Individual Discrimination Within, but Not Between, Two Vocalization Types of the Black-Capped Chickadee
Funding: This work was supported by Natural Sciences and Engineering Research Council of Canada. Canada Foundation for Innovation. Canada Research Chairs.
ABSTRACT
Many songbird species use individual vocal recognition in their social behaviors. Researchers commonly use individual discrimination tasks, such as operant conditioning Go/No-go tasks, to assess individual vocal recognition. Several black-capped chickadee (Poecile atricapillus) vocalizations contain individually distinct features which may be used for individual discrimination. However, not all such vocalizations have been tested for individual recognition with live birds. Additionally, cross vocalization generalization of learned individual discrimination has not been tested. Such generalizability would be advantageous for chickadees, as chickadees often communicate outside of visual contact and use vocal communication to guide their social interactions. Here we test whether black-capped chickadees can discern individual identity of callers in black-capped chickadee chick-a-dee calls. We also aim to answer whether chickadees can generalize learned individual discrimination using chick-a-dee calls to fee-bee songs, and vice versa. Black-capped chickadees were trained to discriminate several chick-a-dee calls or fee-bee songs produced by one male and one female chickadee from those produced by a different male and female in an operant conditioning Go/No-go experiment. We then tested for generalization across vocalization types by presenting birds with recordings from the same four individuals, this time of the opposing vocalization type. Chickadees were able to discriminate between individuals using either chick-a-dee calls or fee-bee songs but were unable to generalize this learning to the opposing vocalization type. While our findings suggest that chickadees can employ individual discrimination within at least two vocalization types, the mechanism by which songs and calls are recognized as belonging to the same individual remains unclear. External contextual cues may play an important role in bridging identity information across those vocalization types.
Individual vocal recognition (IVR) refers to the ability of signal receivers to glean identity information from the vocalizations of conspecific signal producers, such that the receiver may distinguish one producer from another (Carlson et al. 2020). IVR is thought to benefit receivers by allowing them to quickly determine whether, how, and to what extent the information contained within the producer's vocalization is relevant to them as the receiver (Carlson et al. 2020). Receivers may then modify their behavior in response to the content and context of the information provided by producers accordingly, such as avoid a dominant conspecific or territorial aggressor (Lambrechts and Dhondt 1995), approach a potentially threatening stranger but ignore familiar neighbors (Brooks and Falls 1975), or approach kin or mates in distress (Warrington et al. 2014). In turn, producers benefit from their vocalizations being individually distinct by encouraging familial or group cooperation from the receiver (Carlson et al. 2020; Kastein et al. 2013; Zsebők et al. 2017). Because IVR describes an internal process, it cannot be measured directly (Carlson et al. 2020). Therefore, external observations of individuals' behavior are required to infer IVR, which can be assumed when an individual behaviorally demonstrates the ability to discriminate between two or more individuals based on those individuals' vocalizations (Carlson et al. 2020). Individual vocal discrimination (ID), an observable behavior often used as a proxy for IVR, has been widely observed across the animal kingdom (e.g., Amphibia- Chuang et al. 2017; Feng et al. 2009; Mammalia- Ceugniet and Izumi 2004; Kastein et al. 2013; Lemasson et al. 2013; Aves- Brooks and Falls 1975; Montenegro et al. 2020; Weary and Krebs 1992). Moreover, there are many examples of suspected capacity for ID based on observed inter-individual vocalization variability (e.g., Bertucci et al. 2012; Bloomfield et al. 2004; Charrier et al. 2004; Frommolt et al. 2003; Gasser et al. 2009; Guillette et al. 2010; Mammen and Nowicki 1981; Volodina et al. 2006; Warrington et al. 2014; Zsebők et al. 2017); however, individually distinctive acoustic features, although required for ID, are not sufficient to demonstrate ID, as ID necessitates the perception and use of such distinct individualistic features on the part of the receiver (Carlson et al. 2020).
Songbirds serve as a popular model to study ID and IVR (Carlson et al. 2020), due at least in part to the fact that songbirds often communicate outside of visual contact and rely heavily on vocally relaying information among conspecifics (Ficken et al. 1978; Kondo et al. 2010; Mammen and Nowicki 1981). Accordingly, many songbird species have been shown to discriminate among individuals, and do so using varying degrees of specificity. When a signal receiver is able to identify a producer –such as a familiar neighbor or a mate– among other producers, this demonstrates IVR-singular (Carlson et al. 2020). For example, male white-throated sparrows (Zonotrichia albicollis) approached more closely to playback speakers, and responded more quickly, with higher rates of singing and more flight responses, to strangers' songs than to familiar neighbors in a field playback study (Brooks and Falls 1975). Similarly, female great tits (Parus major) emerged from their nests exclusively in response to their mates' songs but not to strangers' (Lind et al. 1996), and almost exclusively to mates' songs compared to neighbors' songs (Blumenrath et al. 2007). In these cases, a single individual was recognized among others at any given time. In contrast, the ability to discriminate among several individuals demonstrates a much finer recognition system known as IVR-multiple, where a signal receiver is able to recognize several producers within a group, and identify one producer from another (Carlson et al. 2020). For example, jungle crows' (Corvus macrorhynchos) ka contact calls exhibit individual variability which jungle crows used to discriminate among familiar individuals in an operant conditioning paradigm (Kondo et al. 2010). Similarly, brood-dividing black redstart (Phoenicurus ochruros) parents responded more reliably to the begging calls of fledglings they had been preferentially feeding than to fledglings they had not been preferentially feeding (Draganoiu et al. 2006). In these cases, receivers were able to discriminate among multiple individuals' vocalizations, and recognized one specific individual from another.
