Divergent in situ expression of IL-31 and IL-31RA between bullous pemphigoid and pemphigus vulgaris
Ecem Zeliha Ergun and Rui Aoki contributed equally.
Abstract
Bullous pemphigoid (BP) and pemphigus vulgaris (PV) are two major autoimmune blistering skin diseases. Unlike PV, BP is accompanied by intense pruritus, suggesting possible involvement of the pruritogenic cytokine IL-31. However, the underlying mechanisms of the clinical difference between BP and PV in terms of pruritus are not fully understood. To compare the expression levels of IL-31 and its receptor IL-31RA in the lesional skin, including peripheral nerves in BP and PV patients, immunohistochemical staining for IL-31 and IL-31RA was performed in skin samples of BP and PV patients and healthy controls (HC). The IL-31RA-expressing area in epidermis and peripheral nerves was analysed using ImageJ and the percentage of positive cells for IL-31/IL-31RA in dermal infiltrating cells was manually quantified. Quantitative analyses revealed that IL-31/IL-31RA expressions in the epidermis and dermal infiltrate were significantly increased in BP compared to PV and HC. The difference between BP and PV became more obvious when advanced bullous lesions were compared. Peripheral nerves in BP lesions presented significantly higher IL-31RA expression compared to PV lesions. In conclusion, we found significantly augmented expressions of IL-31/IL-31RA in BP lesions, including peripheral nerves, in comparison to PV. These results suggest a possible contribution of IL-31/IL-31RA signalling to the difference between BP and PV in the facilitation of pruritus and local skin inflammation, raising the possibility of therapeutic targeting of the IL-31/IL-31RA pathway in BP patients.
1 INTRODUCTION
Bullous pemphigoid (BP) and pemphigus vulgaris (PV) are two major autoimmune bullous disorders characterized by autoantibodies against hemidesmosomal and desmosomal proteins, respectively.1 BP may begin with a non-bullous, pruritic phase with nonspecific excoriated or eczematous papules and/or plaques which sometimes remain to be the only sign of the disease.2 In its classical form, urticarial lesions and tense blisters appear following this prodromal phase. Severe pruritus accompanying the lesions has been shown to be correlated with disease activity.2, 3 In contrast to BP, pruritus is less frequently present and with lower intensity in PV which is usually painful.4 A recent analysis indicated that multiple factors are potentially correlated with pruritus in BP, including eosinophils, interleukin-31 (IL-31) and its receptors (IL-31RA and OSMRβ), substance P and its receptor, periostin, IL-13 and basophils,5, 6 while there are scarce systematic data on pruritus in PV.3 Enhanced in situ expression of IL-31 and IL-31RA has been shown in pemphigus herpetiformis (PH), a rare pruritic clinical subtype of pemphigus, compared to PV.7
IL-31 was initially defined as a pruritogenic cytokine predominantly produced by activated CD4+ Th2 cells.8, 9 Recent studies support the production of IL-31 in various immune cells other than T cells (e.g. monocytes, macrophages, dendritic cells, eosinophils, basophils and mast cells), as well as non-immune cells (e.g. keratinocytes and dermal fibroblasts).9, 10 IL-31 has been shown to play a key role in inducing pruritus in various chronic inflammatory skin diseases such as atopic dermatitis (AD), prurigo nodularis, allergic contact dermatitis, stasis dermatitis, psoriasis, cutaneous T cell lymphoma and chronic urticaria.11-19 In addition, a novel biologic agent targeting the IL-31 receptor (nemolizumab) showed promising results in the treatment of AD and prurigo nodularis.20, 21 IL-31 functions through binding its heterodimeric receptor, which consists of IL-31RA and OSMRβ,8, 9 and activates JAK/STAT, PI3K/AKT and MAPK pathways,22 leading to a wide range of immune responses. This receptor complex is expressed by a variety of different cell types, including keratinocytes, peripheral nerves and immune cells.9, 10 The diverse distribution enables IL-31 to target epithelial tissues, the nervous system and multiple immune functions. IL-31/IL-31RA signalling thus represents a master regulator of neuroimmune inflammation.23
Although several data suggest a possible involvement of IL-31 in the pruritus of BP,15, 24-26 its role in the pathogenesis of BP is not fully known. In particular, previous studies did not specifically address the contribution of IL-31 receptor on the peripheral nerves in BP skin lesions. The findings regarding IL-31 levels in patient sera also remain controversial.27 Thus, we compared the expression levels of IL-31 and its receptor in the skin including peripheral nerves and the serum IL-31 levels in BP and PV patients to assess the role of IL-31 pathway in the two most common autoimmune blistering diseases.
