Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts
Abstract
This study was undertaken to evaluate whether exosomes from human adipose-derived stem cells (ASC-exo) can stimulate the regeneration of human dermal fibroblasts (HDFs). Immunoblotting and FACS analyses showed that ASC-exo was positive for exosome markers. Fluorescence tracking revealed that the contents of ASC-exo were transferred into the HDFs. ASC-exo treatment also stimulated the proliferation and migration of HDFs in a dose-dependent manner. Similarly, the expression levels of genes involved in skin cell proliferation were increased by ASC-exo. Microarray analysis showed an enrichment of microRNAs that have regenerative function. We suggest that the ASC-exo can stimulate skin cell proliferation.
1 BACKGROUND
It has been well reported that adipose tissue-derived stem cell (ASC) secretes various soluble factors, that is growth factors, cytokines, exosomes that can rescue damaged cells.1 Exosome, a 30- to 150-nm-sized extracellular vesicle that is secreted from most cell types, is formed within endosomal compartments and released into the extracellular milieu.2 It has been demonstrated that exosomes possess similar functional properties of mesenchymal stem cells (MSCs) from which they are derived,3 and due to such functions, exosomes are regarded now as a communicator between tissues.4 Functionally, exosomes can reduce the immune recognition, so that the integrity of cell membrane can be maintained.5 A study of stem cell transplantation therapy demonstrated that the role of MSCs in cell-to-cell communication was exerted through a paracrine mechanism and that exosomes play a major role in this process.6 Other findings also described that the conditioned media of ASC (ASC-CM)7 or exosomes8 were able to promote the migration of dermal fibroblasts during the process of wound healing.7 Although the mechanism of the exosomes’ role on proliferation and migration of fibroblasts was demonstrated,8 no study has been conducted to identify the expressional changes of microRNAs, which play major role in various cellular responses, including proliferation.
2 QUESTIONS ADDRESSED
We asked whether ASC-derived exosomes can stimulate the skin dermal fibroblasts’ proliferation and migration, and by what mechanism these exosomes play such functions.
3 EXPERIMENTAL DESIGN
4 RESULTS
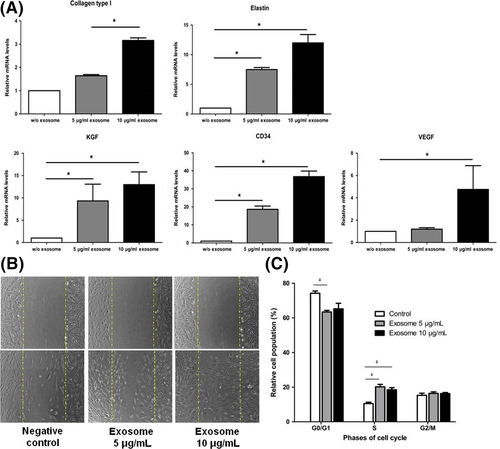
Transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA) showed that ASC-exo isolated from the supernatants of the ASC culture showed the typical morphology of exosomes that are within the range of 70-150 nm (Fig. S1A). Western blot analysis revealed that ASC-exo expresses exosome markers HSP70, CD63 and CD9 (Fig. S1B), while these markers were absent in ASC lysate. Flow cytometry analysis also showed that ASC-exos were positive for CD9, CD63 and CD81 (Fig. S1C). We next asked whether the contents of ASC-exos could be transmitted to HDFs. After co-incubation, for 24 hours, of PKH26-labelled ASC-exos with HDFs, it was found that red fluorescence of PKH26 was localized within the cytoplasm of HDFs (Fig. S1D), showing that ASC-exo can be internalized into HDFs. Also, quantitative real-time PCR showed that the expression of genes related with skin regeneration, such as CD34, collagen type 1, elastin and keratinocyte growth factor (KGF), was increased after being treated with ASC-exos in a dose-dependent manner (Figure 1A). We also found that ASC-exo stimulated the proliferation of HDFs and that such effect was significantly higher in 10 μg/mL compared with those treated with 5 μg/mL (Fig. S2). In line with this finding, we found that the migration of HDFs was increased in a similar manner (Figure 1B). Similarly, cell cycle analysis by FACS demonstrated that ASC-exo treatment significantly increased the population of those within S-phase (Figure 1C).
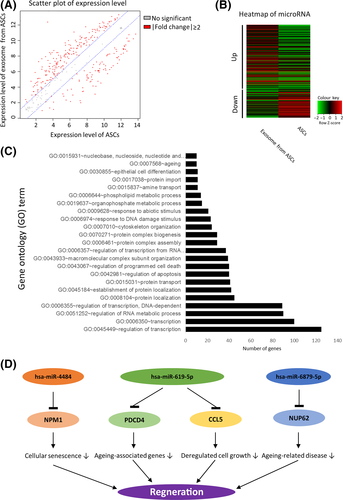
We next conducted a comprehensive analysis of microRNA expression between ASC and ASC-exo by microarray. We selected the microRNAs expressed differentially (>2-folds, Figure 2A) and found that 292 among 333 microRNAs were differentially expressed (199 and 93 upregulated and downregulated, respectively, in ASC-exo) (Figure 2B). These differentially expressed microRNAs were grouped based on function, as referenced with miRTarBase (http://http://mirtarbase.mbc.nctu.edu.tw/). The 718 validated gene targets of top 10 upregulated miRNAs were grouped according to the gene ontology (GOTERM_BP_FAT) with an enrichment EASE score < 0.05 and 10 more than number of target genes (Figure 2C). The role of ASC-exo microRNAs with their corresponding validated target genes was schematically represented in Figure 2D. From this results, it can be concluded that exosomes from ASC contain microRNAs that can inhibit genes including NPM1, PDCD4, CCL5 and NUP62, thereby contributing to the regeneration of skin fibroblasts by stimulating the proliferation of dermal fibroblasts.
5 CONCLUSION
Recent studies have demonstrated that exosomes are involved in the paracrine activity of stem cells.9 This study demonstrates that the exosomes derived from ASCs play a prosurvival role in human dermal fibroblasts by stimulating its cell cycle and that microRNAs that are known to have regenerative potentials were enriched in ASC-exo. Our finding may be of useful for developing novel therapeutic methods for the enhancing wound healing.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
EW Choi performed the research, designed the study, analysed the data and wrote the manuscript. MK Seo, EY Woo, SH Kim and EJ. Park designed the study and performed the research. S Kim edited the manuscript.