Black-capped chickadees (Poecile atricapillus) are cold-tolerant birds that live nearly continuously across the United States and Canada (Smith 1991, 1; Weisman and Ratcliffe 2004), flock exclusively in the fall and winter months, live monogamously in the spring and summer months (Smith 1991, 6), and whose acoustic communication has been extensively studied. Black-capped chickadees are an ideal model species for investigating IVR because much of their territorial and breeding behaviors are mediated by vocal communication, and they rely on the ability of the receiver to distinguish between individuals, such as eavesdropping females distinguishing between two males' songs (Mennill and Otter 2007; Wilson and Mennill 2010). One commonly examined vocalization of the black-capped chickadee is their namesake chick-a-dee call, a relatively acoustically complex and highly variable call most often used in social contexts and in flock coordination, which consists of up to five note types (A, B, C, D, and D-hybrid), any of which may be repeated or omitted (Ficken et al. 1978; Smith 1991, 66–74). Bioacoustical analysis of the chick-a-dee call has found these calls to be more variable between individuals than within (Charrier et al. 2004; Mammen and Nowicki 1981), supporting their function in ID. Specifically, Mammen and Nowicki (1981) found several spectral (D note peak frequency, D note maximum frequency, D note bandwidth), temporal (D note duration, introductory note duration, total call duration, interval between introductory notes, interval between D notes, interval between introductory and D notes), and structural (number of D notes and number of introductory notes) features of the chick-a-dee call to be significantly different between individuals. Similarly, Charrier et al. (2004) found that various acoustic parameters of A, B, and C notes were significantly different among individuals, and D note duration, maximum frequency, and frequency of the first visible harmonic were also significantly different among individuals. To our knowledge, individual discrimination using the chick-a-dee call has not yet been empirically tested with birds.
Another widely investigated chickadee vocalization is the fee-bee song. Unlike most songbirds' songs which are acoustically more complex than their respective calls, the fee-bee song is acoustically simpler than the chick-a-dee call and is primarily used in territorial and reproductive contexts (Charrier et al. 2004; Ficken et al. 1978; Hahn et al. 2013; Smith 1991, 57–61). The fee-bee song consists of two notes, beginning with a high-pitched fee which observes a gradual decrease in frequency known as the fee glissando, followed by a lower frequency bee (Montenegro et al. 2020; Hahn et al. 2013; Smith 1991, 57–61; see review in Weisman and Ratcliffe 2004). As with the chick-a-dee call, bioacoustical analysis has revealed significant individuality within the fee-bee song. Hahn et al. (2013) found that total song duration, fee note proportional duration, fee glissando, bee note start frequency, internote interval ratio, relative amplitude of the fee and bee notes, and the root mean squared amplitude ratio of the fee note to the whole song were all individually distinctive features of the fee-bee song. Wilson and Mennill (2010) also found that male fee-bee songs are individually distinct, but took this one step further by testing the perception of chickadees to these individual differences. In their playback study, Wilson and Mennill (2010) observed that male chickadees remained in close proximity to song playback within their territories longer, and produced more songs, when presented with songs from an unfamiliar male than when presented with songs from an already primed male. Furthermore, Montenegro et al. (2020) were able to train both male and female black-capped chickadees to accurately discriminate among individual females' songs in an operant conditioning paradigm.
Interestingly, none of the aforementioned bioacoustical or behavioral studies tested whether individual vocal variation, or ID by birds, correlated across multiple vocalization types. However, it stands to reason that it would be advantageous to chickadees, which frequently communicate outside of visual contact (Ficken et al. 1978; Mammen and Nowicki 1981), to be able to recognize specific individuals regardless of the particular vocalization type being produced, as has previously been demonstrated in some mammal species (Cheney & Seyfarth), but not in birds. As an example, misidentifying a familiar neighbor calling from near the boundaries of one's territory as a threatening stranger only after that same neighbor starts singing could induce costly and potentially risky behavior which the chickadee may have otherwise avoided. It would therefore be advantageous for chickadees to learn to identify an individual based on one single vocalization type and be able to generalize this learning across other vocalization types. Since chickadees often communicate outside of visual contact (Ficken et al. 1978; Mammen and Nowicki 1981), it would be most advantageous for chickadees to be able to form this IVR generalization on the basis of available information contained within vocalizations.
While bioacoustical analysis suggests both chick-a-dee calls and fee-bee songs are individually distinctive, only the latter has been tested with birds. The first aim of the current study is to answer whether the long-standing assumption of ID in chick-a-dee calls actually holds true in black-capped chickadees. Our second aim is to answer whether black-capped chickadees can generalize learned ID across vocalization types, based solely on the individually-distinctive variation contained within the vocalizations themselves. We predicted that black-capped chickadees trained in an operant conditioning go/no-go paradigm, based on the methods of Montenegro et al. (2020) would be able to successfully discriminate between individuals using either recorded chick-a-dee calls or fee-bee songs. We further predicted that chickadees would continue to discriminate between the same individuals even when presented with recordings of the vocalization type they were not initially trained with.
1 Materials and Methods
1.1 Subjects
Prior to the study, all chickadees were housed in 1/4 individual segments of King's Cages Superior Stack Breeder cages (individual segments: 50.8 × 50.8 × 67.31 cm; King's Cages, East Brunswick, New Jersey) where they had visual and vocal contact with other chickadees within the colony room. Each cage segment was outfitted with three or more perches, and birds were given free access to food (Mazuri Insectivore Diet; Mazuri, St. Louis, MO, USA), water, oyster shell grit, and cuttlebone. Birds were supplemented with six to ten unshelled sunflower seeds and a half peanut daily, hard-boiled egg mixed with chopped greens (spinach or parsley) twice weekly, and with mealworms (Tenebrio molitor) dusted with vitamin solution (Prime Vitamin Supplement; Hagen Inc., Montreal, QB, Canada) three times per week. Colony rooms were lit approximately according to the natural light: dark cycle for Edmonton, Alberta, Canada, and were maintained at 20°C. Twenty black-capped chickadees were included in this operant conditioning study, each randomly assigned to one of four study groups (see Discrimination Training section below). Sixteen chickadees were captured from the E.H. Moss Forest Reserve of the Edmonton River Valley of Edmonton, Alberta, Canada, between February 2020 and February 2023. The remaining four birds were captured from Mill Creek Ravine in the Edmonton River Valley between January 2019 and December 2022. Birds ran variably from October 2023 to April 2024, averaging a little over 2 years in the lab before beginning the study. Earlier captured birds participated in other unrelated studies during this time. All 20 birds were captured as adults (at least 1 year of age) as assessed by examining outer tail retrices (Pyle 1997), and their sex was determined by DNA analysis of blood samples (Griffiths et al. 1998). During the study, chickadees continued on the Mazuri Insectivore Diet via the feeder when responding correctly to operant conditioning trials. Chickadees were also given water, cuttlebone, and oyster shell grit ad libitum. Chickadees were supplemented with two half peanuts and three mealworms daily.