2 MATERIALS AND METHODS
2.1 Sample collection
Paraffin-embedded lesional skin samples of BP (n = 11; mean ± SD age, 76.1 ± 10.2 years) and PV (n = 11; mean ± SD age, 59.9 ± 18.4 years) patients were obtained from the archive of the dermatopathological laboratory of Department of Dermatology and Allergy, University Hospital of Munich LMU. Tissue samples of seven matching patients with histologically normal skin were examined as healthy controls (HC). The patients were diagnosed based on clinical, immunohistopathological and serological findings. All the skin samples were collected prior to the initiation of a systemic treatment. Patient demographic, clinical and laboratory characteristics are displayed in Tables S1 and S2.
2.2 Immunohistochemistry
Immunohistochemistry for IL-31, IL-31RA and S100 was performed on serial paraffin-embedded sections of 5 μm thickness. After standard deparaffinization, antigen retrieval was performed (anti-IL-31 and anti-IL-31RA: heating in a pressure cooker for 15 min with citrate buffer (Target Retrieval Solution pH 6.0; Dako); anti-S100: incubation with protease solution (Sigma-Aldrich) for 10 min at room temperature) with subsequent washing with 0.05 moL/L Tris-buffered saline (pH 7.6). To avoid nonspecific signals, sections were blocked with 0.1% cow milk powder in Tris-buffer for 10 min and then incubated with the following primary antibodies at room temperature for 30 min: polyclonal rabbit antibody that binds to IL-31 (#ab102750; Abcam), polyclonal rabbit antibody that binds to IL-31RA (#ab113498; Abcam), polyclonal IgG rabbit antibody (#31235; Thermo Fisher Scientific) and polyclonal rabbit antibody that binds to S100 (#RB-044-R7; Thermo Fisher Scientific). The alkaline phosphatase based detection system and Fast Red chromogen (Dako REAL Detection System, Alkaline phosphatase/RED, Rabbit/Mouse, K500511-2; Dako) were used for secondary staining and enzyme detection of the labelled antigens. This detection system is based on an indirect streptavidin-biotin method and was employed in a two-step procedure. The first step was the incubation with ready-to-use biotinylated secondary antibody including goat anti-mouse and anti-rabbit immunoglobulins. The second step was incubation with streptavidin conjugated to alkaline phosphatase which reacts with biotinylated antibody molecules. Endogenous alkaline phosphatase was blocked by adding levamisole. The reaction was visualized by a red chromogen. Sections were counterstained with haematoxylin and mounted.
2.3 Quantification of immnohistochemistry
All immunohistochemically stained sections were photographed with Olympus BX51 microscope at 400-fold magnification in three fields that were representative of the stain in epidermis and dermis.
The immunoreactive cells for IL-31 and IL-31RA were manually quantified in three fields of dermis for each section using ImageJ software by two independent investigators. The ratio of positive cells to total infiltrating cells was calculated in each field. The average percentage of these three fields in each section was then calculated.
The percentage of IL-31RA-expressing area in epidermis and peripheral nerves was analysed with ImageJ software (NIH, Bethesda, MD). S100 expression in peripheral nerves was assessed qualitatively. The average of these three fields in each section was calculated.
2.4 Statistical analysis
Statistical analysis was conducted with GraphPad Prism version 9.3.1 (GraphPad Software). The statistical distribution was calculated using Shapiro–Wilk test. If the quantitative variables showed non-normal distribution, Kruskal–Wallis test followed by Dunn's multiple comparisons test were used for the comparison of more than two groups. Mann Whitney U test was employed for two groups. The results were represented by the median + interquartile range (IQR). Differences between groups were considered statistically significant at p < 0.05.
