Is the Setal Patch on the Chelae of Hemigrapsus takanoi and Hemigrapsus sinensis (Crustacea, Brachyura, Varunidae) Advantageous in Fighting and Mating?
Abstract
Many animals have unique morphological characters that function in social behavior. Sexual selection can affect the expression of such traits in males and females, leading to sexual dimorphism. We investigated the social function of setal patches on the chelae of two species of varunid crabs, one in which males, but not females, have setal patches (Hemigrapsus takanoi), and one in which both sexes have setal patches (Hemigrapsus sinensis). We experimentally removed setal patches and compared fighting and mating behavior of individuals with and without setal patches. In H. sinensis, males with setal patches removed were inferior fighters compared to intact males. In male H. takanoi and female H. sinensis, setal removal did not influence the outcome of fights. In mating, males lacking setal patches had a similar ability to copulate with females as intact males in both species. However, male H. takanoi with their setae removed tended to take more time to initiate copulation than did intact males. When females were given the opportunity to choose intact males or males without setal patches, females of H. takanoi did not discriminate between the two. Female H. sinensis, however, copulated with intact males more frequently compared to males lacking setal patches. Male H. sinensis showed no preferences for the presence of setal patches or the body size of females. Thus, our results indicate that setal patches have a social function in male H. takanoi and male H. sinensis, but not in female H. sinensis, suggesting that the setal patches of male crabs are a sexually selected trait in both species. However, the social function of male setal patches was more prominent in the species in which both sexes possess setal patches than in the species in which only males bear setal patches.
Introduction
Animals display a variety of conspicuous traits, with some appearing only in males, while others occur in both sexes. Exaggerated conspicuous traits often function in mating behavior and intrasexual competition and are usually considered to have evolved via sexual selection (Andersson 1994; Berglund et al. 1996; Wong & Candolin 2005; Kraaijeveld et al. 2007; Charles & Ord 2012). If one sex needs the exaggerated trait to acquire a mate and the other sex does not, it is a sexually selected trait and will be present in only one sex (Andersson 1994). However, if the trait is required for both sexes to obtain a common resource, it is a naturally selected trait and will be present in both sexes (Kraaijeveld et al. 2007). Furthermore, if both sexes need the trait to acquire a common resource, but one sex also uses it to acquire mates, it is both a naturally and sexually selected trait and will be expressed in both sexes, but on a larger scale in one sex (Kraaijeveld et al. 2007; Worthington et al. 2012; Warren et al. 2013). Thus, species with highly dimorphic traits are expected to use the trait more extensively compared to less dimorphic species (Baeza & Asorey 2012).
In decapod crustaceans, some species bear conspicuous, hairy structures composed of fine setae on the chelae of males or, in some species, on the chelae of both sexes. Previous studies have suggested that setal patches on crustacean chelae are important in social behavior (Kobayashi & Matsuura 1993; Van Maurik & Wortham 2011; Wortham & Van Maurik 2012); however, these studies did not specifically address the functions of the setal patches in social interactions.
To examine the significance of setal patches on chelae, we focused on two species of crabs in the family Varunidae: Hemigrapsus takanoi and Hemigrapsus sinensis. Hemigrapsus takanoi is common under intertidal boulders of inner bays and estuaries of East Asia. Males of this species have setal patches in the gape of their chelae, growing on both the inner and outer surfaces of the pollex (Fig. 1; Asakura & Watanabe 2005), whereas females lack the setal patch (the sexually dimorphic species). Hemigrapsus sinensis dwells in oyster beds and under boulders in the intertidal to subtidal zone of East Asia. Both males and females have setal patches in the gape of their chelae, growing only on the outer surface of the pollex (Sakai 1976) (the sexually non-dimorphic species). Setal patches do not appear to be used during either intrasexual agonistic interactions or the mating behavior of either species (Miyajima et al. 2012). The frequency of intrasexual agonistic interactions is more prominent in the dimorphic species compared to the non-dimorphic species, although no differences have been observed between the sexes of either species (Miyajima et al. 2012). However, when the setal patch is present, individuals engaging in intrasexual agonistic interactions use their chelae more frequently than their walking legs by grasping each other, whereas when the setal patch is absent, this scenario is reversed: Individuals more frequently use their walking legs than their chelae by touching and pushing each other (Miyajima et al. 2012).