1.2 Acoustic Stimuli
A total of 176 stimuli, 88 chick-a-dee calls and 88 fee-bee songs, were used in the study. Of these, each bird was presented with 132 of these stimuli depending on their group (see Procedure section below for specific stimulus allocation). Stimuli were created from recordings taken from two male and two female black-capped chickadees captured in the Edmonton River Valley between January 2009 and January 2014 as adults. None of the stimulus birds were housed in the lab at the same time as those birds used in the study. Stimulus birds were recorded individually within a sound-attenuating chamber (1.7 × 0.84 × 0.58 m; Industrial Acoustics Company, Bronx, NY) using an AKG C1000S (AKG Acoustics, Vienna, Austria) microphone positioned 10 cm above and toward the back of the cage connected to a Marantz PMD670 (Marantz America, Mahwah, NJ) digital recorder (16 bit, 44,100 Hz sampling rate). Recordings were taken between 2012 and 2017. A total of 22 chick-a-dee calls and 22 fee-bee songs produced by each stimulus chickadee were isolated from these recordings using SIGNAL 5.03.11 software (Engineering Design, Berkley, CA, USA). Individual vocalizations were first bandpass filtered (lower bandpass 400 Hz, upper bandpass 1400 Hz) to reduce background noise (Montenegro et al. 2020) using GoldWave version 6.31 (GoldWave Inc., St. John's, NL, Canada), before 5 ms of silence was added to the leading and trailing ends of the vocalization, and the stimulus was tapered to reduce “popping” during playback and normalized (RMS 1) using SIGNAL 5.03.11 software.
1.3 Apparatus
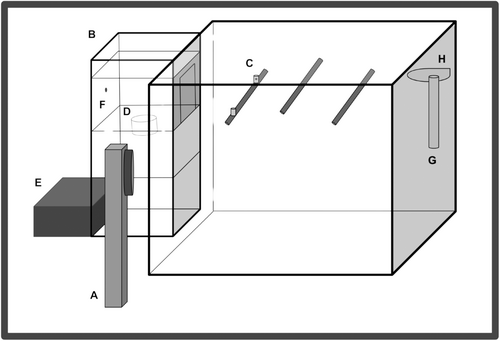
While completing the study, birds were housed in modified Jupiter Parakeet colony cages (30 cm × 30 cm × 40 cm; Rolf C. Hagen Inc., Montreal, QB, Canada) situated within sound-attenuating operant chambers (see Figure 1). The Jupiter cages were modified with an opening (11 cm × 16 cm) on the left side of the cage to permit birds access to the external automated feeder, within which access to feed was conditionally accessible. Jupiter cages were also outfitted with a request perch fitted with infrared light sensors secured 10 cm from the feeder opening, two non-request perches (one in the middle and one in right-hand thirds of the cage), and cuttlebone, a grit cup, a water bottle, and on the first day of pretraining and during periodic “break days” (2 days during pretraining and 1 day following each probe test), a free feed cup all on the right-hand wall of the cage. Also inside the operant chamber was a Fostex full-range speaker (model FE108 ∑ or FE108E ∑; Fostex, Japan; frequency response range: 80–18,000 Hz) located beside the feeder toward the front of the cage. The automated feeder, speaker, and request perch were controlled via a single-board computer (Palya and Walter 1993) connected to a personal computer and a Cambridge Integrated Amplifier (model A300, frequency response rang: 25–60,000 Hz or Azur 640A, frequency response range 4–80,000 Hz; Cambridge Audio, London, England; see Sturdy and Weisman (2006) for a detailed description of a similar set-up) located in an adjacent room. Operant chambers were lit according to the natural light: dark pattern for Edmonton, Alberta, Canada using full spectrum LED bulbs (3 W, 250 lm E26, Not-Dim, 5000 K; Lohas LED, Chicago, Illinois, USA).
1.4 Procedure
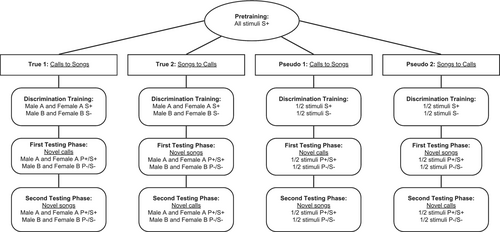
In short, birds in True groups were trained to discriminate either chick-a-dee calls or fee-bee songs based on the individual producer, while birds in Pseudo groups were trained to memorize individual chick-a-dee calls or fee-bee songs. We tested this learning first with a within-vocalization type generalization test, and then with an across-vocalization type generalization test (Figure 2).
1.5 Pretraining
Before beginning any discrimination training, birds were first trained to use the request perch and feeder through successive shaping. Birds learned to land on the request perch, thereby blocking the infrared beam, which would trigger a stimulus to be presented. Birds were initially trained with a visual stimulus (a red cue light) before graduating to a 1 s acoustic stimulus (1000 Hz tone). Birds would respond to these stimuli by leaving the request perch and moving into the feeder, where they would block another infrared beam, triggering a feed cup to rise to the top of the feeder, where it became accessible to the bird for 2 s. Once birds had mastered this routine, the acoustic stimulus was replaced with a set of all 132 stimuli the chickadee would hear throughout the study. This step encourages a high rate of response in successive stages and corrects unintended differential responding. Here, birds triggered the request perch, prompting one of the 132 stimuli to play. All stimuli were played in random order without replacement in each trial block. Chickadees were required to sit on the request perch for 900–1100 ms to trigger the perch and remain on the perch long enough to listen to the entire stimulus (1117–3891 ms) before entering the feeder to receive 2 s access to food. If birds left the request perch before the stimulus was finished playing, they were punished with 30s of darkness. A short period of darkness is commonly used to mark and reduce inappropriate responses (e.g., Montenegro et al. 2020; Sturdy and Weisman 2006). A 30s intertrial period, or 60s if the bird had failed to respond to the stimulus by entering the feeder, was required before birds could re-trigger the request perch. Because high response rates are required to acquire reliable data in later discrimination stages, all chickadees were required to respond to a minimum of 60% of trials for a minimum of 3168 trials. To ensure birds did not enter the discrimination task with any pre-existing bias, each had to have consistently demonstrated, over a minimum of 2112 trials, no more than 10% difference in response rates between calls and songs and between stimuli which would later be reinforced (S+) or punished (S−) during each discrimination stage.
