MyD88-mediated signaling is critical for the generation of seizure responses and cognitive impairment in a model of anti-N-methyl-D-aspartate receptor encephalitis
Abstract
Objective
We previously demonstrated that interleukin-1 receptor-mediated immune activation contributes to seizure severity and memory loss in anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis. In the present study, we assessed the role of the myeloid differentiation primary response gene 88 (MyD88), an adaptor protein in Toll-like receptor signaling, in the key phenotypic characteristics of anti-NMDAR encephalitis.
Methods
Monoclonal anti-NMDAR antibodies or control antibodies were infused into the lateral ventricle of MyD88 knockout mice (MyD88−/−) and control C56BL/6J mice (wild type [WT]) via osmotic minipumps for 2 weeks. Seizure responses were measured by electroencephalography. Upon completion of the infusion, the motor, anxiety, and memory functions of the mice were assessed. Astrocytic (glial fibrillary acidic protein [GFAP]) and microglial (ionized calcium-binding adaptor molecule 1 [Iba-1]) activation and transcriptional activation for the principal inflammatory mediators involved in seizures were determined using immunohistochemistry and quantitative real-time polymerase chain reaction, respectively.
Results
As shown before, 80% of WT mice infused with anti-NMDAR antibodies (n = 10) developed seizures (median = 11, interquartile range [IQR] = 3–25 in 2 weeks). In contrast, only three of 14 MyD88−/− mice (21.4%) had seizures (0, IQR = 0–.25, p = .01). The WT mice treated with antibodies also developed memory loss in the novel object recognition test, whereas such memory deficits were not apparent in MyD88−/− mice treated with anti-NMDAR antibodies (p = .03) or control antibodies (p = .04). Furthermore, in contrast to the WT mice exposed to anti-NMDAR antibodies, the MyD88−/− mice had a significantly lower induction of chemokine (C-C motif) ligand 2 (CCL2) in the hippocampus (p = .0001, Sidak tests). There were no significant changes in the expression of GFAP and Iba-1 in the MyD88−/− mice treated with anti-NMDAR or control antibodies.
Significance
These findings suggest that MyD88-mediated signaling contributes to the seizure and memory phenotype in anti-NMDAR encephalitis and that CCL2 activation may participate in the expression of these features. The removal of MyD88 inflammation may be protective and therapeutically relevant.
Key points
- In contrast to wild-type mice, MyD88 knockout mice did not develop seizures and displayed preserved memory after exposure to patient anti-NMDAR antibodies.
- MyD88 knockout mice did not develop CCL2 activation in the hippocampus, in contrast to wild-type mice.
- Along with interleukin-mediated pathways, TLR/MyD88 signaling may represent a novel target for seizures and neuroprotection in anti-NMDAR encephalitis.
1 INTRODUCTION
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is the most common type of autoimmune encephalitis; it affects 1.5 per million persons per year and develops in previously healthy individuals or patients with early stage tumors.1 The disease manifests with precipitous cognitive decline, psychosis, and seizures, with a mortality rate approaching 25%.1 The acute phase of the disease is marked by severe seizures that are resistant to conventional treatments , and chronic phase sequelae include persistent loss of memory leading to long-term disability.2 The pathogenesis of seizures and memory loss in anti-NMDAR encephalitis is not well understood, which impedes the development of novel treatments and effective rehabilitation strategies for these patients.
