Analysis of optical absorption of photoaged human skin using a high-frequency illumination microscopy analysis system
Abstract
Skin is composed of different layers, including the stratum corneum, epidermal living layer and papillary and reticular dermis. Each has specific optical properties due to differences in their biological components. Alterations in the skin's cutaneous biological components resulting from photoaging caused by chronic exposure to UV light affect the deterioration of appearance associated with the skin's optical properties. Various methods for analysing cutaneous optical properties have been previously proposed, including mathematical models and computer simulations. However, these were insufficient to elucidate changes in each skin layer and comprehensively understand the skin's integrated optical properties. We focused on UV-induced yellowing of the facial skin. We evaluated site-specific optical absorption of human skin tissue sections to investigate the yellowish discoloration, which is suggested to be related to the photodamage process. The method includes our original technique of separating the transmitted and scattered light using high-frequency illumination microscopy, leading to microscopic analysis of the tissue's optical absorption in the regions of interest. In analysing the sun-exposed facial skin tissue sections, we successfully showed that dermal regions of aged skin have increased absorption at 450 nm, where yellowish colours are complemented. Furthermore, we confirmed that elastic fibres with observable histological disorder resulting from photodamage are a prominent source of high optical absorption. We detected changes in the skin's optical absorption associated with dermal degeneration resulting from photodamage using a novel optical microscopy technique. The results provide a base for the evaluation of optical property changes for both yellowing discoloration and other tissue disorders.
1 INTRODUCTION
Human skin is highly sensitive to intrinsic and/or extrinsic stimuli, and its condition is constantly changing. Skin colour is a significant biomarker that provides information on the skin's condition. Although skin is a thin organ, it is made up of multiple layers, each of which has different optical properties. The optical properties result from each individual skin layer's unique structures and contribute to the overall appearance of skin that results from lamination. However, the optical properties of each skin layer remain unclear. Because there are complex microstructures within each layer, the light irradiated into the skin is repeatedly scattered in many directions. As a result, it is difficult to measure and evaluate the optical properties accurately.1, 2 Of all optical properties, optical absorption is the most important indicator for evaluating skin's biological properties because chemical structural or conformational changes in biomolecules often accompany changes in its optical absorption. In 2016, we showed that denaturation of dermal protein could alter its optical absorption of short-wavelength light and cause discoloration of the dermis.3 However, we have yet to fully identify the components responsible for the discoloration because of a lack of techniques to measure site-specific absorption in the microscopic field of view. Therefore, measuring the micro level site-specific optical absorption with high accuracy has been a crucial concern in the optical analysis of skin.
In the past, the optical properties of skin have been analysed by measuring skin specimens formed in a sheet and using the measured value in the mathematical model.4 Several mathematical models have been proposed for optical skin analysis. The theoretical values of optical absorption separated because of scattering can be obtained using the Kubelka–Munk formula,5, 6 diffusion theory,7, 8 adding-doubling method,9-11 and Monte Carlo simulations.12-14 The Kubelka–Munk equation is the most widely used classical analytical model describing the optical properties of thin pigment layers, which simply assumes that the pigment concentration is uniform. Diffusion theory, which assumes that photons undergo multiple scattering and diffuse through the medium, is widely used as a theoretical model for light transport in a turbid medium. The Adding-doubling method combines the Kubelka–Munk and diffusion theory, which makes it possible to model layered media. In addition, Monte Carlo simulations are a relatively new computational method for analysing the transport of photons in complex, heterogeneous media. Although these mathematical models have been used effectively for skin analysis, they assumed optically homogenous tissue layers. Skin is a complex heterogeneous organ where absorbent substances are spatially distributed variably throughout the tissue. Thus the assumption made in these mathematical models often violates and prevents the accurate optical absorption of each skin's microregion with a precise location.
To measure accurate optical absorption of nonhomogeneous skin, it is essential to remove the scattering of light within the skin. In light transport analysis based on computational photography techniques, several methods have been proposed for removing scattering components using high-frequency illumination (HFI) from reflected,15 and transmitting light through an object.16 We extended HFI-based light transport analysis to microscopic image analysis and developed a novel spectroscopic microscope system that can accurately obtain the spatially dependent spectral absorption properties in high spatial resolution.17
This paper aims to assess the process of facial skin photodamage through the HFI-based microscopic light transport analysis. Our light transport analysis is effective for removing the scattering of light within the skin and providing accurate optical absorption properties of the target microregion. Furthermore, we investigate whether spatially dependent optical absorption unveils the skin component that causes the yellowing. The HFI-based approach enabled us to measure the apparent increase in light absorption around the wavelength of 400–500 nm (violet to visible blue region) in denatured elastic fibres after removing scattering components inside the skin, leading to the yellowing of photodamaged skin.