1.6 Discrimination Training
Experimental groups were trained to discriminate either chick-a-dee calls (True 1) or fee-bee songs (True 2) on the basis of individual identity. Control groups were trained to discriminate either chick-a-dee calls (Pseudo 1) or fee-bee songs (Pseudo 2) on a pseudo-randomized basis. Each True group was made up of four males and two females, while each Pseudo group included three males and one female.
During initial discrimination training, chickadees were presented with 44 stimuli, 11 from each of the four recorded stimulus birds. As in pretraining, chickadees operated the apparatus by first landing on the request perch, triggering a stimulus to play. To ensure the entire stimulus was attended to, chickadees were again trained to remain on the perch for the entire duration of the stimulus and were punished with 30s darkness if they left the perch early. Once the stimulus had finished playing, chickadees had 1 s to either enter the feeder (Go) or ignore the stimulus by leaving the perch but not entering the feeder (No-go). When chickadees correctly entered the feeder during S+ trials, they were immediately rewarded with 2 s access to their feed cup, followed by a 30s intertrial interval. When chickadees incorrectly entered the feeder during S− trials, they were immediately punished with 30s lights out. Ignoring the stimulus during either S+ or S− trials resulted in a 0.5 s intertrial interval. Remaining on the perch (neither Go nor No-go) resulted in a 60s intertrial interval to encourage moving to or away from the feeder (i.e., making a choice). For chickadees randomly assigned to either True 1 (calls ID) or to True 2 (songs ID), all stimuli recorded from Male A and Female A were assigned S+, while stimuli recorded from Male B and Female B were assigned S−. For chickadees randomly assigned to either Pseudo 1 (calls control) or Pseudo 2 (songs control), half of each stimulus birds' recorded stimuli were pseudo-randomly assigned as S+ or S−. Because each stimulus bird contributed both S+ and S− stimuli in Pseudo discrimination groups, chickadees could not reliably apply ID to solve the discrimination task, nor could they rely on any other identity features like sex or species, and were thus limited to using rote memorization (Herrnstein 1990). Chickadees were required to achieve a discrimination ratio (DR), which represents the proportion of all correct Go trials out of all Go trials (Go S+ / (Go S+ and Go S−)) of at least 0.80 for a minimum of six trial blocks, with the last two being consecutive, where a trial block consisted of six presentations of each stimulus. The 44 stimuli were played in random order without replacement. Once chickadees had learned the task, their learning was tested using novel stimuli.
1.7 First Testing Phase
Test phases consisted of two tests for generalization, first a probe test, and then a full transfer test. During the probe test, four novel stimuli from each of the four stimulus birds were added to the stimulus pool. These 16 novel stimuli were of the same vocalization type as presented in the initial discrimination phase (calls for True 1 and Pseudo 1, songs for True 2 and Pseudo 2). For True 1 and True 2 groups, stimuli recorded from Male A and Female A were labeled P+, and those recorded from Male B and Female B were labeled P−. These labels were pseudo randomised for Pseudo 1 and Pseudo 2 groups. However, unlike for S+ and S− stimuli, probe stimuli were neither reinforced nor punished, regardless of how the bird responded, to test for generalization of learning without differential reinforcement. Because probe stimuli were neither rewarded nor unrewarded, and to prevent reduced motivation to respond to probe stimuli, chickadees were required to complete two consecutive trial blocks with a discrimination ratio of at least 0.80 while maintaining the initial discrimination parameters, but with a reduced probability of reward during S+ trials from 100% to 85%. By doing so, chickadees would not be unduly biased against responding following the presentation of unrewarded probe stimuli and would not become demotivated by reduced probability of reward during probe testing. Each chickadee completed 6 trial blocks of probe testing, where a trial block consisted of each probe stimulus being played once, and each S+ and S− stimulus being played twice (to maintain a proportion of probe stimuli of about 15%).
Following probe testing, P+ and P− stimuli were converted to S+ and S− stimuli respectively, and an additional seven novel stimuli (calls for True 1 and Pseudo 1, songs for True 2 and Pseudo 2) from each stimulus bird (for a total of 88 stimuli) were also added to the stimulus pool, for a full transfer test. During this test, the probability of reward during S+ Go trials was restored to 100%, and chickadees' responses to novel stimuli were compared to those of trained stimuli. Chickadees passed this transfer test once they had achieved a discrimination ratio of at least 0.80 for at least six trial blocks, the last two of which had to be consecutive. As in the initial discrimination phase, a trial block consisted of six presentations of each stimulus.
1.8 Second Testing Phase
As in the first testing phase, chickadees were first tested with a reduced probability of reward for S+ Go trials from 100% to 85%. Once chickadees achieved two consecutive trial blocks with a discrimination ratio of at least 0.80, four novel probe stimuli from each of the four stimulus birds were introduced to the stimuli pool. Probe stimuli recorded from Male A and Female A were labeled as P+, and those recorded from Male B and Female B were labeled P−. Although S+ and S− stimuli were reinforced or punished depending on chickadees' responses, probe stimuli were neither rewarded nor punished. This time, S+ and S− stimuli were only played once in each trial block to maintain a proportion of probe stimuli of 15%. Also unlike in the first probe test, these novel 16 probe stimuli were of the vocalization type not previously presented in the initial discrimination training and first testing phase (songs for True 1 and Pseudo 1, calls for True 2 and Pseudo 2). Chickadees completed six trial blocks of probe testing, where each stimulus was presented once per trial block.