In the past decade, the role of neuroinflammation as a major factor in epilepsy has been highlighted following the discovery of acute seizure syndromes, including anti-NMDAR encephalitis and new onset refractory status epilepticus (NORSE); in both disorders, seizures are likely either triggered or maintained by central immune activation.3, 4 Consistent with this premise, seizures resistant to anticonvulsants improve in some autoimmune encephalitis patients following the administration of the glucocorticoid methylprednisolone, a wide-spectrum anti-inflammatory agent, as well as agents directed against specific interleukins.5, 6 Specifically, administration of anakinra and tocilizumab, interleukin-1 (IL-1) and IL-6 receptor antagonists, respectively, improved symptoms of autoimmune encephalitis and seizures in some patients resistant to other treatments.5, 7 Our previous studies showed that neuroinflammation perpetuates seizures in anti-NMDAR encephalitis and that blocking IL-1 receptor (IL-1R)-mediated signaling attenuates seizures and alleviates memory loss in mice exposed to antibodies derived from anti-NMDAR encephalitis patients.8, 9 Furthermore, the antagonism of IL-1R-mediated signaling reduced the expression of glial and astrocytic markers of inflammation.9
In addition to interleukin signaling, another major contributor to the inflammatory responses during seizures is the Toll-like receptor (TLR) pathway.4, 10 In the brain, TLRs are expressed in astrocytes, oligodendrocytes, microglia, and neurons. In the absence of pathogens, TLR signaling can be activated by molecules released during tissue injury or intense seizures, thereby providing a potential new therapeutic target.10 The interactions between the TLR and IL-1R systems in seizures are bidirectional. Following engagement of the TLR system, microglial cells release IL-1β, which is a potent proconvulsant that may trigger recurrent seizures.10 Similarly, the sustained seizures in status epilepticus cause neuroinflammation via the IL-1β system and trigger the downstream activation of TLRs, thereby promoting tissue reorganization and epileptogenesis.11 Although activation of TLR signaling has been demonstrated in several animal seizure models,12 its role in the persistence of autoimmune seizures has not been characterized. Studies in patients with NORSE and other acute severe autoinflammatory seizure syndromes found increased levels of relevant cytokines and chemokines in the cerebrospinal fluid (CSF).13-15 Identifying the role of specific inflammatory pathways in the development of autoimmune encephalitis-associated seizures would uncover new targets for seizure attenuation.
Myeloid differentiation primary response gene 88 (MyD88) is a key adapter protein of TLR signaling.16, 17 Following activation of any TLR except for TLR3, MyD88 recruits IL-1 receptor-associated kinases (IRAK 4 and 1) and tumor necrosis factor receptor-associated factor 6 (Traf 6). Upon phosphorylation of IRAK1, the IRAK-Traf 6 complex dissociates from the TLR complex, leading to the downstream activation of nuclear factor kappa B (NF-κB), which promotes the transcription of relevant inflammatory mediators.17, 18 Recent studies have demonstrated that inhibition of MyD88 significantly suppressed seizures in animal models19, 20; however, the role of MyD88 in the pathogenesis of autoimmune seizures has not been previously assessed. In the present study, we investigated the possible involvement of MyD88 in the development of seizures and cognitive impairment as well as the recruitment of brain inflammatory mediators in anti-NMDAR encephalitis using a mouse model previously developed in our laboratory.8
2 MATERIALS AND METHODS
2.1 Animals
Male and female MyD88−/− mice (B6.129P2(SJL)-Myd88tm1.1Defr/J; stock #009088) and C56BL/6J mice (8–12 weeks old) used as a control were obtained from Jackson Laboratory. The genomic library screen using the Mini–Mouse Universal Genotyping Array on MyD88−/− and wild-type (WT) mice revealed >99.9% resemblance between the two strains (Figure S1). Animals were housed in groups of five initially and individually after the surgery; they were maintained on a 12-h light cycle (light on/off at 7 a.m./7 p.m.) with ad libitum access to normal chow and water. The diet sterilization procedure has not been used. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Review Committee for the Use of Animals at the University of Nebraska Medical Center. The principles outlined in the ARRIVE principles, including the 3R concept, were followed during experimental planning.21
2.2 Antibodies
IgG1λ monoclonal antibodies (2G6) specific for GluN1 were derived from memory B cells of a patient with anti-NMDAR encephalitis and control 6A IgG1λ human monoclonal antibodies that do not bind GluN1 receptors were prepared as previously described.9, 22 Antibodies were dissolved in phosphate-buffered saline (PBS) and administered intracerebroventricularly via subcutaneous osmotic minipump at a concentration of .2 μg/μL.
2.3 Stereotaxic surgery and osmotic minipump insertion
Mice were stereotaxically implanted with an injector guide cannula into the lateral ventricle, a head mount for two electroencephalographic (EEG)/two electromyographic channels (Pinnacle Technology, Lawrence, KS) and two subdural cortical screw EEG electrodes as previously described.8, 9, 23 After a 7-day recovery period, mice were implanted with osmotic minipumps filled with experimental or control antibody solution, and the pumps were connected to the guide cannula (Figure 1A). The continuous EEG recording was started while the IgG solution was continuously perfused at a flow rate of .25 μL/h for 14 days. Upon completion of the experiments, the contents of the pumps were examined for residual IgG solution. The residuals were <.05%.
