50%) and women at 60 years old (30%). On the other hand, the different European and American scientific societies have emphasized the importance of improving lipid control in people with a high CVD risk (Climent et al., 2020; Pokharel et al., 2017; Virani et al., 2016).
Dyslipidemia treatment to reduce cholesterol–formally, to achieve a low level of low-density lipoprotein (LDL) particles in blood or low LDL-cholesterol (LDL-C)–, can prevent CVD (Baigent et al., 2005), but this treatment among primary care patients is generally insufficient (Masana, 2019; Tahkola et al., 2020). Moreover, the results of the EUROASPIRE V study (De Backer et al., 2019) show that in Spain, <49% of patients with CVD meet the recommended cholesterol levels according to the European Society of Atherosclerosis criteria. On the other hand, there is no single implementation strategy for FH (Jones et al., 2021), and clinicians need support due to the lack of comprehensive guides. The FH treatment is suboptimal and often starts late (Nordestgaard et al., 2013).
In that regard, clinical decision support systems (CDSS) have been shown to improve the performance of health care processes in primary care (Pflugfelder, 2020), as well as in all of the health care agents (K. H. Yu, Beam, & Kohane, 2018). Early works by Sim et al. (2001) highlight the importance of such systems to reflect the current state of knowledge regarding evidence-based medicine, maintaining clinician awareness that may result from new evidence, as well as the need to include models of individualized patient decision-making in real-world settings. Hasnie et al. (2018) state that the demand for this type of CDSS is still valid. They show effectiveness and efficiency, as well as indicators for errors (Hieb & Handler, 2007), safety and performance speed, and warnings and adaptability of new clinical knowledge (Knols et al., 2020). In particular, CDSS for implementation of guidelines have been proven useful regarding system use, user satisfaction, system quality, information quality, and service quality (Kilsdonk et al., 2017).
This work concerns a CDSS, named HTE 3.0,1 for FH detection and dyslipidemia treatment recommendation; it has been developed with the supervision of lipidologists. It aims to help the decision-making process in the diagnosis and treatment of dyslipidemia in general and, in particular, FH. HTE 3.0 tries to reproduce the optimal decisions that would be made by an expert lipidologist given a particular patient that is, customizing the treatment with the best scientific evidence and effectiveness, clinical safety, and cost-effectiveness criteria while not interfering with clinical practice.
HTE 3.0, on the one hand, supports the detection of candidates at risk of FH from blood analysis requested by any health care service, and primary care general practitioners are alerted about such patients, so that in the next scheduled visit with the patient, the FH diagnosis could be confirmed. On the other hand, HTE 3.0 provides a personalized management of people identified as having high cholesterol by supporting the process of setting individualized lipid level targets and next prescribing the corresponding dyslipidemia treatment. The personalization in dyslipidemia diagnosis and treatment is important due to the highly heterogeneous conditions at the clinical level (Benincasa et al., 2020). This is of paramount importance when considering drug–drug interactions due to comorbidities, drug allergies, and other individual conditions that constrain the treatment (Berner, 2009; Tolley et al., 2018), as addressed in HTE 3.0. Another important contribution of HTE 3.0 is that it is not constrained to the prescription of a single drug recommendation, but it considers combinations of medicines, as a way to reach the target health outcomes in people with complex health conditions (Climent et al., 2020). Due to the different stages of diagnosis and therapy recommendation, HTE 3.0 is designed as a set of knowledge-based systems (KBS) in cascade. Four KBS are implemented: FH detection, lipid level target determination, personal therapy selection, and final recommendation based on treatment prioritization criteria (including drug combination). HTE 3.0 is a first attempt to provide personalization of dyslipidemia and FH target and treatment according to the state-of-the-art knowledge and the local health protocols of a given region, as suggested by Martens et al. (2008) regarding regionally adapted evidence-based tools.
HTE 3.0 is designed as a web service (WS), which facilitates its integration into any hospital information system (HIS) and enables patient data analysis from electronic health records (EHR), so that, depending on the reference hospital, the general practitioner in primary care has access to HTE 3.0 through their desktop. Primary care tools are claimed to be important to deal with the generally insufficient treatment of people at risk of CVD with all of their complexities (especially due to comorbidities) (Tahkola et al., 2020). Integration represents a key feature regarding CDSS to support the clinician in obtaining the appropriate target and treatment in a unique point of access (their desktop). The integration of CDSS in the clinical workflows is one of the challenges of artificial intelligence (AI) (K. H. Yu, Beam, & Kohane, 2018), and the integration of EHR has been tested previously in New Zealand (Piepoli et al., 2016), showing an increase in the risk assessment screening by several points (from 4.7% to 53.5%). HTE 3.0 is also expected to increase the detection of people with uncontrolled cholesterol as well as to improve the management of their disease.
This article is structured as follows. In Section 2, we review related work on CDSS. In Section 3, the novel HTE 3.0 system is introduced. In Section 4, we give a detailed description of the data and the setup used in our experiments, as well as the results achieved, and in Section 5 a discussion about them is presented. Finally, Section 6 draws some conclusions from this work.
2 RELATED WORK
The context of the research has been analysed in this section according to CDSS for dyslipidemia. Moreover, a literature review has been performed regarding CDSS linked to the top illnesses causing death according to WHO (World Health Organization, 2020), in addition to COVID-19, responsible for the recent global pandemic.
2.1 CDSS for dyslipidemia
There are few CDSS dedicated to dyslipidemia. HTE 3.0 has been developed from the experience of HTE-DLP® (Zamora et al., 2013, 2015). HTE-DLP was the first CDSS developed to monitor dyslipidemia treatment in Spain and the first to be validated in clinical practice in Europe (Zamora et al., 2013). HTE-DLP is a tool for dyslipidemia management that includes a mechanism for determining the target lipid levels of a patient and recommending a treatment to achieve such targets following the 2012 European Guidelines for CVD prevention in clinical practice. It was released in 2012 with excellent results regarding the improvement of the recommended treatments of those clinicians using the system when compared with those who did not (Zamora et al., 2013). However, HTE-DLP cannot handle FH, but HTE 3.0 can. Indeed, HTE 3.0 handles both dyslipidemia (following the updated European Guidelines for CVD prevention) and FH (following the NL guidelines) due to the known underdiagnosis of citizens with this disease (Nordestgaard et al., 2013). Moreover, HTE 3.0 has incorporated new medical evidence regarding dyslipidemia treatments (new drugs, drug interactions, and intolerances, etc.) since 2012.