For the final stage of the study, chickadees entered a final full transfer, where the 16 probe stimuli were converted from P+ and P− to S+ and S− respectively, and an additional seven stimuli from each stimulus bird, also of the vocalization type not presented during initial discrimination training (songs for True 1 and Pseudo 1, calls for True 2 and Pseudo) were also added. The probability of reward during S+ Go trials was again restored to 100%, and chickadees' responses to novel stimuli were once again compared to those of trained stimuli. Chickadees passed this full transfer test once they had demonstrated a discrimination ratio of at least 0.80 for at least 6 trial blocks, the last 2 of which were consecutive, where a trial block consisted of six presentations of each stimulus.
1.8.1 Fail Criteria
In order to not prolong experimental birds' time in operant chambers, and to ensure that we allowed adequate time to assess whether birds were going to learn a task, we used specific fail criteria which were designated for each training stage. In Montenegro et al.'s (2020) study, chickadees trained to memorize individual fee-bee songs (a Pseudo control group similar to those described above) took fewer than 18,000 trials on average to reach pass criterion during initial discrimination training, and fewer than 14,400 trials to reach pass criterion during a generalization transfer test. Based on these values and allowing additional trials for the possibility of slower learning in the chick-a-dee call groups, birds in our study were required to reach an initial DR = 0.80 within 100 trial blocks (26,400 trials), and reach all pass criteria within 115 trial blocks (30,360 trials). During the first transfer training stage, birds failed if they did not achieve DR = 0.80 within 50 trial blocks (26,400 trials), and all pass criteria within 65 trial blocks (34,320 trials). In the final training phase, birds were retired if they failed to reach DR = 0.80 within 33 trial blocks (26,136 trials) and pass within 41 trial blocks (32,472 trials). Additionally, birds were retired from the study after 100 days in the chamber. Most birds completed the study well before this cutoff period, averaging 67.2 days in study (SD = 18.66).
A total of five Pseudo group birds reached fail criteria (i.e., were removed from the study before reaching criterion level performance). Of these, two birds from Pseudo 1 reached 100 days in study between the two testing phases and were retired early. An additional three birds from Pseudo 2 failed to reach DR = 0.80 within 100 trial blocks during the initial discrimination stage and were subsequently retired. For these latter three birds, a placeholder of 100 trial blocks to initially reach DR = 0.80 and 115 to complete to pass discrimination training respectively were used for the purpose of analysis. Because five of the eight total Pseudo birds failed during, or soon after initial discrimination training, and this represented a significant relationship between condition (True vs. Pseudo) and outcome (ꭓ2 (1) = 6.94, p = 0.008), we chose to exclude Pseudo groups from all subsequent analyses save for those comparing the number of trial blocks required to reach DR = 0.80 and to pass the initial discrimination training (where all eight Pseudo birds could be included in the analysis) among groups. We believe that the exclusion of these control groups is justifiable as we can reasonably assume that since five of the Pseudo group birds failed, while none of the True group birds failed, the Pseudo groups' rote memorization task was substantially more difficult than that of True groups' discrimination learning. However, summary statistics for these birds are included (see Table 1). Additionally, during initial discrimination training, one bird from True 2 took substantially longer than any other bird in either True group to learn the task. We discovered that this operant chamber had audio malfunctions during some trials. Because the malfunction led to the bird being an extreme outlier, this bird was also removed from all analyses.
| Discrimination training | |||
|---|---|---|---|
| N | M | SD | |
| True 1 | 6 | 0.65 | 0.11 |
| True 2 | 5 | 0.62 | 0.07 |
| Pseudo 1 | 4 | 0.51 | 0.01 |
| Pseudo 2 | 4 | 0.50 | 0.01 |
| First Transfer Test | |||
|---|---|---|---|
| True 1 | 6 | 0.93 | 0.03 |
| True 2 | 5 | 0.91 | 0.05 |
| Pseudo 1 | 4 | 0.51 | 0.03 |
| Pseudo 2 | 1 | 0.62 | NA |
| Second Transfer Test | |||
|---|---|---|---|
| True 1 | 6 | 0.53 | 0.13 |
| True 2 | 5 | 0.48 | 0.22 |
| Pseudo 1 | 2 | 0.49 | 0.06 |
| Pseudo 2 | 1 | 0.49 | NA |
- Note: Discrimination ratios represent the proportion of correct feeder approach (Go) responses out of all Go responses. Sample sizes decrease where birds have reached fail criteria in prior stages.
2 Statistical Analysis
All statistical analyses were conducted using R v4.3.1 (R Core Team 2023). We performed a mixed-model Analysis of Variance (ANOVA) using the afex package (Singmann et al. 2023) to compare the average DRs for novel and previously trained stimuli within the first six trial blocks of the two transfer tests within and among groups. We also used mixed-model ANOVA to compare the proportion of Go responses to S+, S−, P+ and P− stimuli among groups and across probe tests. The afex package automatically applies the Greenhouse–Geisser sphericity correction where sphericity is violated. Where significant main effects and interactions were found, Tukey's post hoc comparisons were conducted using the emmeans package (Lenth 2023). Normality was assessed using the Shapiro–Wilk test and ggplot2 qq plots (Kassambara 2023; Wickham 2016). Where normality was a concern (comparing the number of trials blocks to criterion in the initial discrimination stage) a Kruskal-Wallis rank sum test followed by post hoc wilcoxon-rank sum tests with Benjamini-Hochberg corrections were used in place of ANOVA. Homogeneity of variance was assessed using Levene's test. In all cases, an alpha cutoff of p = 0.05 was used to mark statistical significance. Results figures were made with the ggplot2 package (Kassambara 2023).