In both species, as in other varunid crabs, copulation occurs during the intermolt period without pre-copulatory courtship behavior, and only females whose gonopores are decalcified can copulate (Fukui 1994; Brockerhoff & McLay 2005; Nara et al. 2006; Sal Moyano et al. 2014). Some varunid species exhibit pre- and post-copulatory guarding (Brockerhoff & McLay 2005; Nara et al. 2006; Sal Moyano et al. 2014), but no mate guarding has been observed in the dimorphic species, H. takanoi: A male simply approaches the female, grasps and handles her with his chelae and walking legs, and maneuvers her into the copulatory posture, during which the male and female embrace with the female on top (Miyajima et al. 2012). However, in the non-dimorphic species H. sinensis, males sometimes guard females after copulation, and unlike the dimorphic species, the initiation of mating involves the approach of either the male or female, and males rarely capture females with their chelae (Miyajima et al. 2012).
In this study, we hypothesized that the setal patches of chelae of the two species of Hemigrapsus function in intrasexual fighting and mating behavior and that the social function of the setal patches is more prominent in dimorphic than in non-dimorphic species. The functions of the setal patches were evaluated by comparing social behavior between individuals with their setae removed and intact individuals.
Methods
Collecting and Rearing Crabs
Males and females of H. takanoi (dimorphic species) were collected under boulders in intertidal areas of Samusaura, Shirahama, Wakayama Prefecture (33°41′N, 135°23′E), throughout their breeding season from April to September 2012 and 2013. Crabs were maintained in tanks (60.0 × 100.0 × 40.0 cm height) with running sea water (temperature: 26–28°C). Males and females of H. sinensis (non-dimorphic species) were collected from intertidal oyster beds of the Nakano River Estuary, Mie Prefecture (34°53′N, 136°35′E), from October to December 2012 and from January to May 2013 and 2014. Hemigrapsus sinensis crabs were reared in tanks (48.0 × 48.0 × 50.0 cm height) (ARD 9.5-101A, AQUA Co., Tokyo) with simulated tides of artificial sea water (Premium Salt, Gex Co., Osaka), which was diluted to 16–23 ppt (temperature: 20–25°C). All crabs were kept individually in plastic cups (6 cm in diameter × 13.5 cm in depth) to avoid interactions between crabs. Once every 3 d, the dimorphic species was fed TetraMin (Tetra Japan Co., Ltd., Tokyo), and the non-dimorphic species was fed crushed bait for shrimps and crabs (Kyorin Co., Ltd., Hyogo). Each crab was used in experiments within 3 wk after capture in the field. The mean carapace widths (±SD) of males and females of the dimorphic species were 18.96 ± 3.06 mm (n = 476) and 14.93 ± 2.42 mm (n = 238), respectively. The mean carapace widths for males and females of the non-dimorphic species were 9.02 ± 1.86 mm (n = 96) and 9.48 ± 1.72 mm (n = 147), respectively.
Intrasexual Fights
To examine whether setal patches function in intrasexual agonistic interactions, we observed fights between crabs with intact setal patches and those whose setal patches had been removed (hereafter referred to as seta+ and seta−, respectively). Males of the dimorphic species and both sexes of the non-dimorphic species were tested. Setal patches were removed by cutting them off with a scalpel. Removal of setal patches took 2–4 min per crab. We also moved the blunt edge of the scalpel over the setal patches of seta+ individuals for an equivalent amount of time without cutting off the setae. We confirmed that setal patch removal did not negatively influence crab behavior by observing seta− crabs after setal removal: seta− crabs interacted with other crabs in the same manner as did seta+ crabs.