With regard to the business approaches, HTE-DLP is designed to run on a desktop computer, in an isolated environment, while HTE 3.0 has been developed following the paradigm of software as a service, enabling the integration and interoperability of HIS. HTE 3.0 can be run on any web navigator, and on any PC, laptop, tablet, or mobile phone, providing a multi-platform solution.
There are some other previous CDSS that help with dyslipidemia management (Chen et al., 2010; Gilutz et al., 2009; van Wyk et al., 2008). HTE 3.0 differs from these because none consider FH. HTE 3.0 also considers a personalized target for lowering cholesterol, instead of risk groups (RG). With respect to the medical guidelines upon which the works are based (van Wyk et al., 2008), uses the European Guidelines, as does HTE 3.0, while Chen et al. (2010) and Gilutz et al. (2009) follow the US National Cholesterol Education Program-Adult Treatment Panel III, and Gilutz et al. (2009) implements the Israeli guidelines.
Another interesting task of CDSS for dyslipidemia is reminder (Martens et al., 2008). There are two types of reminder systems: reactive and proactive. Reactive systems are designed to provide reminders of interventions after a patient has been detected. On the other hand, proactive systems help to alert about new patients and find a diagnosis for them. In this regard, HTE 3.0 is a proactive system. Nevertheless, reactive systems with calendars have been analysed (Márquez Contreras et al., 2007); those authors conclude that such models are important for patient follow-up. Therefore, in a future version of HTE 3.0, such a follow-up mechanism should also be incorporated to provide the best health care quality to patients, including a combination of interventions, which according to Petrilla et al. (2005) leads to the most effective and improved patient compliance.
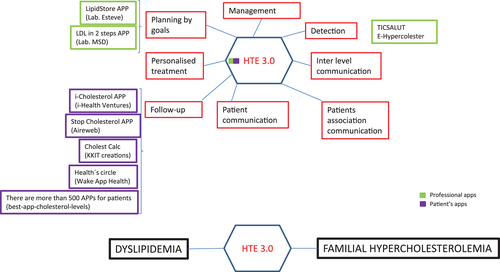
Regarding applications for cholesterol management, it is interesting to distinguish between professional and patient applications (see Figure 1). The first type of application includes tools for risk detection, follow-up, and treatment planning—for example, LipidStore from Esteve labs, LDL in two steps from MSD lab, or E-Hypercolester from TicSalut (green boxes in Figure 1). The second group is dedicated to support patients for following-up the recommended treatment (purple boxes in Figure 1). This group has more available applications (Smartheraphy, 2016), some of which are reminder systems (Olson et al., 2009), including the follow-up by clinical staff. Very few apps are about FH, as HTE 3.0 is. Moreover, as shown in Figure 1, HTE 3.0 covers a wide range of functionalities (red boxes), in contrast to the applications that are particular for some tasks (e.g., the majority of applications for citizens are designed for following-up activities).
2.2 CDSS for other diseases
The use of CDSS is progressively being extended as a common practice in health care. Dyslipidemia is an example, but any other non-communicable disease or communicable disease can be subject of a CDSS implementation. For example, there are examples of CDSS for each of the top diseases causing death according to the WHO (World Health Organization, 2020), by order: ischemic heart disease, stroke, chronic obstructive pulmonary disease (COPD), lower respiratory infections, neonatal conditions, cancer (tracheal, bronchial, and lung), Alzheimer's disease and other dementias, diarrheal diseases, diabetes mellitus, and kidney diseases.
First, Foldyna et al. (2018) propose a CDSS to manage non-invasive systems as the coronary computer tomography angiography, for heart disease diagnosis support. In this same illness, Gudadhe et al. (2010) propose support vector machines and neural networks for disease classification. Second, Lee et al. (2015) present a CDSS to predict thrombolysis after suffering an ischemic stroke, using a multivariate logistic regression approach. Third, Anakal and Sandhya (2018) provide an approach to interpret the data from new devices, such as spirometers, to manage COPD. Fourth, Carr et al. (2020) present a CDSS for the treatment of community-acquired pneumonia, which improves adherence and decreases mortality. Fifth, López et al. (2013) and Mordvanyuk et al. (2019) present a premature baby monitoring system, in which a rule-based system is combined with a case-based reasoning approach to provide personalized alerts to caregivers from the information gathered from sensors (pulse oximeter and scale) and entered by the parents (food intake and sleep behaviour). Sixth, Walsh et al. (2019) point out that CDSS is the path to deal with multi-factorial dimensions in cancer (clinical, biologic, genetic, etc.), because human experts have a limited capacity to manage a given number of variables (the study reports up to five) when making decisions. Seventh, Bucholc et al. (2019) propose a CDSS for predicting the severity of Alzheimer's disease. Eighth, Khan et al. (2020) present a CDSS to decide between rehydration and antibiotics for acute diarrheal disease management, with the aim to avoid misprescription of antibiotics, a phenomenon that has important consequences on antimicrobial resistance, while improving the volume of intravenous fluid. Ninth, Torrent-Fontbona and Lopez (2019) describe a personalized insulin dose recommender system for people with Type 1 diabetes mellitus, using case-based reasoning. Finally, Hamedan et al. (2020) present a fuzzy logic expert system to diagnose chronic kidney disease.
While predictive approaches are based on machine learning approaches, most CDSS for disease management use KBS (rule-based, case-based, and fuzzy), as HTE 3.0 does. In that regard, Gonzalez-Ferrer et al. (2018) propose analysing the data generated by those KBS to complement the information available in EHR and to improve the medical evidence. Moreover, CDSS about several diseases at a time should also be addressed in health care services, for example in triage activities to make emergency services efficient (Arancibia et al., 2019)—as well as the involvement of different care agents in a CDSS tool (primary care and hospitals) (Tamblyn et al., 2010). In this regard, HTE 3.0 offers the detection from primary care services (i.e., blood analysis) and posterior individualized targets and personalized treatment from clinicians, contributing to a comprehensive process model of clinical information integration (Veinot et al., 2018).