2 METHODOLOGY AND MATERIALS
2.1 High-frequency illumination microscopy analysis
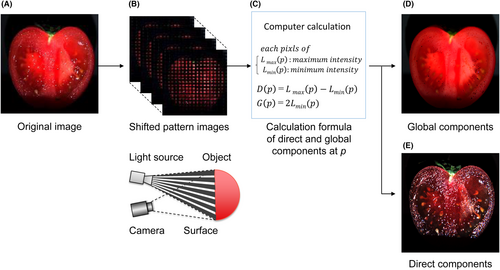
By projecting a high-frequency spatial pattern onto an object and digitally processing the captured image, it is possible to separate the transmitted light and scattered light and sharpen the object from an unclear turbid image.
For example, Figure 1 shows the analysis of an image of a tomato taken with normal lighting. As shown in the figure, the image of the internal structure of the tomato is not clear because of scattered light; however, the image of the direct components, captured and calculated using the HFI system is clear and separated from overlapping light. Therefore, the transmitted light is separated from the major scattered light. As shown in Figure 1D, the tomato's internal structure can be clearly shown. We applied this HFI analysis to the field of transmission light microscopy to de-scatter the light from the skin tissue section images, as described before.17
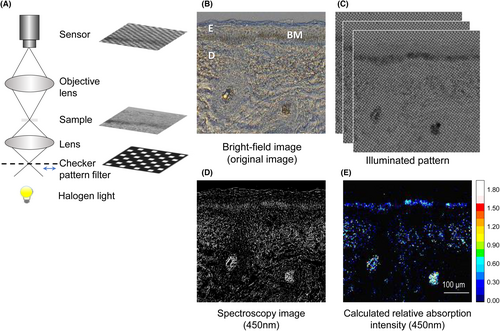
Briefly, as shown in Figure 2A, the experimental setup consisted of a transmission microscope (Olympus BX53), a halogen light for the light source, a high-frequency checker pattern filter (size of the pattern is 16 × 16 μm) and an illumination lens, which was set above the filter to focus the illumination pattern. The XYZ-axes motorized stage (Suruga Seiki KXT04015-LC) was attached to move the filter repeatedly. In addition, to evaluate the discoloration, under a microscope, we used a hyperspectral camera (EBA Japan NH-8) with a spectral range of 380–780 nm, spectral sampling interval of 5 nm, camera resolution 1280 × 1024 pixels, and a dynamic range of 12 bits, which has been recently introduced to enable spectral measurements under a microscope18, 19 Because the target of this study was yellowing, image acquisition was adjusted to provide optimal conditions for the 450 nm channel. At each position, images were captured 23 times. The transmitted, D(p) and scattered lights, G(p), were calculated (3), (4) for each pixel, and the relative absorption intensities of 450 nm were also calculated from each pixel of D(p) according to the Beer–Lambert law.20
2.2 Quantitative evaluation of relative absorption intensities
For quantitative evaluation, ImageJ software (http://imagej.nih.gov/ij) was used. The epidermis, as well as epidermal appendages, were excluded from the calculation fields. Thus, the values obtained represent the mean values of absorption of 450-nm light in each targeted dermis.
2.3 Preparation of cadaveric human skin samples
Normal face and buttock skin specimens, taken from Caucasian female cadavers (Fitzpatrick skin types21 I to III, ages 28–95 years old), were kindly provided by Obio, LLC, complying with all applicable laws, rules, and ethical regulations. It was confirmed prior to the study that Obio had obtained written informed consent. All specimens were identified with randomly assigned codes during the experiments. The use of human skin specimens was approved by the Shiseido Ethics Committee. After the skin was isolated, the specimens were immediately embedded in an OCT compound (Sakura finetek), frozen in liquid nitrogen, and stored at −80°C. The specimens were cut into frozen serial sections (thickness = 7 μm) using a microtome. The unstained sections were washed three times in PBS for 5 min to remove the OCT compound, immersed in PBS, and enclosed with a cover glass. These sections were observed using a transmission microscope with the HFI analysis function.