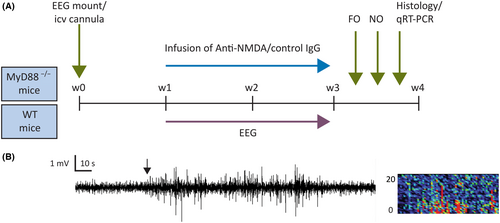
2.4 EEG acquisition and analysis
EEG signals were recorded from the subdural screw electrodes as previously described (Sirenia Seizure Pro 1.8.4., Pinnacle Technology).8, 9, 23 EEG and video analysis to identify electrographic and behavioral seizures was carried out retrospectively and verified visually without knowledge of the treatment status (Figure 1B). Seizures were defined as >5 s of sustained, rhythmic high-amplitude activity in both EEG leads; the events had to be separated by at least 10 s of the background.8 The modified Racine scale was applied to characterize the corresponding behavioral component of seizures.9 The analysis was performed manually by an investigator blinded to the genotype or treatment status of the mice.
2.5 Behavioral tests
The behavioral tests, including the open field and novel object recognition tests, were conducted as previously described.8, 9, 23 Briefly, mice were habituated in the testing room for 30 min before being placed into the chamber and were allowed to move freely during a 20-min trial during which the total distance traveled and percent time spent in the center were recorded. In the novel object test, after a 30-min habituation period, mice were placed into the acrylic chamber and allowed to explore a pair of identical “familiar” objects (FOs), positioned approximately 21 cm apart within the chamber, for 10 min (acquisition phase). After a 2-h retention interval in which mice were isolated from the test environment, one FO was replaced with a “novel” object (NO), and the time spent in texploration of each object was recorded for 5 min (test phase).9 Mice that spent <3 s in total exploration time with both objects were excluded. By this criterion, four mice were excluded from the analysis. The assessment was performed automatically (Any-maze, Stoelting) by an investigator blinded to the treatment status of the mice.
2.6 Preparation of brain tissue, immunohistochemistry, and image processing
Histological evaluations were carried out as previously described.8, 9, 23 Briefly, upon completion of experiments, animals were deeply anesthetized with isoflurane and transcardially perfused with an ice-cold PBS solution. Brains were collected, and the two hemispheres were separated. One hemisphere was immersed in 4% paraformaldehyde in PBS for 48–72 h, transferred to 70% ethanol solution, and stored at +4°C until it was paraffin-embedded for histology studies. The contralateral hippocampus was dissected out, flash frozen, and stored at −80°C for further studies.
Paraffin-embedded coronal sections (4 μm; between bregma −1.55 and −2.03; at least three sections per mouse) were processed for immunohistochemistry for glial fibrillary acidic protein (GFAP; astrocytes) and ionized calcium-binding adaptor molecule 1 (Iba-1; microglia). Briefly, sections were blocked with 2.5% horse serum for 30 min and were incubated at 4°C overnight with polyclonal rabbit anti-GFAP antibodies (1:500, N 1506, Dako) or anti-Iba-1 antibodies (1:500, 019-19741, Fujifilm Wako Chemicals). Following extensive washing, horse antirabbit secondary antibodies (Vector Laboratories) were used for signal detection.
Slide specimens of the CA1 region of the hippocampus were scanned with a Nuance Multi-Spectral Imaging System (Cambridge Research Instruments) fitted to a Nikon ECLIPSE 55i microscope with a 20× objective (1392 × 1040 pixels, .4975125 μm2/pixel). The absorbance profiles of immunostaining for GFAP or Iba-1 were determined, and quantitative grayscale images corresponding to these profiles were extracted from the scanned images. Grayscale images were transferred to the Nuance environment for quantification; the abundancies per event were computed as the product of mean pixel intensity and area (grayscale units [gsu] ● μm2).9, 23
2.7 RNA isolation and quantitative real-time polymerase chain reaction
Total mouse hippocampus RNA isolation and cDNA analysis were carried out as previously described.23 Primers used for quantitative real-time polymerase chain reaction are listed in Table 1.
| Genes | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| GAPDH | TGTCCGTCGTGGATCTGAC | CCTGCTTCACCACCTTCTTG |
| ACTB | CCAACCGTGAAAAGATGACC | ACCAGAGGCATACAGGGACA |
| HPRT1 | GGTCCATTCCTATGACTGTAGATTTT | AATCAAGACGTTCTTTCCAGTT |
| TNF | TCTTCTGTCTACTGAACTTCGG | AAGATGATCTGAGTGTGAGGG |
| COX-2 | CTCCACCGCCACCACTAC | TGGATTGGAACAGCAAGGAT |
| CCL2 | CATCCACGTGTTGGCTCA | GCTGCTGGTGATCCTCTTGTA |
| Iba-1 | TCGATATCTCCATTGCCATTCAG | GATGGGATCAAACAAGCACTTC |
2.8 Statistical analysis
The median seizure counts, and the mean locomotor activity and anxiety and memory scores as well as immunopositivity for GFAP and Iba-1 in the hippocampus were compared for each genotype using t-tests (GraphPad Prism 8.4, Boston, MA, USA). The median fold change of hippocampal mRNA in mice treated with anti-NMDAR antibodies from the corresponding mediator levels in mice treated with control inactive antibodies was compared for all inflammatory mediators using analysis of variance followed by Sidak multiple comparison tests.