It is interesting to highlight the advances in CDSS due to the COVID-19 pandemic. On the one hand, detection of COVID-19 has been empowered by lung image processing (Sedik et al., 2021). The complexity of the images to be analysed requires deep learning approaches that have proven to be useful for image feature learning (Wang et al., 2020). Moreover, the often necessary requirement of remote assistance has involved the development of secure communication protocols and systems (Masud et al., 2020; C. Yu, Li, et al., 2018), boosting initiatives such as blockchain for decentralized health data (Esposito et al., 2021), multi-agent systems (Khiat & Djamila, 2019), as well as the use of non-specialized devices (Alsmirat et al., 2019). HTE 3.0 has also been designed with secure communication protocols and systems, although the service is internal to the health corporation in which it has been installed. In that regard, the new advances in blockchain and multi-agent systems offer a possibility to deploy the system in a remote setting and a collaborative working mode for a future version of the tool.
2.3 Added value of HTE 3.0
- EHR interoperability: This feature indicates the CDSS is able to manage EHR.
- HIS integration: This characteristic indicates if the CDSS has been integrated with a HIS or health care corporation system.
- Guidelines: This factor refers to the scope/region of the guidelines used to develop the system. When the CDSS is built upon data, the DT code is used.
- Personalization or group risk: This feature refers to whenever the CDSS provides personalized assessment (P) or recommendations based on RG. Regarding systems based on machine learning approaches, RG has been assumed (as machine learning learns patterns), unless specified otherwise.
- Reactive or proactive: This feature indicates whether the systems reacts (R), by providing some solution when a health condition has been already identified, or proactively (P) looks for the risk of developing the health condition.
- Agents: This factor refers to the kind of setting where the system is deployed: primary care (PC) or hospitals (H); the latter including any other non-ambulatory service.
- Task: This characteristic indicates what the CDSS does: classification or diagnostic (C), detection (D), management of the disease (M), reminder (R), and treatment recommendation (T).
- Domain: This feature refers to the disease for which the CDSS has been designed.
- FH: This factor reflects whether the system considers FH. This feature only applies for systems dedicated to dyslipidemia.
| Reference | EHR interoperability | HIS integration | Guidelines | Personalisation (P)/group risk (GR) | Reactive (R)/proactive (P) | Agents | Task | Domain | FH |
|---|---|---|---|---|---|---|---|---|---|
| Sedik et al. (2021) | DT | P | P | H | D | COVID-19 | |||
| Foldyna et al. (2018) | DT | RG | P | H | C | Ischemic heart attack | |||
| Gudadhe et al. (2010) | DT | RG | P | H | C | Ischemic heart attack | |||
| Lee et al. (2015) | DT | P | H | T | Stroke | ||||
| Anakal and Sandhya (2018) | ✓ | DT | RG | P | C, T | COPD | |||
| Carr et al. (2020) | ✓ | ✓ | US | RG | R | H | D, T | Pneumonia | |
| López et al. (2013) and Mordvanyuk et al. (2019) | ✓ | DT | P | P | H | M | Neonatal | ||
| Walsh et al. (2019) | ✓ | N/A | P, RG | R, P | H | C, M, T | Cancer | ||
| Bucholc et al. (2019) | DT | P | P | H | D | Alzheimer | |||
| Khan et al. (2020) | WHO | RG | R | H | T | Diarrheal diseases | |||
| Torrent-Fontbona and Lopez (2019) | DT | P | P | PC, H | T, M | Diabetes | |||
| Hamedan et al. (2020) | UK, United States | RG | P | H | C, P | Kidney | |||
| Zamora et al. (2013) | European | P | P | H | M, T | Dyslipidemia | |||
| Chen et al. (2010) | ✓ | United States | RG | P | PC, H | M | Dyslipidemia | ||
| Gilutz et al. (2009) | ✓ | Israel | RG | P | H | M | Dyslipidemia | ||
| van Wyk et al. (2008) | ✓ | European | RG | R | PC, H | M | Dyslipidemia | ||
| Martens et al. (2008) | European | RG | R | PC | R | Dyslipidemia | |||
| Márquez Contreras et al. (2007) | United States | P | P | PC | R | Dyslipidemia | ✓ | ||
| This work | ✓ | ✓ | European | P | P | PC, H | D, M, T | Dyslipidemia | ✓ |
- Note: DT has been used for machine learning (data-driven) approaches.
- Abbreviations: C, classification or diagnosis; D, detection; H, hospital; M, management; PC, primary care; R, reminder; T, treatment.
In Table 1, when a feature is empty, it means that it is not available in the published work. It is possible to observe that the majority of CDSS do not consider EHR interoperability or HIS integration explicitly, while it seems to be an indispensable feature for the success of the systems (Piepoli et al., 2016; Tahkola et al., 2020; K. H. Yu, Beam, & Kohane, 2018). This is not the case for HTE 3.0, which has been designed to work in an integrated way on the clinicians desktop. Moreover, from the information summarized in Table 1, HTE 3.0 is a unique system offering personalized treatment for dyslipidemia, with detection functionalities for FH, managing the setting of the lipid target, and recommending a treatment for reaching the target.
3 HTE 3.0 CLINICAL DECISION SUPPORT SYSTEM
HTE 3.0 is a CDSS whose aim is to aid clinicians by following a four-step process (see Figure 2): (i) the detection of dyslipidemia and FH, (ii) the calculation of the CVD risk and the therapeutic objectives, (iii) the personalization of lipid-lowering treatments taking into account patient comorbidity and concomitant drugs, and (iv) the treatment recommendation, taking into account several political, and clinical criteria. A KBS has been developed to respond to the requirements of each step of the CDSS as described below. HTE 3.0 is integrated with the HIS according to the diagram in Figure 2.
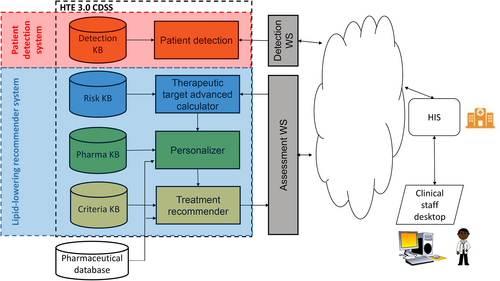
As it is possible to observe in Figure 2, the different KBS are organized in a cascade. The detection KBS (red box in Figure 2) receives information from the HIS about blood analysis, the results of a routine ambulatory request. The KBS analyses the dyslipidemia and FH conditions, and then returns to the HIS a flag with the result (positive or negative). If the result is positive, an alarm button is displayed on the clinical staff's desktop linked to the patient record. Thus, in the next scheduled visit with the patient—for example to get the results of the blood analysis—the clinician could visualize the alert and proceed with the remaining lipid-lowering recommender system (blue box in Figure 2). First, the risk KBS computes the lipid level target according to the patient's characteristics. The result (LDL-C target) is used by the personalizer KBS to obtain (from the pharma KB) available treatment options for the patient according to their individual conditions (personalization). Finally, the available treatments are ranked in the treatment KBS to achieve the particular criteria of the health care environment. Of note, the FH detection KBS and the remainder KBS work asynchronously—the physician needs to activate the process—while the lipid-lowering recommender KBS operates synchronously—once the process is activated, all the KBS are operated in sequence.