We selected size-matched (<0.5 mm difference in carapace widths) same-sex pairs of crabs, one seta+ and one seta−, for observations of intrasexual agonistic behavior. Observations were conducted from April to May 2013 for the dimorphic species and from January to March 2013 and 2014 for the non-dimorphic species. Crabs were placed in an observation tank (dimorphic species: 18.5 × 29.0 × 17.0 cm height; non-dimorphic species: 13.5 × 21.0 × 13.0 cm height) with a gravel-covered bottom (1 cm depth) filled with sea water (10 cm depth). One stone (ca. 5 × 8 × 5 cm height) was placed in the center of the tank. We observed and video-recorded interactions for 10 min (Everio Hi-Vision Hard Disk Movie GZ-HD320, JVCKENWOOD Co., Kanagawa). For fights during which grasping occurred within the 10-min observation period, we documented the process and noted which crab retreated in the fight. The crab that retreated was recorded as the loser. Grasping is the most aggressive fighting element in both species; therefore, we assumed that dominance would typically be determined by these aggressive fights. In addition, dominance reversals rarely occurred during the 10-min observation period. We observed fights between 24 pairs of males in the dimorphic species and 22 pairs of males and 23 pairs of females in the non-dimorphic species.
Occurrence of Copulatory Behavior
To determine whether males lacking setae can copulate with females as successfully as intact males, we observed the pairing behavior of the same individual female with both seta+ and seta− males of each species during their breeding season (dimorphic species: April to August 2013; non-dimorphic species: February to March 2013). We confirmed that females of both species could copulate with all types of male during their receptive period. By having the same female encounter seta+ and seta− males separately, we aimed to compare the mating behavior of each type of male with the same female. Tested seta+ and seta− males were size-matched (<0.5 mm difference in carapace width). Receptive females (confirmed by operculum mobility) were selected from rearing tanks and placed in an observation tank (dimorphic species: 18.5 × 29.0 × 17.0 cm height; non-dimorphic species: 13.5 × 21.0 × 13.0 cm height) with either a seta+ or a seta− male. The size difference between the female and the male was <4.5 mm carapace width. We observed the pair and video-recorded their interactions for 30 min. The first male was then removed and replaced with the other male of the pair; the behavior of the male and female was again observed and recorded for 30 min. The seta+ and seta− males were presented to the female in a random order. Each receptive female encountered one male of each type (seta+ and seta−). The observation tank was emptied, washed, and refilled with fresh sea water between each trial. Each individual was used for only one trial, and 30 and 16 replicate trials were conducted for the dimorphic and non-dimorphic species, respectively.
Mate Choice
To investigate whether males or females discriminate against the presence/absence of setal patches on the chelae of a potential mate, we conducted female choice experiments for both species and male choice experiments for the non-dimorphic species. During their breeding season, two crabs of the same sex, one seta+ and one seta−, were presented to an intact mate (Fig. 2). For the female choice trials, we presented an intact female with a pair of size-matched males, one seta+ and one seta−. For the male choice trials, we presented an intact male with a pair of females, one seta+ and one seta− (we were unable to size-match the females due to the low number of receptive females available per day). In the non-dimorphic species, the presented crabs were tethered separately to similar-sized stones (ca. 4 × 6 × 2 cm height) by tying one ambulatory leg with sewing yarn. The tied crabs could move freely without any harmful effects, except that they could not contact one another. In the dimorphic species, fishing line was used to tether the crabs to the stones, with one end affixed to the carapace of each male using an adhesive agent (Aron Alpha, Toagosei Co., Tokyo) (after checking that the glue did not adversely affect crab behavior). The stones were placed in opposite corners of the observation tank (dimorphic species: 18.5 × 29.0 × 17.0 cm height; non-dimorphic species: 13.5 × 21.0 × 13.0 cm height). After acclimation, the focal crab (chooser) was introduced to the tank, and their behaviors were observed and video-recorded for 30 min. All other conditions, including tank cleaning, were the same as in the experiment examining the occurrence of copulatory behavior. We conducted 22 replicates for female choice of the dimorphic species and 15 and 16 replicates for female choice and male choice in the non-dimorphic species, respectively, using each individual only once.
Data Analyses
Data for intrasexual fights were analyzed by comparing the frequency of wins between seta+ and seta− individuals using a binominal test against an expected equal proportion of wins. The results of the first grasping fight in each pair were used to determine which crab had won.
