Topical silver and gold nanoparticles complexed with Cornus mas suppress inflammation in human psoriasis plaques by inhibiting NF-κB activity
Funding information
M.C., L.O. and A.F. were funded by “Partnerships in priority areas-PN II” (frame project no 147/2012) of ANCS, CNDI-UEFISCDI, Romania.
Abstract
New biomaterials based on nanoparticles (NPs) carrying polyphenols-rich extracts (Cornus mas) recently showed promising anti-inflammatory activity in psoriasis. We aimed to understand how topically delivered silver and gold nanoparticles complexed with Cornus mas (Ag-NPs-CM, Au-NPs-CM) modulate inflammation in psoriasis at cellular and molecular level. The impact on psoriatic inflammation was assessed in vitro on pro-inflammatory macrophages, by clinical score, high-frequency ultrasonography and immunohistology of psoriasis plaques treated with Ag-NPs-CM, Au-NPs-CM or control. Incubation of pro-inflammatory macrophages with nanoparticles significantly decreased the release of NO, IL-12 and TNF-α. Immunofluorescence confirmed that nanoparticles significantly reduced CD68-positive macrophages and their IL-12 and TNF-α production in human psoriasis plaques. NPs-CM appear to repress NF-κB activation in macrophages, inhibiting the production of pro-inflammatory factors with causal role in psoriasis. Ag and Au NPs-CM represent a novel nanoparticle-based “green” technology which may provide an efficient tool for modern psoriasis therapy, circumventing immunosuppression-related side effects of biologicals.
1 BACKGROUND
TNF-α and IL-12 represent key pathogenic mediators in human psoriasis and first-line targets of effective therapies; their systemic use, however, is limited by immunosuppression-related side effects.1 Selective TNF-α and IL-12–producing pro-inflammatory macrophage inhibition in psoriasis skin lesions by topical drug delivery would represent a safe alternative, but was not reported so far.
New metallic nanoparticles (NPs) carrying polyphenols-rich extracts recently showed promising anti-inflammatory activity.2 Metal particles alone may exert toxic effects by reactive oxygen species induction; the green method of synthetizing nanomaterials with anthocyanin molecules from natural extracts counteracts these potential toxic effects, making nanomaterials stable and safe.3
2 QUESTIONS ADDRESSED
We aimed to understand the effect of silver and gold nanoparticles complexed with polyphenols-rich Cornus mas extract (Ag-NPs-CM, Au-NPs-CM) on inflammatory macrophages in vitro and human psoriatic plaques.
3 EXPERIMENTAL DESIGN
Ag-NPs-CM and Au-NPs-CM nanoparticles were previously synthesized (frame project no. 147/2012, “Partnerships in priority areas-PN II,” of ANCS, CNDI-UEFISCDI, Romania)4, 5 and characterized by in vivo and in vitro toxicity studies (Data S1, Figures S1a,b and S2).6
To investigate their anti-inflammatory effect, bone marrow-derived murine macrophages, pro-inflammatory stimulated with LPS (10 ng/mL) and IFNγ (50 IU/mL), were incubated with NPs (Au-NPs-CM concentration: 0.165 mg/mL, Ag-NPs-CM concentration: 0.19 mg/mL, dilutions 1:10, 1:100), and their release of NO, IL-12 and TNF-α was measured by ELISA.7 After obtaining approval of the Ethics Committee (Medical University Cluj-Napoca, Romania) and informed consent, 24 patients exhibiting similar disease severity were treated with 2% Ag-NPs-CM/Au-NPs-CM–based ointments or positive control 0.5% hydrocortisone 1× daily for 6 weeks. Immunohistology was performed on skin cryosections of plaques treated with nanoparticles-based ointments/control, to assess the variation of TNF-α and IL-12–expressing macrophages.8 As TNF-α perpetuates macrophage activation in psoriasis by NF-κB pro-inflammatory pathway activation, NF-κB was investigated in in vitro-stimulated macrophages and macrophages infiltrating psoriasis plaques upon treatment with NPs-based/control ointments.9 Western blot for phosphorylated-IκBα was performed with lysates from nanoparticles-treated M1 macrophages and skin biopsies. With high-frequency ultrasound, we investigated the dermal echogenicity variation in the treated plaques. The statistical significance was determined by two-tailed Student's t test. P values <0.05 were considered statistically significant.