3.1 Patient detection
The HTE 3.0 patient detection KBS aims to detect patients with dyslipidemia as well as patients with FH. The patient detection KBS is a rule-based system that implements the European Guidelines on CVD prevention in clinical practice for dyslipidemia detection (Piepoli et al., 2016), complemented by the NL guidelines for HF detection (Nordestgaard et al., 2013). The KBS works at two different levels to detect both types of patients. First, the system checks the patient's blood tests, age, and previously diagnosed CVD to see if they are a candidate for dyslipidemia. For example, if the patient's age is between 18 and 30 years and the LDL-C level is >230 mg/dl, the patient is considered a candidate for dyslipidemia (see Rule 1).
Rule 1. Dyslipidemia detection
IF 18 ≤ (patient age) ≤ 30 AND LDL-C > 230 mg/dl
THEN dyslipidemia = TRUE.
On the other hand, the patient detection KBS uses other patient information to detect patients who might have FH. For example, when a patient has an LDL-C >200 mg/dl and a triglyceride level <400 mg/dl, the patient is considered to possible have FH (see Rule 2). When some FH risk is detected, the application alerts the clinician of this fact and offers the opportunity to perform additional actions to enrich the information about the patient. Table 2 shows the four actions currently available in the system.
| Perform the Dutch lipid clinic network test (Mickiewicz et al., 2016) |
| Check other cardiovascular disease risk factors |
| Get additional information about FH |
| Consult reference information of lipid units |
Rule 2. FH detection
IF LDL-C >200 mg/dl AND triglycerides < 400 mg/dl
THEN FH = TRUE.
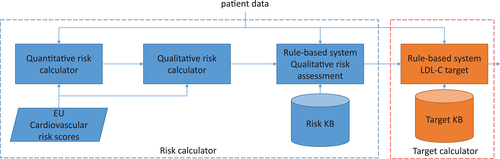
1 Therapeutic target advanced calculator
The objective of the therapeutic target calculator is to estimate the CVD risk of the patient, and then define the LDL-C target accordingly (see Figure 3). The KBS used for the therapeutic target calculator is a model-based system that interprets the charts of the European Society of Cardiology combined with a rule-based system that modifies the risk according to lipidologist expert knowledge, and a second one that supports the determination of the LDL-C target.
Algorithm 1. Risk calculator
Output: Qualitative risk level Input: Patient data
Step 1: Quantitative value
If patient's country  high epidemiological risk region Then
high epidemiological risk region Then
If sex is female Then
riskTable = findTable(chart(HighEpidemiologicalRisk,Women))
Else riskTable = findTable(chart(HighEpidemiologicalRisk,Men))
Else
If sex is female Then
riskTable = findTable(chart(LowEpidemiologicalRisk,Women))
Else riskTable = findTable(chart(LowEpidemiologicalRisk,Men))
quantitative_risk = riskTable(systolic blood pressure, cholesterol)
Step 2: Qualitative value
qualititative_risk = score(quantitative_risk)
Algorithm 2. findTable
Output: Risk table
Input: Chart (subset of tables) according to the country and sex
Case (age):
40 ≤ age ≤ 50:
If smoker Then riskTable = tableAssignment(chart,age4050,smoker)
Else riskTable = tableAssignment(chart,age4050,non-smoking)
50 ≤ age ≤ 55:
If smoker Then riskTable = tableAssignment(chart,age5055,smoker)
Else riskTable = tableAssignment(chart,age5055,non-smoking)
55 ≤ age ≤ 60:
If smoker Then riskTable = tableAssignment(Chart,age5560,smoker)
Else riskTable = tableAssignmente(Chart,age5560,non-smoking)
60 ≤ age ≤ 65:
If smoker Then riskTable = tableAssignment(Chart,age6065,smoker)
Else riskTable = tableAssignment(Chart,age6065,non-smoking)
age ≥ 65:
If smoker Then riskTable = tableAssignment(Chart, age > 65,smoker)
Else riskTable = tableAssignment(Chart, age > 65,non-smoking)
First, HTE 3.0 determines a quantitative value of the risk followed by a qualitative value (very high, high, moderate, and low), as shown in Algorithm 1. To compute the quantitative cardiovascular risk score (Step 1 of Algorithm 1), the therapeutic target advanced calculator uses the charts of the European Society of Cardiology. There are two charts: one for high epidemiological risk countries and another one for low epidemiological risk countries (shown in Figure 4). Based on sex (female and male), two different sets of risk tables are applicable from the chart: women tables are on the left side of Figure 4, and men tables are on the right side of Figure 4. With this decision (subset of tables or chart), Algorithm 2 (findTable) determines the final risk table for the patient. Given the age (centre of Figure 4), two different risk tables could be triggered. Subsequently, according to the smoking condition of the patient (non-smoker, smoker), a single table is selected. With this risk table, the last instruction of Step 1 of Algorithm 1 is to compute the quantitative risk: The systolic blood pressure is used as the key for accessing the row of the selected table, and the total cholesterol is used to identify the corresponding column, so the indexed cell provides the CVD risk for the person. For example, a 57-year-old non-smoking woman, living in Spain, with a 140 mmHg systolic blood pressure, and a total cholesterol of 7 mmol/L, has a cardiovascular risk of 1.