For the observations of copulatory behavior, logistic regression was used to analyze the effects of the presence or absence of setal patches, the order of pairing, and their interaction on the occurrence of copulation (copulated or not). In cases in which copulation occurred, the effects of the presence or absence of setal patches on the time until initiation of copulation (the onset of embracing) and the duration of copulation (the time from the onset of embracing to the separation of the couple) were analyzed with general mixed models using residual maximum likelihood (REML). Fixed factors were the presence of setal patches, the order of pairing, and their interaction. Female identity was included in the analysis as a random factor. Finally, we used a Fisher's exact probability test to compare the relative frequency of the occurrence of chela handling (grasping and/or jerking behavior) between seta+ and seta− males, prior to copulation.
For the female choice experiment, we used a binominal test (against an expected equal proportion) to compare the number of seta+ and seta− males first approached by the female. We used logistic regression to analyze the effects of the presence or absence of the setal patch on the occurrence of copulation (copulated or not). The first approach by the females was also included as a fixed factor. In cases for which the female did not copulate with the male, the effects of the presence or absence of the male setal patches on the time spent in proximity to each male during the observation period were analyzed with general mixed models using REML. Female identity was included in the analysis as a random factor. ‘Proximity’ was defined as the female being next to the stone or in contact with the male.
For the male choice experiment, we used a logistic regression to first analyze the effects of the presence or absence of female setal patches on first approach by the male. We then used logistic regression to analyze the effects of the presence or absence of female setal patches on copulation with the males. The presence or absence of setal patches and carapace width of females were included as fixed factors in comparisons of the first approach. In the comparison of the occurrence of copulation, we also included first approach by males as a fixed factor.
All statistical analyses were conducted using JMP 9 statistical software (SAS Institute Inc., North Carolina). The normality of residuals was checked in all models. Data that did not satisfy the assumption of normality were ln-transformed.
Results
Intrasexual Fights
Of the 24 pairs tested in the intrasexual competition trials in the dimorphic species, 23 exhibited agonistic behaviors. Of the first interactions between these males, 12 fights were won by seta+ males and 11 were won by seta− males, with no significant difference between seta+ and seta− males (binominal test: p = 0.50). In the experiment with males of the non-dimorphic species, 21 of 22 pairs exhibited agonistic behaviors. Of the first interactions between these males, 15 fights were won by seta+ males and 6 were won by seta− males, with seta+ males winning significantly more frequently than seta− males (binominal test: p = 0.04). Among 23 pairs of females of the non-dimorphic species, 12 exhibited agonistic behaviors. Seven seta+ females and five seta− females won the first interaction, with no significant difference between seta+ and seta− females (binominal test: p = 0.39).
Occurrence of Copulatory Behavior
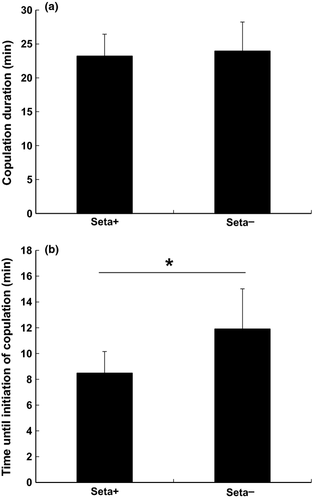