3 Results
3.1 Discrimination Training
During initial discrimination training, True group birds were trained to discriminate among 44 stimuli on the basis of individual discrimination, while Pseudo group birds were trained to discriminate among 44 stimuli using rote memorization. On average, birds in True 1 took M = 8.17 trial blocks (SD = 4.75) to reach a DR = 0.80; True 2 M = 7.6 trial blocks (SD = 1.67); Pseudo 1 M = 36.8 trial blocks (SD = 24.6); and Pseudo 2 M = 87.5 trial blocks (SD = 25; see Figure 3). There was a significant difference among groups in the number of trial blocks required to reach DR = 0.80 (ꭓ2 (3) = 13.627, p = 0.003). Post hoc pairwise comparisons revealed that birds in each True group took significantly fewer trial blocks than either Pseudo group (all ps = 0.037). There were no significant differences between True groups (p = 0.713) or between Pseudo groups (p = 0.065).
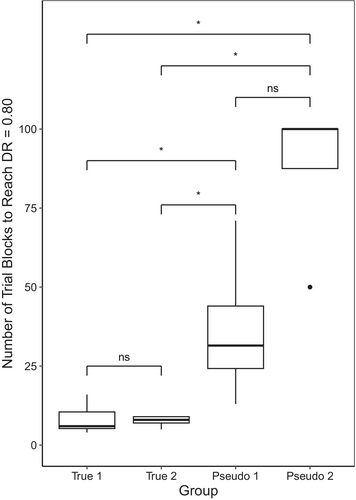
Birds in True groups also took fewer trial blocks to reach pass criteria, with True 1 birds taking an average of 15.5 trial blocks (SD = 9.29), True 2 birds M = 13.4 trial blocks (SD = 1.34), Pseudo 1 birds M = 46.5 trial blocks (SD = 29.6) and Pseudo 2 birds M = 102 trial blocks (SD = 26.5). There was also a significant difference among groups in the number of trial blocks required to pass this stage (ꭓ2 (3) = 13.313, p = 0.004). True 1 birds took significantly fewer trial blocks than Pseudo 2 birds (p = 0.038), but not than Pseudo 1 birds (p = 0.063). True 2 birds took significantly fewer trial blocks than birds in either Pseudo group (ps = 0.038). Neither True group birds (p = 0.408) nor Pseudo group birds (p = 0.065) significantly differed from each other.
3.2 Testing Phases
During probe tests, we compared birds' average proportion of Go responses to S+, S−, P+, and P− stimuli. If birds in True groups had learned the individual discrimination task, we would expect them to approach the feeder at higher rates following P+ presentations than following P− presentations. On average, during the first probe test, True 1 birds responded by entering the feeder (Go) on 81.2% of S+ trials, 9.1% of S− trials, 74% of P+ trials, and 8.2% of P− trials. True 2 birds exhibited a Go response to 91.8% of S+ trials, 27.5% of S− trials, 95.9% of P+ trials, and 30.5% of P− trials. During the second probe test, True 1 birds responded by entering the feeder (Go) on 84.3% of S+ trials, 7.6% of S− trials, 28.2% of P+ trials, and 54% of P− trials. True 2 birds performed a Go response to 88.2% of S+ trials, 5.1% of S− trials, 10.9% of P+ trials, and 15% of P− trials. There was a significant main effect of probe test (first or second; F(1,9) = 13.534, p = 0.005, η2G = 0.228) and stimulus type (S+, S−, P+ or P−; F(2.56,23) = 147.952, p < 0.001, η2G = 0.792), and a significant interaction of these factors (F(1.81,16.33) = 44.240, p < 0.001, η2G = 0.523) on the proportion of Go responses. There was no significant effect of group membership (F(1,9) = 0.147, p = 0.710, η2G = 0.006). In the first probe, True 1 birds performed significantly more Go responses to S+ compared to S− trials (t(9) = 7.371, p < 0.001) and to P+ compared to P− trials (t(9) = 8.205, p < 0.001). Similarly, during the first probe test True 2 birds responded more to S+ compared to S− trials (t(9) = 5.931, p = 0.003) and to P+ compared to P− trials (t(9) = 7.475, p < 0.001). However, during the second probe test True 1 birds responded significantly more only to S+ than S− trials (t(9) = 20.203, p < 0.001), and actually responded significantly more to P− than to P+ trials (t(9) = 4.067, p = 0.037). In the second probe test, True 2 birds also responded significantly more to S+ than to S− trials (t(9) = 19.966, p < 0.001), but did not respond significantly differently to P+ or P− trials (t(9) = 0.589, p = 0.998).
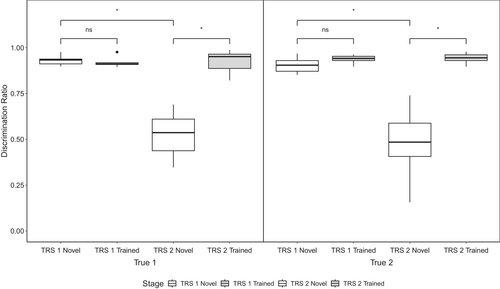
During full transfer tests, we compared birds' average DRs for novel stimuli and trained stimuli within the first six trial blocks of each transfer test (see Figure 4). If True birds learned the individual discrimination task, they should be able to generalize this learning in the first transfer test to novel stimuli, as evidenced by a high and roughly equal DR for novel stimuli as for trained stimuli. In the first transfer test, True 1 birds had an average DR of 0.921 (SD = 0.029) for trained stimuli and 0.931 (SD = 0.028) for novel stimuli. True 2 birds had an average DR of 0.937 (SD = 0.026) for trained stimuli and 0.905 (SD = 0.046) for novel stimuli in the first transfer test. If True birds are able to generalize learned individual discrimination across vocalization types, then we would expect birds to demonstrate high and roughly equal DRs for novel stimuli as for trained stimuli in the second transfer test. During this second transfer test, True 1 birds had an average DR of 0.925 (SD = 0.065) for trained stimuli and 0.525 (SD = 0.129) for novel stimuli. True 2 birds had an average DR of 0.942 (SD = 0.031) for trained stimuli and 0.475 (SD = 0.217) for novel stimuli. Overall, there was a significant effect of test phase (first or second; F(1,9) = 69.796, p < 0.001, η2G = 0.595), and of stimulus type (novel or trained; F(1,9) = 55.286, p < 0.001, η2G = 0.629), and a significant interaction of these factors (F(1,9) = 40.342, p < 0.001, η2G = 0.606) on average DRs. There was no significant effect of group membership on average DRs (F(1,9) = 0.174, p = 0.686, η2G = 0.004). In the first transfer test, True 1 birds did not significantly better discriminate trained stimuli over novel stimuli (t(9) = 1.106, p = 0.695). The same was found for True 2 birds (t(9) = 3.030, p = 0.057). However, in the second transfer test, both True 1 (t(9) = 4.718, p = 0.005) and True 2 (t(9) = 5.032, p = 0.003) birds displayed significantly poorer discrimination of novel stimuli than for trained stimuli. Both True 1 birds (t(9) = 5.289, p = 0.002) and True 2 birds (t(9) = 5.113, p = 0.003) were significantly worse at discriminating novel stimuli in the second transfer test than in the first.