3 RESULTS
3.1 IL-31 and IL-31RA immunoreactivities differentiate between BP and PV skin
We investigated in situ expression of IL-31 and IL-31RA in BP, PV lesions and HC skin samples based on immunohistochemical staining. Representative images of immunoreactivity for IL-31 and IL-31RA in the epidermis and the upper dermis of each group are demonstrated in Figure 1. IL-31 and IL-31RA staining was observed in a predominantly cytoplasmic pattern in both keratinocytes and dermal infiltrate.
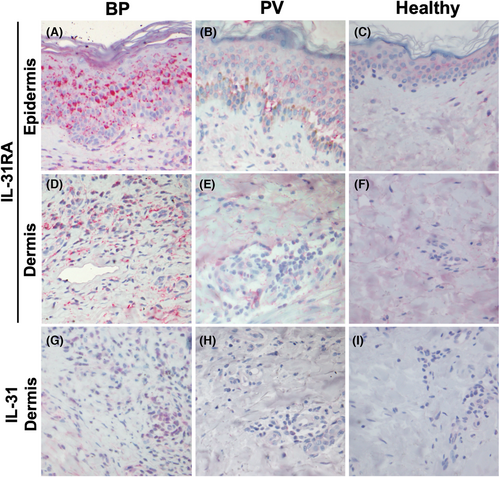
Early erythematous lesions of BP showed a strong and broad expression of IL-31RA in the epidermis (Figure 1A). In contrast, the expression of IL-31RA in PV epidermis was weak, although a few strongly positive acantholytic keratinocytes were observed (Figure 1B). Similar to the epidermis, BP lesions presented more abundant immunoreactivity of IL-31 and IL-31RA in the upper dermal infiltrate, especially in perivascular area (Figure 1D,G), compared to PV lesion (Figure 1E,H) and HC skin (Figure 1F,I).
To quantify the immunoreactivity for IL-31RA in the epidermis, the percentage of IL-31RA-expressing area in the epidermis was analysed using ImageJ software. The expression level of IL-31RA in the epidermis was significantly higher in BP lesions (median, IQR; 34.9%, 28.6%–47.4%, respectively) than in PV lesions (16.7%, 9.2%–25.4%, respectively) (p = 0.0496) and in HC skins (2.7%, 0.9%–7.1%, respectively) (p = 0.0007) (Figure 2A). There was no significant difference between the PV and HC group (p = 0.3321).
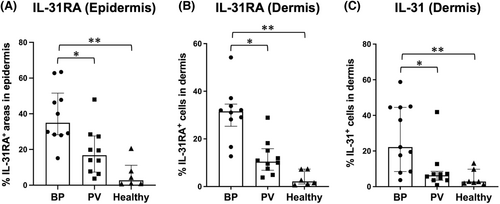
We additionally analysed the percentage of the dermal infiltrating cells immunoreactive for IL-31 and IL-31RA, respectively. IL-31RA expression in the dermal inflammatory cells was significantly enhanced in lesional BP skin (median, IQR; 31.5%, 28.5%–33.4%, respectively) in comparison to PV (10.4%, 8.0%–14.3%, respectively) (p = 0.0215) and HC (2.1%, 1.3%–6.2%, respectively) (p < 0.0001), while no significant difference was detected between PV and HC (p = 0.1694) (Figure 2B). In line with the IL-31RA immunoreactivity, IL-31 expression was also significantly increased in the dermal infiltrate of BP (median, IQR; 22.2%, 13.0%–44.5%, respectively) compared to PV (6.3%, 3.9%–7.2%, respectively) (p = 0.0291) and HC biopsies (2.9%, 2.2%–6.4%, respectively) (p = 0.0030) (Figure 2C). The difference was not significant between PV and HC (p > 0.9999).