Laboratory observations of mating behavior revealed that seta− males copulated as frequently as did seta+ males in both species (Table 1). Logistic regression indicated no effect of setal patches on copulation frequency (dimorphic species: likelihood ratio χ2 = 0.24, df = 1, p = 0.63; non-dimorphic species: likelihood ratio χ2 = 0.64, df = 1, p = 0.42). In the dimorphic species, males that were presented first were more successful in copulating with females compared to males that were presented second, regardless of the presence or absence of setal patches (logistic regression, likelihood ratio χ2 = 4.49, df = 1, p = 0.03), although the order of pairing did not affect copulation frequency in the non-dimorphic species (logistic regression, likelihood ratio χ2 = 1.87, df = 1, p = 0.17). The interaction between the presence of setal patches and the order of pairing did not affect copulation frequency in either species (logistic regression, dimorphic species: likelihood ratio χ2 = 0.24, df = 1, p = 0.63; non-dimorphic species: likelihood ratio χ2 = 1.87, df = 1, p = 0.17). In addition, copulation duration did not differ between seta+ and seta− males in either species (Table 2, Figs. 3 and 4) or between first males (dimorphic species: mean ± SD, 22.39 ± 14.48 min, n = 16; non-dimorphic species: 11.92 ± 6.54 min, n = 13) and second males (dimorphic species: 25.83 ± 8.08 min, n = 8; non-dimorphic species: 12.47 ± 5.49 min, n = 15) (Table 2). However, in the dimorphic species, the presence of setal patches and the order of pairing with a female influenced time until initiation of copulation; that is, seta− males or males presented second (regardless of setal patches) took more time (mean ± SD, 13.70 ± 9.49 min, n = 8) to initiate copulation than did seta+ males or males presented first (8.22 ± 7.29 min, n = 16) (Table 2, Fig. 3). In the non-dimorphic species, the time until initiation of copulation did not significantly differ between seta+ and seta− males (Table 2, Fig. 4); however, the order of pairing with a female affected time until initiation of copulation, in that males presented second (6.57 ± 5.17 min, n = 15) took more time to initiate copulation than did the male presented first (3.22 ± 4.99 min, n = 13), regardless of the presence of setal patches (Table 2). The relative frequency of the occurrence of pre-copulatory grasping or jerking did not differ between seta+ and seta− males in either species, although there was a tendency that seta− males performed pre-copulatory grasping or jerking more frequently than seta+ males in the non-dimorphic species (Fisher's exact probability test: dimorphic species, seta+: 11/13, seta−: 11/11, p = 0.28; non-dimorphic species, seta+: 3/14, seta−: 8/14, p = 0.06).
| Species | Male | Order | Not copulated | Copulated |
|---|---|---|---|---|
| Hemigrapsus takanoi | Seta+ | First | 6 | 9 |
| Second | 11 | 4 | ||
| Seta− | First | 8 | 7 | |
| Second | 11 | 4 | ||
| Hemigrapsus sinensis | Seta+ | First | 1 | 7 |
| Second | 1 | 7 | ||
| Seta− | First | 2 | 6 | |
| Second | 0 | 8 |
| Species | Factor | numDF | denDF | F | p | |
|---|---|---|---|---|---|---|
| Copulation duration | Hemigrapsus takanoi | Setal patch | 1 | 4.41 | 0.15 | 0.71 |
| Order | 1 | 4.41 | 0.06 | 0.82 | ||
| Setal patch×Order | 1 | 18.50 | 0.09 | 0.76 | ||
| Hemigrapsus sinensis | Setal patch | 1 | 12.33 | 0.36 | 0.56 | |
| Order | 1 | 12.33 | 0.17 | 0.69 | ||
| Setal patch×Order | 1 | 14.38 | 1.96 | 0.18 | ||
| Time until initiation of copulation | H. takanoi | Setal patch | 1 | 1.95 | 145.10 | 0.008* |
| Order | 1 | 1.95 | 146.08 | 0.008* | ||
| Setal patch×Order | 1 | 17.96 | 1.41 | 0.25 | ||
| H. sinensis | Setal patch | 1 | 9.19 | 1.67 | 0.23 | |
| Order | 1 | 9.19 | 13.21 | 0.005* | ||
| Setal patch×Order | 1 | 11.69 | 0.08 | 0.79 |
- Significant values were shown with asterisk.

Mate Choice
Among 22 replicates of female choice for the dimorphic species, the number of occasions during which a male was first approached by the female did not significantly differ between seta+ (n = 13) and seta− males (n = 9) (binominal test: p = 0.26). Five females copulated with seta+ males, and seven females copulated with seta− males (10 did not copulate). Logistic regression analysis indicated no effect of setal presence on female choice (likelihood ratio χ2 = 0.67, df = 1, p = 0.41). In addition, the female's first approach was not related to the occurrence of copulation (likelihood ratio χ2 = 0.67, df = 1, p = 0.41), and the time spent by females in proximity to the male did not differ between seta+ males (6.09 ± 3.52 min, n = 10) and seta− males (6.44 ± 3.39 min, n = 10) (mixed effects ANOVA: F1,9 = 0.06, p = 0.81).