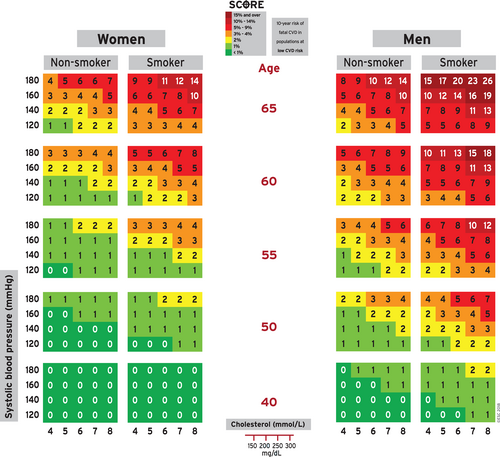
- Red is considered a very high risk.
- Amber is considered a high risk.
- Yellow is considered a moderate risk.
- Green is considered a low risk.
In the previous example (57-years-old, non-smoking woman), the colour associated to the cell of the retrieved chart is green, so a low-risk score is set.
Although the low- and high-risk charts have been elaborated according to epidemiological conditions that are often associated with countries (i.e., low risk: Spain, Austria, Denmark; high risk: Bulgaria, Russia, Latvia), charts should be used in light of the lipidologist's knowledge. In that regard, HTE 3.0 takes into account the latest medical evidence by means of rules gathered from the lipidologist's team (see example in Rule 3). By applying such rules, each patient is reviewed and a new label could be generated.
Rule 3. Risk qualitative determination
IF disease = hypertension OR
familial hypercholesterolemia OR
diabetes mellitus OR
LDL > 240 OR
total cholesterol > 320 OR
30 ≤ glomerular filtration rate ≤ 60
THEN qualitative_risk = high
Finally, the therapeutic target calculator computes the LDL-C target, that is, the desired LDL level to achieve with the treatment. For example, an LDL-C target of 70 mg/dl is set if there are any markers of high CVD risk (see Rule 4). For a moderate risk, a 115 mg/dl LDL-C level can be generated (see Rule 5).
Rule 4. LDL-C target
IF CVD risk is high
THEN target= 70 mg/dl.
Rule 5. LDL-C target
IF CVD risk is moderate
THEN target= 115 mg/dl.
1 Personalizer
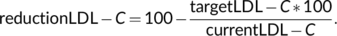 (1)
(1)HTE uses the dataset of drugs (Figure 2) to recover all drugs that enable the desired reduction. Next, several rules are applied according to the patient's condition to filter out intolerances, concomitant treatments, renal function, risk of diabetes, and risk of myopathy (Figure 5).
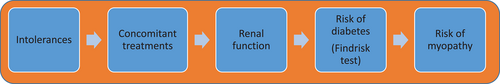
First, the personalizer filters out treatments that have been shown to cause intolerances to the patient in the past (first block of Figure 5). Next, HTE 3.0 analyses the patient's other ongoing (concomitant) treatments to remove lipid-lowering medications with potential interactions with other treatments of the patient (second block of Figure 5). The personalizer continues gathering information regarding the glomerular filtration rate, which is used to prioritize treatments with fluvastatin or atorvastatin (third block of Figure 5). Regarding the risk of diabetes, the Findrisk test—Finnish Diabetes Risk Score (Lindström & Tuomilehto, 2003)—is applied to evaluate a patient's 10-year risk of diabetes (fourth block of Figure 5). If the patient has some risk of diabetes in the next 10 years, the least diabetogenic drugs are prioritized. Finally, in relation to the risk of myopathy, the personalizer checks whether the patient has any disease that increases the risk of myopathy (last block of Figure 5). If that is the case, the recommended dose is cut in half. Two example rules of the personalizer (Rule 6 and Rule 7) are shown.
Rule 6. Concomitant treatment
IF current patient medication is cyclosporine OR
tacrolimus OR
everolimus OR
sirolimus
THEN remove pitavastatin.
Rule 7. Renal function
IF glomerular filtration rate ≥ 60
THEN remove ezetimibe with doses higher than 10.
1 Treatment recommender
The treatment recommender is a rule-based system developed with the aim to generate a sorted list of candidate treatments according to the criteria shown in Table 3. They are applied in cascade: First the safest drugs regarding renal function (glomerular filtration rate), next diabetes, and so on. Treatments can be simple or a combination of several drugs, because combinations of medicines are required to reach the target health outcomes in a person with complex health conditions (Climent et al., 2020). Combinations of drug doses must fulfil one of the following criteria (European Panel of Experts, 2016): (a) a single dose of a statin, (b) a dose of a statin and a dose of ezetimibe, or (c) a dose of a statin, a dose of ezetimibe, and a dose of a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor. Moreover, only combinations that reach the therapeutic target are chosen, and if there is no treatment that fulfils this requirement, only those reducing the LDL-C level by >50% are chosen in very high CVD risk patients.
| Prioritize combinations with the safest drugs (when there is an unbalanced glomerular filtration rate) |
| Prioritize the least diabetogenic combinations (in the case of a 10-year future diabetes risk) |
| Prioritize shorter combinations |
| Prioritize lower doses when comparing equal active ingredients |
| Prioritize cheaper combinations |
| Prioritize combinations (considering only those capable of reaching the target) with a higher cholesterol reduction |
Finally, the physician is free to choose a treatment from among all of those suggested with the advantage that the best treatments will be at the top of the list. Table 4 shows an example of a possible output. Each entry in the output list refers to a treatment and consists of the active ingredient(s) with the corresponding dose(s), the LDL-C reduction (in %), the expected LDL-C value, the CVD risk reduction (in %), and the price for a 28-day treatment. The first two recommendations are equivalent, the second and third are more expensive, while the fifth one offers less effectiveness and safety outcomes.
| Active ingredients and doses | LDL-C reduction | Expected LDL C value | CVD risk reduction (%) | Price |
|---|---|---|---|---|
| Atorvastatin 80.0 mg, Ezetimibe 10.0 mg, Alirocumab 150.0 mg | 84.96 | 37.6 | 108.92 | 549.27 € |
| Atorvastatin 80.0 mg, Ezetimibe 10.0 mg, Evolocumab 140.0 mg | 84.96 | 37.6 | 108.92 | 549.27 € |
| Rosuvastatin 20.0 mg, Ezetimibe 10.0 mg, Alirocumab 150.0 mg | 84.96 | 37.6 | 108.92 | 551.36 € |
| Rosuvastatin 20.0 mg, Ezetimibe 10.0 mg, Evolocumab 140.0 mg | 84.96 | 37.6 | 108.92 | 551.36 € |
| Pitavastatin 4.0 mg, Ezetimibe 10.0 mg, Alirocumab 150.0 mg | 83.04 | 42.4 | 106.47 | 555.23 € |
- Abbreviations: CVD, cardiovascular disease; LDL-C, low level of low-density lipoprotein—holesterol.