3 RESULTS
3.1 MyD88 knockout mice did not develop seizures during exposure to anti-NMDAR antibodies for 2 weeks
In contrast to our previous findings of frequent seizures in >80% of WT mice infused with anti-NMDAR antibodies,8, 23 only three of 14 MyD88−/− mice (21.4%) of both sexes (n = 6 male, n = 8 female) developed seizures (median = 0, interquartile range [IQR] = 0–.25 in 2 weeks; Figure 2). Consistent with our previous reports,8, 9, 23 eight of 10 (80%) WT mice (n = 8 male, n = 2 female) treated with monoclonal patient-derived anti-NMDAR antibodies developed seizures (median = 11, IQR = 3–25 in 2 weeks; Figure 2). The seizure counts in WT mice treated with anti-NMDAR antibodies were significantly higher than in mice of the same phenotype treated with control antibodies, but such difference was not present in MyD88−/− mice receiving the same treatments (p = .002 and p = .99, respectively; t-tests). Consistent with our previous findings, there were rare seizures in both MyD88−/− (n = 8 male, n = 6 female) and WT mice (n = 10 male, n = 3 female) treated with control antibodies; the total median counts in 2 weeks were 0 (IQR = 0–.5) in each group.
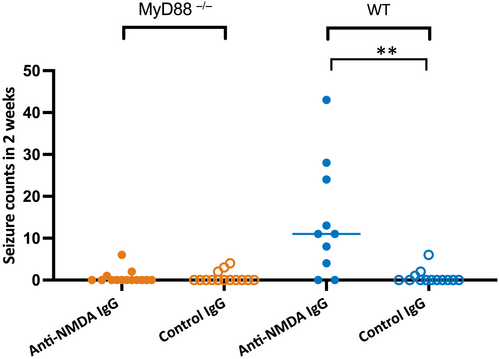
Of 198 seizures recorded in all four groups of mice, 185 (93%) were electrographic,8 whereas 11 seizures (5.7%) were accompanied by minor behavioral changes or myoclonic jerks, and two (1%) had an unclear presentation. The median of total seizure duration over 2 weeks in the MyD88−/− mice treated with anti-NMDAR and control antibodies was 388 (IQR = 44–732) and 213 (IQR = 210–575) s, whereas the duration of seizures in the corresponding groups of WT mice was 1514 (IQR = 224–3393) and 178 (IQR = 84–795) s, respectively.
3.2 MyD88 knockout mice do not develop memory impairment following the infusion of patient antibodies
In an open field chamber, the overall distance traveled by mice was similar in MyD88−/− of both sexes treated with anti-NMDAR antibodies (n = 5 male, n = 7 female) and those treated with control antibodies (n = 7 male, n = 5 female; Figure 3A). Specifically, the mean travel distances of MyD88 mice were 88.3 ± 4.3 and 91.3 ± 6.6 m, respectively (p = .7, t-test). Consistent with our previous reports,8, 9, 23 the measures of distance were not different in WT mice treated with anti-NMDAR (n = 8 male, n = 2 female) and control antibodies (p = .1, t-test; n = 9 male, n = 2 female). During the session, they traveled 50.7 ± 8.1 and 66.4 ± 6.9 m, respectively (Figure 3A).