Among 15 replicates of female choice for the non-dimorphic species, the number of instances during which the male was first approached by the female did not significantly differ between seta+ males (n = 6) and seta− males (n = 9) (binominal test: p = 0.85). Similar to the occurrence of copulation, seven females copulated with seta+ males, and two females copulated with seta− males (six did not copulate). Logistic regression analysis indicated that females of the non-dimorphic species copulated with seta+ males significantly more frequently compared to seta− males (likelihood ratio χ2 = 5.93, df = 1, p = 0.01). However, the female's first approach was not related to the occurrence of copulation (likelihood ratio χ2 = 3.24, df = 1, p = 0.07). The time spent by females in proximity to the male did not differ between seta+ males (19.28 ± 9.82 min, n = 6) and seta− males (5.80 ± 6.66 min, n = 6) (mixed effects ANOVA: F1,5 = 4.38, p = 0.09).
Among 16 replicates of male choice (non-dimorphic species only), the presence of setal patches did not affect the first approach by males: Seta+ females were approached first in nine cases, and seta− females were approached first in seven cases (logistic regression: likelihood ratio χ2 = 0.77, df = 1, p = 0.38). For the occurrence of copulation, nine males copulated with seta+ females, and seven males copulated with seta− females. Logistic regression revealed no effect of setal patches on female chelae on male mate choice (likelihood ratio χ2 = 0.33, df = 1, p = 0.57). However, males were more likely to copulate with the female that they first approached (logistic regression: likelihood ratio χ2 = 6.21, df = 1, p = 0.01). Female carapace width and its interaction with the presence of the female setal patch did not affect male choice (effect of female carapace width in first approach: likelihood ratio χ2 = 1.75, df = 1, p = 0.19; effect of interaction in first approach: likelihood ratio χ2 = 2.26, df = 1, p = 0.13; effect of female carapace width on occurrence of copulation: likelihood ratio χ2 = 0.20, df = 1, p = 0.66; effect of interaction on occurrence of copulation: likelihood ratio χ2 = 2.98, df = 1, p = 0.08).
Discussion
A setal patch on the chelae affected the outcome of intrasexual fighting in only males of the non-dimorphic species. Fights between crustaceans often result in damage to the chelae (Jones 1980; Barki et al. 1997; Claverie & Smith 2007; Rypien & Palmer 2007; Thiel et al. 2010; Rojas et al. 2012; Bauer et al. 2014), and injuries may affect an animal's fighting abilities and its general health (Berzins & Caldwell 1983; Claverie & Smith 2007; Thiel et al. 2010). Crabs close their chela by contracting the closer muscle on the propodus (Warner 1977). Thus, setal patches on the chelae could be advantageous in protecting the chela from fighting damage, as setal patches can function as a cushion to soften damage caused by grasping by the opponent (Miyajima et al. 2012). This function of setal patches would likely operate to a greater extent in males of the non-dimorphic species, for which seta+ males were superior to seta− males.
We found that males lacking setal patches copulated with females as often as did intact males, indicating that setal patches are not required for copulation. However, males lacking setal patches of both species have difficulty in maneuvering females prior to copulation. In the female choice experiment, moreover, the females preferred to copulate with males with setal patches in the non-dimorphic species, but there found no such a preference in the dimorphic species. Female copulatory preferences for certain male characteristics have also been reported in other decapod crustaceans (Fukui 1995; Oliveira & Custodio 1998; Diaz & Thiel 2003; Aquiloni & Gherardi 2008). The difference between the dimorphic and non-dimorphic species in the effect of setal patches on female mate choice could be associated with interspecific differences in mating behavior. For example, males of the dimorphic species often grasped females to initiate copulation, whereas males of the non-dimorphic species rarely grasped females to initiate copulation (Table 1, see also Miyajima et al. 2012). Therefore, females are more likely to be free to refuse males in the non-dimorphic species than in the dimorphic species, which could result in lower copulation frequencies for seta− males compared to intact males in the non-dimorphic species.
In males of the brush-legged wolf spider Schizocoza ocreata, which have tufts of bristles on the tibiae of their forelegs, the tufts do not play an obvious role in mating success, whereas they have been hypothesized to increase the efficacy of visual displays (Scheffer et al. 1996). However, neither the dimorphic species nor the non-dimorphic species in the present study exhibit any visual courtship behavior prior to copulation (Miyajima et al. 2012). In addition, our trials examining the occurrence of pairing behavior and female choice demonstrated that females changed their behavior depending on the presence or absence of the male setal patch during copulation. Thus, male setal patches are not used to attract females in the same way as behaviors such as courtship waving, but they may serve as information for females to trigger copulation with males.