Complementary to the drug recommendation, the physician could eventually receive additional recommendations regarding other kinds of interventions. For example, Rule 8 is used to warn about the recommendation to intensify the effect of the treatment with style life behavioural change.
Rule 8. Alternative intervention recommendations
IF cardiovascular risk is low and LDL ≤ 190
THEN warning “In this patient with this lipid profile, it is recommended to intensify actions on lifestyles.”
1 HIS integration
HTE 3.0 has been encapsulated as a service in order to use cloud computing and storage to integrate such an innovative component into the HIS. This integration strategy is a trend in public health care systems (Cosialls, 2015). Two services have been defined (Figure 2): a WS for patient detection, and a WS for assessment (remaining HTE 3.0 components, which work in a cascade). Patient detection does or does not generate an alarm, which is highlighted in the physician's desktop at the patient's planned visit. The physician has the option to answer the alarm, and then the assessment service is activated.
- The therapeutic target assigned to the patient;
- The value of cardiovascular risk;
- The results of the survey of the Dutch lipid clinic network;
- The top five recommended treatments.
Furthermore, HTE 3.0 has been designed with educational purposes.2 Thus, the HTE 3.0 user interface has various optional information panels to help the user understand what is happening and to take the correct action. For example, the user can find information about scientific knowledge of CVD risk factors, atherogenic dyslipidemia, and FH. Moreover, the user interface enables the user to quickly access the lipid reference centres, by providing their contact information, an endeavour that ease the communication among the different health care levels.
HTE 3.0 has been developed under the Ubuntu operating system using Java and Angular 2.0, which provides a short response time when the user interacts with the interface. The HTE 3.0 user interface languages are English, Catalan, and Spanish. HTE 3.0 uses information from a database that includes the efficacy, interactions, and price of lipid-lowering treatments. The treatment database is implemented with postgreSQL. Drug interactions are automatically read from the DrugBank database (Law et al., 2014), which is periodically updated by the University of Alberta. However, dose prices and dose efficacies are not automatically updated. Therefore, some connectivity services should be developed in the near future to update the list of treatments and their prices automatically. Another approach to be explored in the future is to learn dose efficiency from new scientific evidence.
The system is currently deployed, in an integrated way, at CSMS-Hospital of Blanes. Moreover, HTE 3.0 is also available free of charge for non-commercial use at http://hte.udg.edu:3000. However, this version is not integrated with an HIS and cannot connect to the hospital to obtain patient information; thus, it has to be manually provided by the user.
4 EXPERIMENTAL RESULTS
Two pilot tests have been conducted: one for the detection of FH and another for dyslipidemia treatment.
4.1 Detection of FH
Regarding the detection of FH, all the analytical procedures performed between the years 2010 and 2016 in the CSMS-Hospitals of Blanes and Calella (Catalonia), covering a patient population of 200,000 inhabitants, have been analysed.
The system detected 1,013,310 blood tests with LDL-C values corresponding to 99,667 patients. A total of 467 patients with inclusion criteria were identified and corresponded to the FH phenotype. There were 81 patients registered at the Lipid Clinic with the diagnosis of FH during the study period (2010–2016). Therefore, the use of HTE 3.0 would mean increasing the detection capacity of HF by 5.7 times compared with usual clinical practice (Ascaso et al., 2015).
4.2 Dyslipidemia treatment
For dyslipidemia treatment assessment, up to 20 cases have been defined, including complex situations like patient comorbidities and the presence of multiple (concomitant) treatments for a single patient. We followed an agreement validation methodology (Verdaguer et al., 1992) and a grader consensus methodology (Krause et al., 2018; Rebholz-Schuhmann et al., 2010) to test the system. These methodologies are recommended to be applied in knowledge areas where a gold standard is not readily available, as is the case for dyslipidemia and FH. To that end, the 20 cases were presented to seven lipidologists (EX1–EX7), none of whom had participated in the design of the system, with the aim to obtain the treatment they would prescribe to the patient. The outputs were compared to the three first recommendations of HTE 3.0 (HTE_1, HTE_2, and HTE_3). According to the validation methodologies (Krause et al., 2018; Rebholz-Schuhmann et al., 2010; Verdaguer et al., 1992), we aimed to show that HTE 3.0 works as well as any other clinician, keeping in mind that it is very difficult to have two human experts provide identical answers.
Among the received answers, two patients were removed because the answers provided were not considered in the system being evaluated. Next, to analyse the similarity or dissimilarity among the answers, distances among them were computed. Due to the number of drugs available, and combinations of them, computing such distance is not easy, unless we reduce the number of combinations. To that end, we mapped the possible answers into three labels: incorrect, acceptable, and the best according to the expert lipidologist who collaborated in the HTE 3.0 design.
Figure 6 shows the distribution of the answer categories (incorrect, acceptable, and best) per participant (EX1–EX7 and HTE_1–HTE_3). EX1 had the highest score of best answers (up to nine), and the fewest incorrect answers (just one); EX2 exhibited a very poor behaviour (up to eight incorrect answers). EX3 had a similar poor behaviour. On the other hand, HTE_1, HTE_2, and HTE_3 provided correct answers; HTE_3 was scored as the best answer, in the second position after EX1.
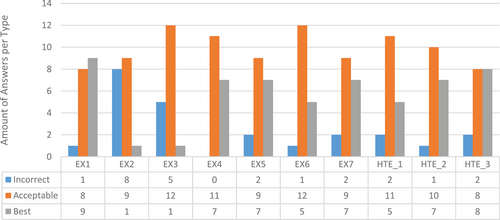
From the information provided in Figure 6 we can say that the success rates of each HTE 3.0 recommendation were as follows: HTE_1, 88.89; HTE_2, 94.33; and HTE_3, 88.89. The second recommendation, HTE_2, was the most successful one; however, such results should be analysed in the context of the recommendations that could be provided by human experts, according to the above-mentioned agreement methodologies. To that end, pairwise distances among experts were computed according to the answer ordinal categories (incorrect < acceptable < best) for all of the 18 patients.