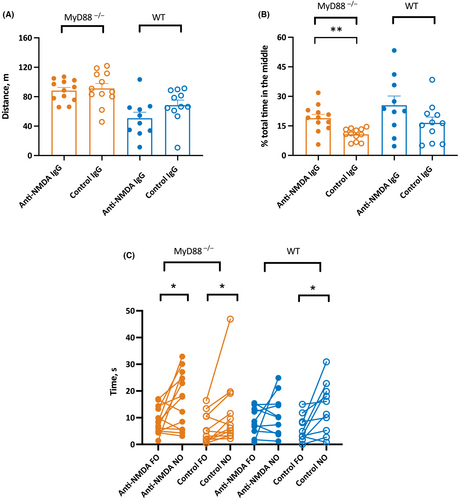
The mean percent time spent in the center of the arena was significantly higher in MyD88−/− male and female mice treated with anti-NMDAR antibodies compared to those infused with control IgG (p = .001, t-test; Figure 3B). This was consistent with previous reporting on the higher level of anxiety in MyD88−/− mice.24 Thus, the mean percent time in the anti-NMDAR antibody-treated group (n = 6 male, n = 7 female) and control antibody-treated groups (n = 7 male, n = 5 female) in MyD88−/− were 18.9 ± 1.9 and 10.7 ± .9 percent, respectively. The mean percent time spent in the center of the arena was similar in WT mice treated with anti-NMDAR and control antibodies (p = .11, t-test; Figure 3B). The WT mice treated with anti-NMDAR antibodies (n = 8 male, n = 2 female) and control antibodies (n = 9 male, n = 2 female) spent on average 25.5 ± 4.7 and 16.3 ± 3 percent, respectively, of time in the center (Figure 3B).
Similar to our previous findings in WT mice,9 in the measure of memory, MyD88−/− mice treated either with anti-NMDAR antibodies or with control antibodies demonstrated a preference for exploring NOs over FOs, consistent with intact spatial memory (p = .03 and p = .04, paired t-tests; Figure 3C). The times spent on FOs and NOs in the MyD88−/− group treated with anti-NMDAR antibodies were 9.0 ± 1.4 and 16.4 ± 3.0 s, respectively (n = 7 male, n = 6 female), whereas the corresponding latencies in the groups infused with control antibodies were 5.8 ± 1.6 and 11.5 ± 3.6 s, respectively (n = 7 male, n = 5 female). In the WT group treated with anti-NMDAR antibodies, consistent with our previous report,9 there was no difference with respect to time spent exploring the FOs and NOs, which suggests impaired memory (p = .41, paired t-test; Figure 3C). The latencies for the FOs and NOs in the WT mice treated with anti-NMDAR antibodies were 8.6 ± 1.6 and 11.3 ± 2.4 s, respectively (n = 8 male, n = 2 female). As expected, WT mice receiving control antibodies had intact memory and spent significantly less time exploring the FOs compared to the NOs (6.0 ± 1.5 and 13.8 ± 3.0 s, p = .02, paired t-test; n = 8 male, n = 2 female).
3.3 Expression of astrocytic and microglial markers of inflammation in the hippocampus of MyD88 knockout mice
Although we did not previously find evidence of astrocyte proliferation in WT mice with antibody-induced seizures during the infusion of patients' CSF and purified patient antibodies,8 it was important to confirm our finding with monoclonal anti-NMDAR antibodies and determine the histopathological changes in MyD88−/− mice in response to the antibodies. The expression of GFAP in the hippocampal CA1 region of MyD88−/− mice treated with anti-NMDAR antibodies (n = 7 male, n = 4 female) was similar to that in mice of the same genotype treated with control antibodies (p = .70, t-test; Figure 4A; n = 5 male, n = 4 female). The expression (mean ± SEM) of GFAP in these groups was 9.7 ± 1.4 and 8.9 ± 1.6 gsu ● μm2 × (103), respectively. The expression of GFAP in WT mice treated with anti-NMDAR and control antibodies was 15.3 ± 1.7 and 15. 6 ± 2.2 gsu ● μm2 × (103), respectively; there was no difference between the two groups (p = .9, t-test; n = 5 male, n = 3 female [anti-NMDAR]; n = 7 male, n = 3 female [control]).
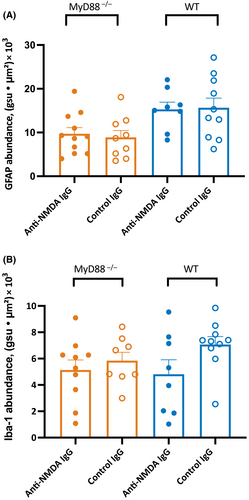
The expression of Iba-1 in the hippocampal CA1 region of MyD88−/− mice treated with anti-NMDAR and control antibodies was 5.1 ± .8 and 5.8 ± .6 gsu ● μm2 × (103), respectively (n = 6 male, n = 4 female [anti-NMDAR]; n = 5 male, n = 3 female [control]). There was no change in the expression of microglial activation in response to the administration of patient antibodies (p = .51, t-test; Figure 4B). The expression of Iba-1 in the WT group treated with the control antibodies was not different from those treated with anti-NMDAR antibodies (4.8 ± 1.1 and 7.1 ± .6 gsu ● μm2 × 103, respectively; p = .08, t-test; n = 5 male, n = 3 female [anti-NMDAR]; n = 7 male, n = 3 female [control]). The representative images of histology for GFAP and Iba-1 are shown in Figures S3A, B.