4 Discussion
Our study set out to confirm whether black-capped chickadees can use chick-a-dee calls for individual discrimination and answer whether learned ID can be generalized from chick-a-dee calls to fee-bee songs and vice versa. We trained two groups of chickadees to discriminate among four individuals' vocalizations, using either calls or songs as discriminative stimuli (True discrimination groups), and compared learning acquisition in the True groups to two control groups wherein birds were trained using rote memorization of the same stimuli (Pseudo discrimination groups). We next tested for generalization across vocalization types by presenting chickadees with stimuli recorded from the same birds but this time of the opposing vocalization type than that used during initial discrimination training. Chickadees in True groups learned the discrimination task significantly faster than those in Pseudo control groups, suggesting the discrimination rule True group birds employed provided these birds with a substantial advantage compared to learning via rote memorization. In fact, ID provided such an advantage that while a total of five Pseudo birds failed the study, three of which failed during the initial discrimination stage, no True birds (even one bird who's learning was disrupted by equipment failure) failed to complete the entire study. Birds in Pseudo 2 seemed to be especially disadvantaged, particularly during the initial discrimination stage. This may reflect greater difficulty in discriminating among the more stereotyped fee-bee song iterations (Ficken et al. 1978) than the more variable chick-a-dee call iterations presented to Pseudo 1 birds. We acknowledge that our sample sizes are limited. However, where statistically significant differences were detected, the accompanying effect sizes are consistently large. While future studies with larger sample sizes would be welcome, the findings we have reported here are bolstered by their large effect sizes.
Chickadees in True groups were able to generalize their learning to novel stimuli of the same vocalization type, with no significant reduction in DR to novel vs. trained stimuli. Thus, we were able to demonstrate that chickadees can employ ID using chick-a-dee calls in an operant conditioning paradigm, and were also able to replicate Montenegro et al.'s (2020) findings of individual discrimination using fee-bee songs. However, when presented with stimuli of the opposing vocalization type (e.g., fee-bee songs to chick-a-dee calls), chickadees were not able to generalize their learning. There was a significant decrease in DR for these novel stimuli as compared to previously trained stimuli. Both True groups responded to these stimuli at about chance (DR = 0.50), which would suggest there was no advantage to True birds based on prior learning for discriminating these novel stimuli. Though we did not include Pseudo group birds in any generalization test phase analyses due to a remarkably high failure rate, those that did complete the second testing phase also, predictably, responded to these novel stimuli at about chance.
Interestingly, although chickadees in True groups were not able to generalize successfully learned ID across vocalization types, True 1 birds were about 2.5 times more likely to respond to novel female songs during the second probe test than to novel male songs. Since sex was balanced between the rewarded and unrewarded stimulus categories (one male and one female each rewarded and punished), and any pre-existing biases were controlled for during pre-training, we would not have anticipated a substantial discrepancy in responding to one sex over the other. Such a discrepancy was also not observed in True 2 birds, which responded to novel male and female chick-a-dee calls about equally. We first assumed this behavior observed within True 1 birds might be a function of males (which made up four of the six birds in True 1) responding to potential breeding partners. However, the four males themselves were only about twice as likely to respond to females than males, while the two females were a little more than three times as likely to respond to females than males. Given the high preference for female songs over male songs, we instead posit that this bias reflects an increased willingness to approach when the singer is likely to be a less dominant, and less threatening individual, since female black-capped chickadees tend to be subordinate to males (Desrochers 1989; Odum 1941), and males tend to play a more active role in territorial defense, often preceded by singing (Baker et al. 2012; Stefanski 1967). Authors of future studies implementing both male and female songs as stimuli should be cautious when interpreting sex-mediated biases, particularly concerning cognitive tasks where altered performance may otherwise be attributed to hindered learning acquisition for one sex over the other.
The lack of cross-vocalization generalization observed in our study leaves us with two main questions. First, why might chickadees not be able to generalize learned ID across vocalization types? On its face, the lack of generalizability of individual recognition across vocalization types seems an inefficient system, though this is not an inherently unique finding. Elie and Theunissen (2018) found that zebra finches (Taeniopygia guttata) can discriminate between individuals using each call type in their repertoire, but the zebra finches likely memorize the individual signature markers of each call type independently. Similarly, song sparrows operantly conditioned to discriminate between individuals were not able to generalize their learning among song types (Beecher et al. 1994). Elie and Theunissen (2018) propose that while memorizing vocal signatures for each vocalization type independently is a less efficient system, it is likely a result of limited passive frequency filtering. In other words, the anatomy of small birds like zebra finches, and perhaps chickadees, is so limited in its capacity for inter-individual variation that there is little opportunity for resonant frequencies to be reliably used as a cross-vocalization identity cue. In support of Elie and Theunissen's (2018) theory regarding limited passive frequency filtering in small avian species, current evidence for cross-vocalization identity generalization, or modeling of such generalization, is readily found in mammals (Cheney and Seyfarth 1988; Pisanski et al. 2020; Reby et al. 2006), but not in birds. The memorization of vocalization-specific individual signatures would explain the inability of chickadees in our study to generalize learned ID across calls and songs. However, this brings us to our second question: if chickadees do memorize individual vocal signatures independently for each vocalization type, how then do chickadees come to understand that these vocalization-specific signatures originate from the same producer?