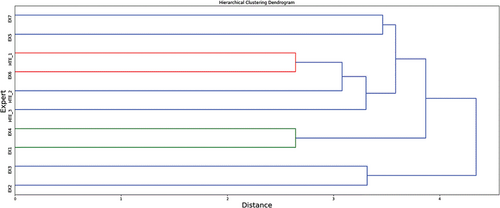
According to Verdaguer et al. (1992), pairwise distances enable the application of hierarchical clustering, which condenses the information about the differences among KBS regarding any human expert. To that end, we have applied hierarchical clustering, using the Euclidean distance, to compute a distance matrix among all of the experts, and the average linkage method to combine clusters.
The results are shown in the dendrogram in Figure 7. The differences among the answers provided by HTE 3.0 and human lipidologists are smaller than the differences between human experts. It is possible to observe a subset of lipidologists, {EX1, EX4}, that corresponds to the ones that have performed best according to the outcomes of Figure 6, and a cluster of poor lipidologists, {EX2, EX3}. Regarding HTE 3.0, HTE_1 is similar to EX6, and EX1 is similar to EX4. HTE_2 and HTE_3 are the nearest experts to {HTE_1, EX6}. Finally, poor lipidologists comprise the farthest cluster; therefore, they both provided treatments that are very different from the ones provided by other lipidologists or HTE 3.0.
Pairwise distances among experts according to the ordinal categories (incorrect < acceptable < best) enable the application of other complementary consensus methodologies to analyse the results achieved: the Copeland method, and the Borda rule. The Copeland method (Baharad & Nitzan, 2003) is used in social science to select the winner of an election according to the position of the candidates expressed in the votes. This method consists of counting, for each pair of candidates, the number of votes that one candidate is preferred over the others. Next, the candidate that wins the most times is selected. The application of the Copeland method to evaluate HTE 3.0 was conducted as follows: When an expert has a label higher than another one regarding one patient (voter), they are considered the winner of the vote. Table 5 shows the matrix regarding the pairwise analysis among experts. Unfortunately, the Copeland methods presented ties when summing up the overall values (how many times one has beaten the others). In particular, all of the experts and HTE_1, HTE_2, and HTE_3 beat each other equally, except EX2, who performed the poorest compared with all others.
| EX1 | EX2 | EX3 | EX4 | EX5 | EX6 | EX7 | HTE_1 | HTE_2 | HTE_3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| EX1 | 18 | 16 | 17 | 15 | 12 | 14 | 13 | 15 | 14 | 13 |
| EX2 | 5 | 18 | 13 | 6 | 8 | 9 | 8 | 6 | 8 | 6 |
| EX3 | 9 | 15 | 18 | 7 | 9 | 9 | 10 | 10 | 9 | 8 |
| EX4 | 14 | 17 | 17 | 18 | 11 | 14 | 13 | 14 | 14 | 12 |
| EX5 | 9 | 17 | 16 | 11 | 18 | 14 | 13 | 14 | 14 | 12 |
| EX6 | 10 | 18 | 16 | 12 | 13 | 18 | 12 | 15 | 12 | 11 |
| EX7 | 12 | 17 | 18 | 12 | 14 | 13 | 18 | 13 | 14 | 12 |
| HTE_1 | 9 | 15 | 15 | 11 | 13 | 14 | 11 | 18 | 12 | 12 |
| HTE_2 | 11 | 16 | 17 | 13 | 14 | 14 | 14 | 15 | 18 | 12 |
| HTE_3 | 11 | 16 | 15 | 12 | 13 | 13 | 12 | 15 | 12 | 18 |
The Borda rule produces fewer ties (Baharad & Nitzan, 2003). It works as follows: Given n candidates, the candidate in the first position receives a value of n, the next candidate a value of n − 1, and so on. These values are summed up, and the candidate who receives the highest aggregated outcome is the winner. The results are shown in Table 6. The Borda scores corroborated the results of hierarchical clustering: there was a subset of excellent lipidologists, {EX1, EX4}, and a subset of very poor lipidologists, {EX2, EX3}, while the answers provided by HTE 3.0 were in the middle. In particular, HTE_2 and HTE_3 were in the third position.
| Borda | |
|---|---|
| ID | Score |
| EX1 | 26 |
| EX4 | 25 |
| HTE_2, HTE_3 | 24 |
| EX7, EX5 | 23 |
| EX6 | 22 |
| HTE_1 | 21 |
| EX3 | 14 |
| EX2 | 10 |
4.3 Adding drug prices
The results regarding the quality of the recommended treatments were complemented with the information about drug prices, as shown in Figure 8. The lipidologist with the highest medical score, EX1, recommended drugs with a higher cost than HTE 3.0 (HTE_1, HTE_2, and HTE_3). This is due to the fact that HTE 3.0 is designed to take into account the cost of the treatment besides effectiveness and security. Thus, HTE 3.0 tries to recommend the cheapest treatments that achieve maximum effectiveness and guarantee the patient's security. This procedure provides a good balance between the three objectives.
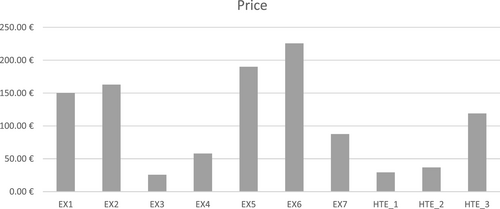
EX4 also achieved a good balance between medical score and economic cost—the best among the human experts. EX4 was in the second position in the Borda rank regarding the medical scores of the recommended treatments. However, the average cost achieved by EX4 doubles (or almost doubles) HTE_1, HTE_2, and HTE_2 was ranked third in the Borda rank, with just one point less than EX4. These results prove the excellent balance achieved by the HTE 3.0 decision-making methodology based on KBS operated in cascade.
5 DISCUSSION
HTE 3.0 as a FH detection support system could be used to minimize the gap in misdiagnosed FH, identifying up to 5.7 times more cases than the current practice. Therefore, the risk of early CHD could be diminished to a similar extent if the risk could be treated early. On the other hand, the increase in the number of patients involved in health care systems would increase the cost in the short term, but it should be argued to the political authorities that there would be marked benefits in the long term (including fewer people who would die young). The latest versions of CDSS on the dyslipidemia field (Chen et al., 2010; Siskind et al., 2000; Zamora et al., 2013) show not only that its use improves the achievement of lipid targets, but also how likely they would be to have a beneficial impact on the health care costs of coronary disease (Zamora et al., 2015).