2.4 Histochemistry
The degree of actinic elastosis (also known as actinic keratoses caused by photodamage) was demonstrated using resorcin-fuchsin stains. The degree of actinic elastosis was scored as described by Kligman,22 that is, G0, no change; G1, increase in number without thickening; G2, greater hyperplasia with thickening and curling; G3, marked hyperplasia with thickening and curling and frequent branching; and G4, complete replacement of the dermis by a dense tangle of thickened, disorderly fibres accompanied by disorganization into murky amorphous masses.
For the detection of carbonylation, a marker of tissue degeneration, acrolein adduct was immunohistochemically detected using a monoclonal anti-acrolein antibody (5F6, Nippon Oil & Fats) as a primary antibody and followed by serial reactions with anti-mouse Ig conjugated to peroxidase polymer (DAKO) and 3,3′-diaminobenzidine (DAB) staining.
3 RESULTS
3.1 Applying HFI analysis to human skin tissue
To confirm that the HFI analysis could also work under the microscope to distinguish D(p) (mainly transmitted light) from G(p) (mainly scattered light), we compared how skin tissue sections were imaged using a facial skin specimen. Figure 2B shows the unstained facial skin section under bright-field mode, which shows the obvious melanin deposition in the epidermal basal layer and turbid dermis with scattering from the extracellular matrix. Figure 2C shows the pattern-illuminated transmission images which were used for the calculations. Figure 2D is the spectroscopy image of transmission at 450 nm using a hyperspectral camera imaging. It shows the scattering and apparent shadows resulting from dermal fibre blockage. Because de-scattering was not performed, absorption signals were unclear, just as with the bright-field image in Figure 2B. In contrast, in Figure 2E, the apparent shadows from fibres are not considered absorption signals. In particular, the signal from the fibres in the subdermal layer that appear to be collagen fibres (judging from subsequent EVG staining; data not shown) has been eliminated. Furthermore, hardly any absorption signal was detected from the epidermis (other than in the basal layer, including melanin), which is known to have low scattering.1, 23 These results suggest that the system is able to separate the scattering and remove apparent absorption from real absorption.
3.2 Histological distribution of optical absorption in photo-aged facial skin
The dermis is thought to degenerate with long-term UV exposure, causing yellow discoloration in the skin.3, 24 We focused our analysis on the facial cheek skin of people exposed to long-term irradiation and those who were not. First, we selected specimens that were significantly degenerated, which were expected to have yellow discoloration, and specimens that were expected to have less sun exposure and, therefore, little or no discoloration. To confirm the degeneration, acrolein adduct staining was performed to indicate the denaturation of proteins following aldehyde modification.
We selected specimens from a 31-year-old subject as the benchmark for little or no degeneration (native case). A 62-year-old subject was selected as the significantly degenerated case. As the staining from anti-acrolein adduct (brown area) in Figure 3B shows, the specimen from the degenerated case had a prominently stained dermis. The specimen from the native case in Figure 3A was not stained at all.
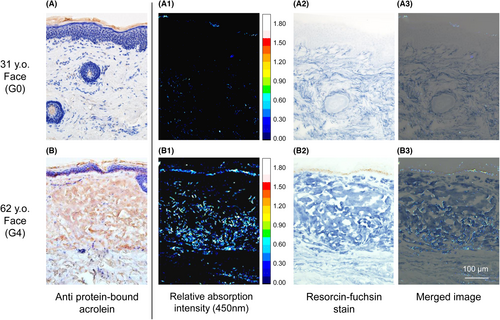
Second, we measured the unstained skin sections of both the native and degenerated cases for 450 nm absorption with a HFI microscopy analysis system. The results show the distribution of relative absorption intensity (Figure 3A1,B1). Elastic fibres were stained subsequently with resorcin-fuchsin staining in deep blue area (Figure 3A2,B2). Figure 3A3,B3 are the merged images of the distribution of elastic fibres and absorption at 450 nm. These images clearly showed that the region with relatively high absorption at 450 nm coincided with the distribution of elastic fibres from a degenerated dermis. In the dermis from the native case, in which no degenerated elastic fibres were observed, no noticeable absorption was seen.
Relatively high absorption was also observed from melanin, which is one of the most important chromophores (pigment) in the skin. As shown in Figure 3B3, the specimen from a 62-year-old showed significant melanin deposition in the basal layer of the epidermis. It also coincided with the area where clear optical absorption was observed. From the specimen from a 31-year-old, no melanin deposition was observed histologically, and thus no absorption at 450 nm was seen (Figure 3A3).