As for the male mate choice, the males of the non-dimorphic species did not exhibit a preference for setal patches on female chelae or for female body size. Instead, the males were more likely to copulate with the females that they approached first. Males of another varunid crab Gaetice depressus also mated indiscriminately with regard to female body size but frequently copulated with the first females they encountered (Fukui 1995). In the hermit crab Pagurus filholi, males do not prefer females of larger size, of higher fecundity, or who have less time remaining until spawning, suggesting that male hermit crabs may also adopt a mating strategy of pairing with the first ripe female they encounter (Goshima et al. 1998). Fukui (1995) attributed a male-biased operational sex ratio as the cause for an indiscriminative mating strategy by males. In crustaceans, the operational sex ratio is generally biased toward males, as the potential reproductive rate of males is greater than that of females (Duffy & Thiel 2007). Alternatively, crab density may also affect male behavior. Individuals of the non-dimorphic species tend to hide alone in oyster beds, and density is extremely low (A. Miyajima, own data). A low density may make it difficult for males to encounter receptive females; thus, males may prefer to attempt to copulate with the first female they encounter regardless of the presence of setal patches or body size. A similar pattern has been documented in the fiddler crab, Uca mjoebergi (Reading & Backwell 2007).
The present study is the first to demonstrate that setal patches on the chelae affect the owner's success in social behaviors; that is, setal patches of male chelae are advantageous for mating in the dimorphic species and in both male–male fighting and mating in the non-dimorphic species. Contrary to our expectations, the social function of male setal patches was more prominent in the non-dimorphic species, in which both sexes bear setal patches, than in the dimorphic species, in which only males bear setal patches. This difference appears to arise from an interspecific difference in social behavior, as mentioned above. On the other hand, the setal patch of females of the non-dimorphic species did not confer any advantages in the social interactions examined here, raising the question of why do both sexes bear setal patches in the non-dimorphic species? One possible reason is that they serve another function that is naturally selected in both sexes (Kraaijeveld et al. 2007), while the social function evolved only in males. For example, in antelope and deer species for which both sexes carry horns, male horns are used for contests with rivals, whereas female horns are used primarily in defense against predators or in competition for resources (Andersson 1994; Clutton-Brock 2009). Bright colors in male and female poison-dart frogs are thought to signal distastefulness in both sexes, but the colors of males are also used in female choice (Kraaijeveld et al. 2007; Stevens & Ruxton 2012). When females bear the same ornaments as males as a result of correlated selection and sexual selection also occurs on the ornament of the males; then, these traits are expected to be more exaggerated (e.g., larger in size) in males than in females (Worthington et al. 2012). In the non-dimorphic species, the setal patch on chela is sometimes used for feeding in both sexes (A. Miyajima, own data), suggesting correlated selection; however, the male-biased functions for fighting and mating as well as the larger increase in setal patch size with growth in males than in females (Miyajima et al. 2012) indicate that the male setal patch of the non-dimorphic species is also a sexually selected trait.
Species bearing setal patches on their chelae abound in the family Varunidae. Although there are a variety of patterns of sexual differences in the appearance of setal patches and their growth, most species have the setal patches on male chelae but not on female chelae, as in the dimorphic species. The present study provides evidence that setal patches on male chelae affect both fighting success and mating behaviors, suggesting that the setal patches of males are sexually selected traits. However, we did not observe an advantage of the female setal patch in a social context. Overall, our results imply that setal patches on chelae of decapod crustaceans function in the social interactions of males but not females.
Acknowledgements
We thank Professor Y. Yusa of Nara Women's University and Professor Y. Fukui of Osaka University of Arts Junior College for valuable advice and support of this study. We also thank the members of the Laboratory of Population and Community Ecology, Nara Women's University and of the Seto Marine Biological Laboratory, Kyoto University, as well as Mr. R. Murakami for their support and encouragement. Dr. P. Backwell, Dr. J. Christy, and Dr. M. Thiel, and an anonymous reviewer kindly reviewed the manuscript. This work was supported in part by Nara Women's University Intramural Grant for Project Research to K. Wada.