Regarding the HTE 3.0 lower-lipid treatment assessment, and in the absence of gold standards, the differences between treatments provided by HTE 3.0 and human lipidologists are smaller than the differences between human experts. Therefore, HTE 3.0 behaves as any human lipidologist, being closer to the good ones than the less expert ones. Nevertheless, the lipidologist ultimately chooses the adequate treatment, so HTE 3.0 supports the human decision. In so doing, and according to the analysis of the first three recommendations provided by HTE 3.0, we can conclude that these recommendations are not just good treatments, but conform to a set of treatments that describe the balance between cost, clinical safety, and effectiveness.
5.1 Limitations
HTE 3.0 has been tested in a semi-real scenario: Hypercholesterolemia detection has been tested with real data, while the treatment recommendation has been performed using a set of 20 cases. While seven human experts have contributed to the validation, the number of cases is small, especially considering the high variability of health conditions. Therefore, a future clinical trial should be conducted that provides additional statistical power relative to the results obtained with this in-silico scenario.
HTE 3.0 is derived from guidelines, which require continuous updates.3 In that regard, alternative approaches that automate the acquisition of guidelines should be considered to guarantee that the tool is always using the last evidence in medicine based on, for example, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (Sanabria et al., 2015). Nevertheless, the validation of such automated systems is still a challenge.
Finally, HTE 3.0 provides recommendations as a single expert opinion, while human experts usually consult their colleagues for complex situations (Cai et al., 2019). In that regard, multi-agent systems as described by Khiat and Djamila (2019) could be explored in future research. This approach includes multiple criteria to negotiate and to agree on a health outcome between human experts and systems, or different systems with the same aim.
6 CONCLUSIONS
This paper describes a CDSS called HTE 3.0, which aims to aid clinicians in the detection and treatment of dyslipidemia and FH. It consists of several KBS that operate in a cascade to support clinicians. A patient detection system checks all patients' blood analyses to identify patients who might have dyslipidemia; a therapeutic target calculator system supports the estimation of the desired LDL level; a personalized KBS recommends drugs according to the individual's health status (e.g., comorbidities); and, finally, a treatment recommender system provides the clinician with a sorted list of recommended treatments (including combinations of medicines) according to efficacy, security, and price among other criteria. All the systems comprise HTE 3.0, which can be integrated in a HIS as a service.
HTE 3.0 has been validated with the collaboration of seven lipidologists. The results show that, in the absence of a gold standard, the differences between recommendations provided by HTE 3.0 and the ones provided by the lipidologists are smaller than the differences between human experts themselves. Moreover, HTE 3.0 recommendations provide a good- and perhaps be the best-balance between the cost of the treatments and their effectiveness and security.
As a future work, HTE 3.0 should go one step further regarding the formal description of patient comorbidities and the presence of multiple (concomitant) treatments for a single patient. In this regard, the inclusion of AI methods based on constraints as performed by Kovalov and Bowles (2016) could be a starting point. Moreover, and to avoid CDSS obsolescence, it could be interesting to explore the use of AI techniques to analyse and incorporate new medical evidence automatically.
ACKNOWLEDGEMENTS
Funding: This work has been developed with the support of the research group SITES awarded with distinction by the Generalitat de Catalunya (2017 SGR 1551). The development phase of HTE 3.0 and clinical validation has been developed independently. The dissemination phase has been supported by Sanofi laboratories. We would like to thank the following people for their collaboration: Eduard Feliu, Alejandro Pozo-Alonso, and Oscar Rivas in the implementation of the HTE 3.0 software and in its deployment into the HIS of CSMS-Hospital of Blanes; Guillem Paluzie and Joan Garcìa-Vilches for their participation in FH screening tests; and the lipidologists who participate in the validation process: Nuria Plana, Daina Ibarretxe, Alex Vila, Cristina Soler, Juan Carlos Sevilla, Gemma Estrada, and Rafa Cuenca.
CONFLICT OF INTEREST
Alberto Zamora, Beatriz López, and Ferran Torrent-Fontbona have the intellectual property register of HTE 3.0. The authors have no relationship with people or organizations that could inappropriately influence the design and development of HTE 3.0.
Endnotes
Biographies
Beatriz López is a senior lecturer at the Department of Electrical, Electronics and Automation Engineering of the University of Girona, director of the Biomedical Engineering Degree, member of the Health campus of the University of Girona, and Collaborator of the Biomedical Research Institute of Girona. B. López leads the research line on Artificial Intelligence applied to Medicine and Health in the eXiT research group of the University of Girona. Her main research interest is the development of new artificial intelligence (case-based reasoning, optimization) and machine learning methods to advance in the progress of Medicine and Healthcare.
Ferran Torrent-Fontbona is the lead data scientist in Aimsun SLY – a Siemens business. Previously he was a research associate of the group eXiT of the University of Girona. He received the Telecommunications Engineering degree (2011) from the Polytechnique University of Catalunya (UPC), the M.Sc. in Artificial Intelligence (2012) from the University of Girona, and the Ph.D. degree (2015) in the Department of Electrical, Electronic Engineering and Automation of the University of Girona.
Luis Masana-Marin is professor of Medicine at Rovira i Virgili University (Reus-Tarragona). Former Head of the Internal Medicine Service at “Sant Joan” University Hospital (Reus) (1992–2006). Director of the Lipids and Atherosclerosis Research Unit and Vascular Medicine and Metabolism. Past-President of the Spanish Society of Arteriosclerosis. Former Member of the Executive Committee of the European Lipoprotein Club. and of the Executive Committee of the European Atherosclerosis Society. Full Member of the Royal Academy of Medicine of Catalonia since 2002. Author of more than 350 publications on lipids and arteriosclerosis.
Alberto Zamora is a Specialist in Internal Medicine (Ph.D.), member of the Spanish Interdisciplinary Committee for Vascular Prevention (CEIPV), Coordinator for the Risk-Vascular and Lipid Unit of Corporacio de Salut del Maresme i la Selva; Barcelona, Spain. Associate Professor of the Faculty of Medicine of the University of Girona, Spain. Researcher at the TransLational Medicine and Decision Sciences Group at the University of Girona and Researcher at the Center for Biomedical Research in Cardiovascular Diseases Network of Spain. Her main research interest is massive data analysis and the development of decision support systems in the field of cardiovascular disease and familial hypercholesterolemia.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