In Figure 3A1,B1, a tiny signal from the stratum corneum in the top layer of the skin (considered as artefacts from scattering elements that could not be separated because of optical absorption in the stratum corneum or very strong scattering) was detected.
3.3 Evaluation of the photoaging process using HFI microscopy analysis
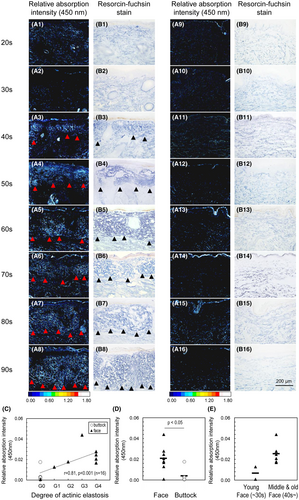
To confirm that this analysis is able to evaluate the process of photoaging, the changes in optical absorption distribution were measured using sun-exposed and sun-protected skin specimens from subjects aged in their 20s to 90s. The grade of degeneration of elastic fibre, a photoaging marker, was classified according to Kligman's method.22 G0 represented no denaturation (thin normal elastic fibres). G4 represented a progressing case (thickening and prominent deposition in the upper dermis). Immunohistochemical examination with anti-acrolein antibody revealed that the denaturation of protein, where positive staining can be seen, was observable in the upper dermis of sun-exposed facial specimens in subjects from their 40s (Table 1). Figure 4A1–A16 shows the distribution of optical absorption (450 nm). Simultaneously performed resorcin-fuchsin staining Figure 4B1–B16, photoaging (abnormal elastic fibre deposition, as shown in the black triangles in Figure 4B1–B8) was only detected in sun-exposed areas of the upper dermis, beginning from approximately 40 years of age, as with previously reported. Notably, an increase in absorption at 450 nm was also observed at the same site, as shown in the red triangles in Figure 4A1–A8. In addition, we quantified the mean absorption intensity in the region of the upper reticular dermis (from the basal layer to a depth of 200 μm). This region is known to develop elastosis, which is the benchmark of photoaging in the dermis with advancing age.22, 25 Subsequent analysis showed a statistically significant correlation between the quantified values and grade of actinic elastosis (Figure 4C). Spearman's rank correlation was used for this analysis, and the resulting correlation coefficient, r, was 0.81 (p < 0.001, n = 16). We also examined the differences between the non-exposed areas (buttocks) and the exposed areas (faces), using the non-parametric Wilcoxon signed-rank test, and confirmed that the non-exposed areas had significantly lower absorption intensity (p < 0.05) than the exposed areas, and an absence of abnormal elastic fibre deposition (Figure 4D). Furthermore, an increase in optical absorption from the exposed facial area was observed in the specimen taken from the middle and old aged donors (older than the 40s) (Figure 4E), similar to the age-related onset of abnormal elastic fibre deposition (Figure 4B1–B8).
| Case No. | Age | Degree of actinic elastosisa | Reactivity with anti-acrolein adduct antibodyb | Degree of actinic elastosisa | Reactivity with anti-acrolein adduct antibodyb |
|---|---|---|---|---|---|
| Site | Sun-exposed | (face) | Sun-protected | (buttock) | |
| 1 | 28 | G1 | − | G0 | − |
| 2 | 31 | G0 | − | G0 | − |
| 3 | 49 | G2 | ± | G0 | − |
| 4 | 54 | G3 | + | G0 | − |
| 5 | 62 | G4 | ++ | G0 | − |
| 6 | 77 | G4 | ++ | G0 | − |
| 7 | 83 | G4 | ++ | G0 | − |
| 8 | 95 | G4 | ++ | G0 | − |
- a Degree of actinic elastosis was scored according to the Kligman criteria (1969).22
- b ++, strongly positive; +, moderately positive; ±, partially positive; − negative.