3.2 The difference between BP and PV is more obvious in the advanced bullous lesion
To determine whether IL-31 RA expression is affected by the stage of BP and PV, we analysed IL-31 RA expression of the epidermis and the dermal infiltrates in nonbullous and bullous lesions of each disease. As for the classification, we defined it according to the pathological findings as follows: non-bullous, superficial perivascular inflammatory infiltrate often accompanied by intracellular spongiosis as a nonbullous lesion (n = 5) and subepidermal blistering with a dense inflammatory infiltrate as a bullous lesion (n = 5) in BP (Figure 3A,B); intercellular edema with loss of intercellular attachments, not showing any epidermal cleavage and blister formation as a nonbullous (n = 3) and clefting and blister formation containing inflammatory cells and acantholytic cells as a bullous lesion (n = 7) in PV (Figure 3C,D).
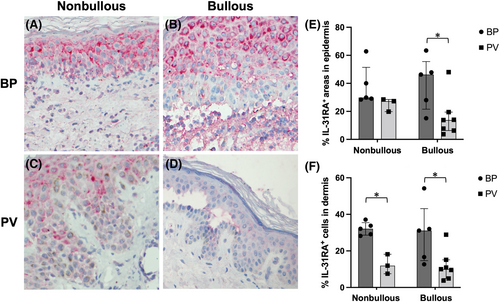
In the nonbullous lesion, PV epidermis revealed the same level of IL-31RA expression (median, IQR; 27.3%, 23.5%–27.9%, respectively) as BP epidermis (29.9%, 29.1%–40.0%, respectively) (p = 0.0714) (Figure 3E). However, epidermal IL-31RA expression tended to decrease in the bullous lesion of PV, while it increased in the bullous lesion of BP. No significant differences were observed between nonbullous and bullous lesions of both diseases. However, bullous lesions showed a significant difference in IL-31RA expression between BP (median, IQR; 46.3%, 28.0%–47.8%, respectively) and PV epidermis (13.8%, 7.0%–16.7%, respectively) (p = 0.0480) (Figure 3E).
The advanced bullous lesion of BP also revealed strong IL-31RA expression in the intensive lymphocytic and eosinophilic infiltrates in the blister cavity and the upper dermis (median, IQR; 31.1%, 16.6%–32.0% respectively), comparable to the level in the dermal infiltrates of nonbullous BP lesion (32.1%, 29.1%–33.8% respectively) (p = 0.5476) (Figure 3F). In connection with this receptor expression, IL-31 was also expressed in the epidermis and dermal infiltrates of BP lesion (data not shown). In contrast with BP, scarce immunoreactivity was found in the dermal infiltrates of PV nonbullous/bullous lesions both for IL-31 and IL-31RA (Figure 3F).
3.3 IL-31RA expression on peripheral nerves predominates in BP compared to PV
Considering the relevance of pruritus in BP patients, we assessed IL-31RA immunoreactivity on the peripheral nerves in lesional BP and PV samples to evaluate the possible direct interaction of IL-31 with peripheral nerves. Initially, we identified the peripheral nerves in six BP and four PV sections with S100 staining. Representative images of staining with S100 and IL-31RA are shown in Figure 4, demonstrating apparent difference in IL-1RA expression between BP (Figure 4C,D) and PV (Figure 4G,H).
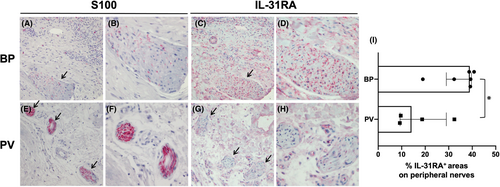
We then calculated the percentage of IL-31RA-expressing area within the peripheral nerves. The median (IQR) of IL-31RA expression levels were 38.9% (33.9%–39.3%) in BP and 14.1% (9.5%–22.1%) in PV. Thus, BP lesions presented significantly augmented expression of IL-31RA on the peripheral nerves compared to PV lesions (p = 0.0381) (Figure 4I).
3.4 Serum levels of IL-31 are not different between BP and PV patients
In addition, we evaluated serum levels of IL-31 in BP and PV patients using ELISA. Although some BP patients showed elevated serum concentrations of IL-31, no significant difference was detected as compared to PV or HC group (p > 0.9999) (Figure S1).