4 RESULTS
In vitro, nanoparticles remarkably suppressed NO, TNF-α and IL-12 secretion from pro-inflammatory macrophages in a dose-dependent manner, Au-NPs-CM being more effective than Ag-NPs-CM (Figures 1A and S2). The smaller size of Au-NPs (13-52 nm), compared with Ag-NPs (9-82 nm), allowing better cell penetration\activity, may explain the variable efficacy.10
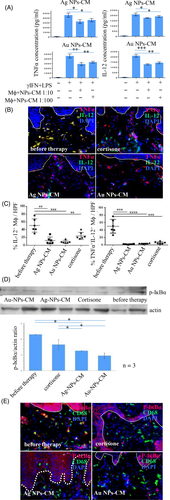
Topical NPs-CM significantly decreased IL-12 and TNF-α–producing CD68-positive cells more efficiently than cortisone in treated plaques, as demonstrated by immunohistology and quantification of double-positive cells (Figure 1B,C). As macrophages are the major cellular source of IL-12 in psoriasis plaques, we considered the IL-12+TNF-α+ double-positive cells as a population predominantly consisting of macrophages. A small-cell population may however be also represented by myeloid dendritic cells which may be activated and release IL-12+TNF-α+ as well.
Phosphorylated-IκBα was assessed as a measure of NF-κB activation in macrophages infiltrating human psoriasis plaques.8 Western blot of skin lysates revealed a significant decrease of phosphorylated-IκBα in psoriasis plaques upon Au-NPs-CM and Ag-NPs-CM treatment (Figure 1D). CD68 and phosphorylated-IκBα immunostainings demonstrated the specific inhibition of NF-κB activation in psoriatic skin-infiltrating macrophages by NPs-CM (Figure 1E). This mechanism probably underlies the sustained pro-inflammatory cytokines suppression from activated macrophages in vitro and psoriasis lesions by NPs-CM application.
NPs-CM treatment resulted in significant subjective and clinical improvement of psoriasis plaques, with reduced scaling, erythema and plaque thickness in patients treated for 6 weeks (Figure 2A). Quantification of low echogenity pixels as measure of dermal inflammatory infiltrate by high-frequency ultrasound showed that NPs-CM suppressed the infiltrating inflammatory cells in psoriatic plaques, with Au-NPs-CM being again more effective than Ag-NPs-CM. We also found a reduction in plaque thickness in NPs-CM–treated psoriatic skin, suggesting a decrease in the hyperproliferative epidermal and massive inflammatory dermal component (Figure 2B).
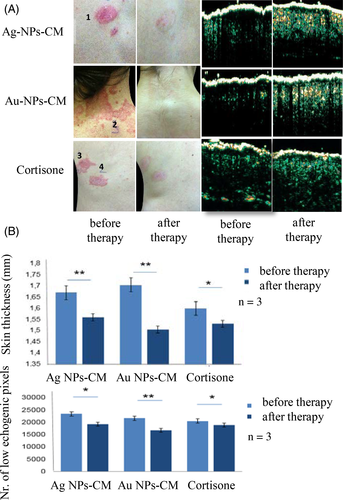
NPs-CM were more efficient in suppressing macrophage activation and cytokine production than hydrocortisone, probably due to the better bioavailability of NPs-CM to the inflammatory infiltrate in psoriatic plaques. Importantly, there were no treatment-related side effects for the NPs-CP ointment. This observation is consistent with reports from other studies in humans where NP therapy showed excellent long-term tolerability.4
As for penetration and toxicity concerns, based on others and our own studies, we suggest that the medium-sized nanoparticles applied at concentrations in the lower published range in this study may have penetrated the superficial epidermal layers and the hair follicles of the inflamed psoriatic skin. Their elimination most probably occurred by epidermal turnover and with some delay by sebum production from the follicular appendages (See Data S2). We suggest that further studies with different concentrations and treatment schedules need to be performed in order to improve the anti-inflammatory effect of the here studied particles.
5 CONCLUSIONS
Nanoparticles proved promising therapeutic tools in preclinical models of skin infection, inflammation and cancer, especially on keratinocytes, as also previously shown by our group.11, 12, 2 We demonstrated for the first time that, apart from suppressing keratinocytes, NPs specifically and efficiently inhibited macrophage activation in vitro and in human psoriasis plaques, eventually resulting in disease resolution. We describe an unreported role of metallic polyphenol-enriched nanoparticles to decisively contribute to suppression of NF-κB activation in macrophages in vitro. This effect appears to be amplified in vivo in inflamed psoriasis skin, inhibiting the production of pro-inflammatory cytokines with causal role in psoriasis pathogenesis (TNF-α, IL-12). The complex mechanism of dampening macrophage activation, eventually resulting in clinical clearance of psoriasis plaques needs to be elucidated in future studies.
Our results highlight the therapeutic potential of an innovative nanoparticle-based “green” technology, which may provide a modern, safe and efficient tool for modulating inflammation as occurring in psoriasis and other autoimmune diseases, and for circumventing often limiting side effects of systemic therapies.
ACKNOWLEDGEMENTS
DC, AS, MC, AH and SS performed research. DC, AS and MC analysed data and wrote the manuscript. AS, MC and LO designed the research study. AS and KSK supervised the research study and critically revised the manuscript. LO, MC, KSK and LAS critically revised the manuscript. LO, AF and AS provided reagents and tools.
CONFLICTS OF INTEREST
The authors have declared no conflicting interests.