4 DISCUSSION
The light irradiated into the skin is absorbed by various chromophores. The main chromophores of human skin are haemoglobin and melanin.1, 2, 26 Melanin is located in the epidermis, and the basal layer contains melanocytes, which produce the melanin pigment. Haemoglobin is a chromophore that exists in the microvascular network of the dermis. Variations in the physiological hemodynamic processes within this vascular network profoundly influence cutaneous coloration. In addition to the major chromophore, minor chromophores such as bilirubin or carotenoid are reported to be the source of yellowish skin discoloration in pathologic conditions. Recently, the fibrous component of the dermis has also been reported to be the source of yellowish discoloration in the older facial skin.3, 24 The development of yellowish skin colour alterations associated with photoaging is noted as one of the biggest aesthetic concerns for elderly East Asian people. In the past, we analysed Japanese bulk skin tissue and found that dermal degeneration represented by carbonyl modification causes yellowish discoloration in elderly people.3 In this study, we described for the first time the yellowish discoloration in Caucasians as well. The yellowing appearance is not often mentioned as a skin concern for elderly Caucasians; however, the degeneration of the dermis and its relation to yellowish discoloration is a common phenomenon. One of the typical concerns regarding skin discoloration in elderly Caucasian people is redness, such as in rosacea. Rosacea is a chronic skin condition that leads to redness with small and superficial dilated blood vessels affecting the face. Because the absorption by chromophores such as haemoglobin is fairly high, the mild yellowish discoloration of the dermis may be masked by discoloration resulting from vasodilation. Furthermore, skin colour is greatly influenced by melanocyte pigments present in the epidermis over the dermis, so there will be individual differences in how dermis yellowness affects whole skin colour.
In investigating the optical properties of tissue, assessing the optical absorption correctly has been a crucial concern. Because living tissue contains scatterers, including cells, the extracellular matrix, and substrates,1, 4, 23 the absorbance by chromophores is masked by scattering, and the identification of substances causing discoloration is distorted. The dermis contains strong scattering components, with abundant collagen, and recently it has been revealed that collagen volume has a certain influence on skin brightness.27 Therefore, overcoming the uncertainty resulting from collagen fibre-related scattering is thought to be key in correctly evaluating skin's optical properties. Thinning the tissue sample is one of the solutions to prevent scattering as this could simplify the absorption assessment. For thin samples, the sensitivity of measuring absorption and the limitations of the detection range are major concerns. Recently, spectroscopic (hyperspectral) camera technology and spectroscopic (hyperspectral) systems have become available for microscopic imaging and have been introduced in the field of skin pathology. The high-resolution images of spectral alteration provided by this technology enable the targeting of morphological and spectral changes, and pathological identification. This is expected to contribute to the development of diagnostic medical technology.18, 19 Meanwhile, our target is the absorption analysis of normal dermal tissue sections without staining to histologically analyse the colour (optical absorption) of samples. However, even under a microscope, our observation of the frail target of dermis coloration revealed turbidity in the images due to scattering from tissue sections making it difficult to measure absorption accurately which made it difficult to measure absorption accurately Figure 2B. When observing skin tissue using a conventional transmission microscope, the skin must be sliced as thinly as possible (≤5 μm). This is to prevent overlapping optical paths and to inspect only the layer that is under observation. It prevents overlapping optical paths and allows for the inspection of only the image of the layer that is under consideration. However, when trying to obtain an optical absorption signal above the detection limit, the tissue section requires a certain sample thickness (>5 μm). Under this condition, as with bulk skin, the transmitted light is scattered, and absorption cannot be measured accurately. Even when analysing skin tissue without staining, separation of scattering and absorption are unavoidable problems. This is because the optical scattering coefficient of native skin tissue is overwhelmingly greater than the optical absorption coefficient.1, 2 Thus, the information contained in the optical absorption (colour) of the tissue cannot be accurately extracted without cancelling out the contribution of optical scattering.
In recent years, in the field of computational photography, the removal of scattered light has been proposed by combining high-frequency illumination and calculation methods. It has been shown that by projecting a high-frequency spatial pattern onto an object and digitally processing the captured image, it is possible to separate transmitted light and scattered light and sharpen the object into a turbid image. The principal of this method was proposed by Nayar et al.,15 and it has been widely used in the field of computational photography.23, 28-31 We applied HFI to the light source of a microscope and extended the functionality of this method, expanding the field of transmission light microscopy. We used the difference in observations between focused and unfocused illumination conditions and succeeded in removing scattering from the transmitted light.17
In conventional optical analysis methods, the change in optical absorption (colour) of microscopic structures could not previously be captured. Therefore, substances that are candidate causes of yellowing could not be narrowed down. In this study, microscopic observation of the optical absorption of each skin component revealed that the region of increased optical absorption at 450 nm is precisely the same as the abnormal deposition site of elastic fibres (Figure 3), clearly indicating that denatured elastin fibres are the cause of the yellowish colour. Until now, with the limitations of absorption analysis sensitivity with a conventional microscope system, it could only be speculated that degenerated elastic fibres in the photodamaged dermis would be yellow. Clear evidence was difficult to obtain under a microscope, and speculations were made using bulk specimen evaluation.3 However, in this study, using an HFI microscopic analysis system, we were able to determine the degeneration of elastic fibre as the main cause of the optical absorption changes (increase in 450 nm absorption, yellowing).