4 DISCUSSION
This study shows increased IL-31/IL-31RA expressions in the peripheral nerves as well as epidermal keratinocytes and dermal inflammatory cells in BP lesions as compared to PV lesions. Recent findings have highlighted a central role of IL-31 in neuro-immune-epithelial crosstalk for the induction and amplification of chronic pruritus.23, 28, 29 This concept was prominently investigated in the setting of AD, but could be adapted to other Th2-driven inflammatory conditions.23, 30
BP is characterized by the presence of IgG autoantibodies targeting two hemidesmosomal proteins (collagen XVII and dystonin-e, also called BP180 and BP230, respectively), with a prominent inflammation consisting of eosinophils and neutrophils targeting basement membrane.2, 31 BP is considered to be induced and sustained by a Th2-driven autoimmune response.28 In the presence of BP180 autoantibodies, BP180-autoreactive T cells produce Th2 cytokines, which induce autoantibody production and activate infiltrating eosinophils and neutrophils, with the subsequent release of proteinases such as matrix metalloproteinase and neutrophil elastase (NE) cleaving the anchoring fibrils and leading to subepidermal blisters.2, 32, 33 In PV patients, anti-desmoglein 1 and 3 (Dsg-1 and -3) IgG autoantibodies are present, and their binding to these desmosomal transmembrane proteins causes acantholysis and intraepidermal cleavage.31, 34 A large number of studies have shown that various T cell subsets exhibit a range of abnormalities and drive immunopathogenesis in both BP and PV.34 The abnormality in Th1/Th2 and Th17/Treg balance may affect the difference in skin inflammation and pruritus between the two diseases.
Cytokine effects are based on their capacity to assemble receptor complexes and thus their expression patterns in target cells determine their ability to respond to specific cytokine signals.23 IL-31 is known to bind predominantly to IL-31RA, but not to OSMRβ in the IL-31 receptor complex,9, 35 and the limiting factor for IL-31 signal transduction appears to be the expression of the IL-31RA chain.23 Thus, we examined IL-31RA expression in epidermal keratinocytes and peripheral nerves, important targets for IL-31, to assess the capacity to induce IL-31/IL-31RA signalling.
In our quantitative analysis of immunohistochemical staining, keratinocytes exhibited a significantly higher expression of IL-31RA in BP lesions compared with PV and HC lesions. IL-31-stimulated keratinocytes were reported to release key proinflammatory cytokines (e.g. IL-1α, IL-1β and IL-6) and chemokines (e.g. CXCL1, CCL1, CCL4, CCL17, CCL19, CCL22 and CCL23), indicating that IL-31 has a proinflammatory role involved in the recruitment of polymorphonuclear cells, monocytes and T cells to a site of skin inflammation.8, 36 Hence, keratinocytes may amplify skin inflammation via IL-31/IL-31RA signalling in BP.
IL-31RA is also constitutively expressed in a subset of peripheral neurons and dorsal root ganglia (DRG).9, 12, 37 In the peripheral nervous system, the first event is binding of pruritogens to a subset of primary afferent C-fibre somatosensory neurons that innervate skin.30 Kato et al. first detected IL-31RA protein in the nerve fibres of the dermis of AD patients as well as in the neurons of normal DRG.38 However, in BP skin lesions, the protein expression of IL-31 receptor on peripheral nerves has not, to our knowledge, been previously described. We demonstrated a significantly augmented expression of IL-31RA on the dermal peripheral nerves, which were also stained positive for S100, in BP compared to PV, indicating the possibility for direct stimulation of sensory neurons via IL-31RA in BP.