It could be that free-flying chickadees approach an individual each time a novel vocalization type is produced, identifying the individual visually before adding this new memorized vocal signature to their “IVR toolbox”. This strategy could be time consuming though, and would present greater risk than identifying a potential threat from afar. Another hypothesis would be that chickadees rely on the use of external cues like spatial and temporal proximity, or other vocal identity markers, like sex or dominance, to link different vocalizations together. The logic to this theory would be that when two vocalizations of different types originate from the same place at the same time and from a similarly dominant individual of the same sex, they can reasonably be attributed to the same individual. Alternatively, individuals may rely on encoded contextual information to simultaneously derive both meaning and identity from an acoustic signal.
Cheney and Seyfarth (1988) found that vervet monkeys (Chlorocebus pygerythrus) are capable of generalizing learned habituation to playback of a specific individual's Wrr calls to the same individual's Chutter calls (two calls which convey similar contextual information and elicit similar behavioral responses), but do not generalize learned individual habituation when the habituated call and the novel call convey different contextual information (such as the leopard alarm call and eagle alarm call which elicit very different behavioral responses). Cheney and Seyfarth (1988) were also able to demonstrate that individual identity information was being attended to during this paradigm, as generalization did not occur when the habituated calls were followed by stimuli produced by a novel individual, regardless of whether the two call types shared or did not share contextual meaning. It seems, then, that vervet monkeys can employ cross-vocalization generalization of individual recognition, but this ability may be limited to vocalizations that convey similar contextual information. Reby et al. (2006) found that red deer's (Cervus elaphus) common roars could be used to train a hidden Markov model to categorize roars by individual. When the model was later tested with harsh roars, chase barks, and barks (vocalizations with overlapping contextual use) recorded from the same individuals, the model was able to correctly identify the producer 63.4% of the time. Red deers' common roars are predominantly used during female herding and conspecific male contests, harsh roars at the conclusion of a roaring contests or during herding, chase barks in the context of conspecific agonistic and breeding/herding behaviors, and barks may precede roars, may signal a nearby threat, or may be used in territorial or mating contexts (Reby and McComb 2003). Similarly, Pisanski et al.'s (2020) acoustic analysis of human recordings found that fundamental frequency could be used to predict identity within human individuals' non-verbal vocalizations. The strength of this correlation was greatest between vocalizations of similar contextual use, such as fearful speech and screams, or aggressive speech and roars.
In considering both Reby and McComb (2003) and Pisanski et al. (2020), while the respective various vocalizations described may serve slightly different purposes, they can occur concurrently and share substantial contextual overlap. In these cases, however, the vocalizations are also more acoustically similar to each other, which may more easily accommodate a shared vocal signature than would be afforded by the vocalizations used in our study. We presented chickadees with chick-a-dee calls and fee-bee songs, two vocalizations that, while sometimes produced in short succession, serve to convey very different contextual information and are very acoustically distinct (Ficken et al. 1978; Smith 1991, 57–74). Had we used two more contextually similar vocalizations, such as the gargle call, used in territorial and agonistic contexts (Smith 1991, 57), and the fee-bee song, we may have seen greater generalizability. If chickadees do attend to the contextual meaning of acoustic signals as much as they do the identity of signal producers, as described in vervet monkeys by Cheney and Seyfarth (1988), perhaps this contextual information aids in facilitating the assimilation of vocalization-specific vocal signatures by signal receivers. Such hypotheses require further investigation to better understand the way that individual vocal recognition is acquired, stored, modified, and retrieved in chickadees and other songbirds.
Author Contributions
Sarah M. L. Smeltz: conceptualization, methodology, investigation, formal analysis, data curation, writing – original draft. Moriah J. Deimeke: conceptualization, investigation, methodology, writing – review and editing. Carolina Montenegro: conceptualization, methodology, writing – review and editing. Prateek K. Sahu: investigation, writing – review and editing. Katharine H. Stenstrom: investigation, writing – review and editing. Andrés Camacho-Alpízar: investigation, writing – review and editing. Christopher B. Sturdy: conceptualization, methodology, resources, writing – review and editing, funding acquisition, data curation, project administration.
Acknowledgements
The authors would like to thank all volunteers, members, and contributors of the Songbird Neuroethology Lab for their support in undertaking this study. We would also like to thank Isaac Lank and Philip May for their technical assistance, and the Science Animal Support Services staff for their exemplary animal care during this and all of our studies. The authors declare no competing interests. C.B.S. received funding from the Canada Research Chairs Program (CRC-2019-00080), Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants (RGPIN-2016-04591, RGPIN-2022-02968), a Discovery Accelerator Supplement (NSERC RGPAS-2016-412311), the Canada Foundation for Innovation, and from the University of Alberta Faculty of Science. Graphical abstract created in BioRender. Deimeke, M. (2025) https://BioRender.com/2yt7w9f.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethics Statement
All procedures pertaining to this study were conducted in accordance with the Canadian Council on Animal Care (CCAC) Guidelines and Policies with approval from the Animal Care and Use Committee for Biosciences for the University of Alberta, which is consistent with the Animal Care Committee Guidelines for the Use of Animals in Research. All birds were captured and research conducted under a Canadian Wildlife Service Scientific permits (13-AB-SC004 (2019,2020,2021,2022), SC-PR-2023-AB-013A (2023)), Province of Alberta Capture and Research permits (#19–020(2019), #20–084(2020), #21–098(2021), #22–017(2022), #23–015(2023)) and a North Saskatchewan River Valley Area Redevelopment Plan (NSRV ARP) permit.
Open Research
Data Availability Statement
The data used to support the findings of this study are available upon request made to the corresponding author (C.B.S.) by email at [email protected] or at: Smeltz et al. (2025) Individual discrimination within, but not between, two vocalization types of the black-capped chickadee [Dataset]. Dryad. https://doi.org/10.5061/dryad.c866t1ghk.