Even in the region of the dermis, where the scattering is strong, the histological distribution of optical absorption is clearly shown. This reveals that the analysis of optical absorption separated from scattering by an HFI microscopic analysis system is useful for microscopic observation of the skin. It will be useful in clarifying the relationship between histological changes and optical absorption using microscopy. We believe that this novel technique may shed light on the evaluation of both histological and optical observations.
No one would deny that appearance is a key indicator in diagnosis. Recently, many efforts have been put into developing artificial intelligence (AI) for image recognition to make diagnoses using appearance data.32-34 They demonstrate the potential of hyperspectral imaging and highlight the importance of using machine-learning and deep-learning algorithms to analyse medical images. Their aim is to develop algorithms and models to process and analyse medical images. In contrast, this paper aims to assess the process of facial skin photodamage through HFI based microscopic light transport analysis. Our focus is removing the scattering of light within the skin and providing accurate optical absorption properties of the target layer. For highly accurate diagnosis, information of accurate optical absorption, which is an essential aspect of appearance and tissue's biochemical properties, may be worth using in machine learning. There is no doubt that histological understanding of optical absorbance will help achieve a comprehensive understanding of discoloration at lesion sites.19 This accumulated knowledge may eventually lead to further improvements in the accuracy of non-invasive AI diagnostics and thus accelerate their practical application. For example, AI algorithms have already been attempted to identify mycosis fungoides, psoriasis, and atopic dermatitis using single shot images,33 and adding the spectral information is expected to increase the identification accuracy. In addition, it may also become possible to non-invasively distinguish keratoacanthomas from non-pigmented lesions or SCCs, which are currently difficult to identify by appearance, to distinguish without biopsy by using information regarding the optical properties of each pathological feature.
In this study, we have shown that an HFI system is an effective tool for understanding the histological distribution of optical absorption and micro-level positional information. Our novel technique is also useful for assessing skin colour and other pathologies. This concept of analysis, which combines not only pathology and optics, but also computer vision, is expected to provide a breakthrough in the accumulation of reliable optical data for various pathological conditions. We hope that our research will contribute to the development of non-invasive and reliable diagnosis in the future.
In summary, we applied the HFI method to evaluate several skin specimens and succeeded in analysing the spatial-dependent optical absorption separate from scattered light. This novel technique combined histological and optical approaches and helped us to assess the optical alterations in the skin's components at the micro-level. Specifically, we were able to determine the degeneration of elastic fibres as being the main cause behind both the increase in optical absorption and discoloration. This result would not have been possibly if only histological or conventional optical methods were being employed. Furthermore, we quantitatively showed a high correlation between the optical absorption and conventional histological grading of cutaneous photodamage of the facial area. In this respect, this method is useful in assessing the process of facial skin photodamage from the increase of discoloration seen through optical absorption, which occurs only in sun-exposed areas of subjects aged more than 40 years. These results highlight the potential of this system as a powerful tool for not only the detection and diagnosis of photoaging but also for comprehensive pathological investigation.
AUTHOR CONTRIBUTIONS
All authors have conceived the idea of the study. Mihoko Shimano, Ryoma Bise and Imari Sato developed the HFI analysis system. Mihoko Shimano and Ryoma Bise conducted computational analyses. Imari Sato revised draft on intellectual content. Yuki Ogura performed the experimental study and drafted the original manuscript. Toyonobu Yamashita drafted and contributed to the interpretation of the results. Chika Katagiri contributed to the interpretation of the results and supervised the conduct of this study. All authors have read and approved the final manuscript.
ACKNOWLEDGEMENTS
This research was supported by the JST (Japan Science and Technology Agency) ImPACT program. We would like to thank Seiichi Sugino for his technical assistance. We would also like to thank Branko Unkovski-Korica, from Ten-Nine Communications, for editing a draft of this manuscript. We thank John Holmes, MSc, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
CONFLICT OF INTEREST STATEMENT
Yuki Ogura, Toyonobu Yamashita and Chika Katagiri are employees of Shiseido Co., Ltd.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