Besides keratinocytes and peripheral neurons, multiple immune cells express IL-31RA in steady state or more importantly under activated conditions.23 Our study showed that IL-31 and IL-31RA were more abundantly expressed within the lymphocytic and eosinophilic infiltrates in the upper dermis and at the dermal-epidermal junction in BP compared with PV lesions. However, the limitations of our study include the lack of the evaluation of their cellular sources. In this regard, previous data revealed eosinophils as the major cellular source of IL-31 in BP.5, 25, 26 Basophils and mast cells have been reported to be involved in BP39-41; however, their potential contribution to IL-31 production in BP is not yet known.27
Notably, our analysis on IL-31RA expression in nonbullous and bullous lesions demonstrated that the difference between BP and PV became more obvious when they were compared in the advanced bullous lesion. Provided that eczema-like, nonbullous form might be the initial stage of a bullous lesion, these results may imply that IL-31RA immunoreactivity is prolonged in BP lesion compared to PV lesion. Th2 cytokines IL-4 and IL-13 have been reported to increase IL-31RA expression in monocytes and macrophages and upregulate IL-31.42, 43 Considering together with the contribution of IL-31-stimulated keratinocytes to skin inflammation, IL-31/IL-31RA signalling may be amplified through the interactions between activated keratinocytes and immune cells especially in the established bullous lesion of BP. PV usually present with pauci-inflammatory blister formations and hence their interactions could be scarce as compared to BP. Owing to this nature, less expression levels of IL-31/IL-31RA might be obtained in PV.
Regarding serum IL-31 levels in BP patients, there are conflicting reports compared with HC.27 Salz et al. observed significant increases,25 whereas Kulczycka-Siennicka et al. reported reduced levels of IL-31 in BP sera.44 Consistent with the study by Rüdrich et al., who showed only minor elevations in BP,26 we could not find a significant difference between BP, PV and HC groups. Our collected skin and serum samples did not belong to the same patient, and the outcomes can vary with the disease activity and severity according to when the samples were collected. Thus, the correlation results could not be obtained between in situ and serum data.
This study is limited by a small sample size reducing the power of the statistical analyses, different sources of skin and serum samples and retrospective nature of the study.
Scratching typically accompanies pruritus in BP.3 Consequently, some patients can develop chronic prurigo lesions due to prolonged scratching.45 However, the current therapy recommendations do not outline specific antipruritic therapies besides the immunosuppressive therapies.44 Recently, preliminary evidence from a small case series has indicated a good clinical response to dupilumab, an IL-4Rα antagonist, in patients with BP.46 Clinical trials using dupilumab are underway to evaluate its efficacy in BP patients.47
In conclusion, we provided evidence that IL-31/IL-31RA expression is significantly augmented in the skin lesions of BP compared to PV; the difference in the intensity of the pruritus and local skin inflammation between the two diseases may be explained in part by the divergent expression of IL-31/IL-31RA. We speculate that enhanced IL-31/IL-31RA signalling may be associated with the induction and persistence of pruritus in BP. In addition, this signalling may contribute to the amplification of the inflammatory cascade in BP skin. Therefore, nemolizumab, a humanized monoclonal antibody targeting IL-31RA, may represent a new additional treatment option for BP.
AUTHOR CONTRIBUTIONS
Thomas Ruzicka and Işın Sinem Bağcı designed this study. Ecem Zeliha Ergun, Rui Aoki and Işın Sinem Bağcı carried out the experiment. Ecem Zeliha Ergun and Rui Aoki analysed and interpreted the data, discussing with Işın Sinem Bağcı. Daniela Hartmann, Michael J. Flaig, Orsolya N. Horváth and Miklós Sárdy provided technical and environmental support. Ecem Zeliha Ergun, Rui Aoki and Laura Calabrese collected information on patients. Ayşe Esra Koku Aksu, Mehmet Salih Gürel and Vildan Manav commented on the project. Ecem Zeliha Ergun drafted the manuscript, and Rui Aoki and Işın Sinem Bağcı critically revised it. Miklós Sárdy, Lars E. French, Thomas Ruzicka, Takashi K. Satoh and Daniela Hartmann provided input for the manuscript and commented on the text. All authors approved and contributed to the final version of the manuscript.
ACKNOWLEDGEMENTS
This study was funded by grants from Friedrich Bauer Foundation 2018 (No. 40/18) and approved by the ethics committee of University Hospital of Munich LMU (#18-659). The patients in this manuscript have given written informed consent to publication of their case details. We would like to thank Ursula Puchta and Johanna Laude for technical assistance with immunohistochemistry. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