3.4 MyD88 knockout mice do not develop enhanced expression of C-C chemokine ligand 2 during exposure to anti-NMDAR antibodies
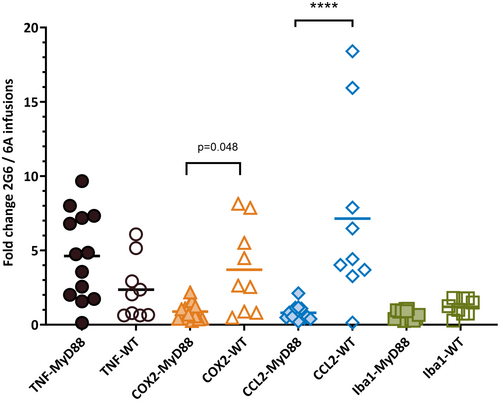
The hippocampal mRNA expression for the five proinflammatory genes most relevant for antibody-induced seizures was examined upon completion of the behavioral testing, approximately 3 days after the infusion of antibodies was completed. The levels of tumor necrosis factor (TNF), cyclooxygenase-2 (COX-2), C-C chemokine ligand 2 (CCL2), and Iba-1 mRNAs in mice treated with anti-NMDAR 2G6 antibodies were normalized to the corresponding levels of mice treated with control antibodies (Figure 5). The fold changes (mean ± SEM) in the expression of these mediators in MyD88−/− groups treated with the patient and control antibodies were 4.6 ± .8, .9 ± .2, .8 ± .1, and .7 ± .1 (n = 5 male, n = 8 [anti-NMDAR]; n = 8 male, n = 2 female [control]). On the other hand, the corresponding values in the WT group were 2.4 ± .7, 3.7 ± 1.0, 7.1 ± 2.0, and 1.2 ± .2 (n = 8 male, n = 6 female [anti-NMDAR]; n = 4 male, n = 2 female [control]). Thus, the expression of CCL2 was significantly higher in the WT mice compared to the MyD88 knockout mice (p = .0001), whereas the expression of COX-2 in the former group was only marginally higher in the former group (p = .048, Sidak tests).
4 DISCUSSION
In the present study, we have demonstrated that mice deficient in MyD88 protein do not develop seizures or memory impairment during exposure to patient-derived anti-NMDAR antibodies, thereby supporting the critical role of MyD88 in the pathophysiology of seizures in autoimmune encephalitis and expression of key behavioral features of this disorder. We also found that MyD88−/− mice had reduced CCL2 and COX-2 activation in the hippocampus compared to WT mice following antibody treatment. Consistent with our earlier findings in WT mice,23 MyD88−/− mice treated with anti-NMDAR antibodies did not show changes in the expression of TNF or Iba-1 and did not develop microglial or astrocytic activation in the hippocampus. Collectively, these data support the critical role of MyD88 signaling in the pathophysiology of autoimmune seizures and are compatible with the notion that CCL2 contributes to both seizure and memory phenotypes in autoimmune encephalitis.
Our previous studies showed that neuroinflammation perpetuates seizures in anti-NMDAR encephalitis and that blocking IL-1R-mediated signaling attenuates seizures and alleviates memory loss in mice exposed to patient-derived anti-NMDAR antibodies.9 In the present study, we have examined TLR signaling, another major pathway that is critically important for neuroinflammatory responses during seizures.17 We took advantage of MyD88 knockout mice that are known to develop seizures in response to chemoconvulsants and during viral infection-induced encephalitis.20, 25 Along with IL-1 signaling, the TLR cascade is dependent on MyD88 protein function; the latter serves as an essential adaptor for the signaling exerted by most TLR subtypes.26 The IL-1/TLR signaling has been shown to contribute to the pathogenesis of autoimmune disorders such as multiple sclerosis, which supports a novel concept that inflammation can regulate autoimmunity27; however, studies on the interplay of neuroinflammation and autoimmunity in epilepsy are just beginning to emerge.9, 28
The altered expression of the MyD88 protein may be an important contributor to the pathogenesis of seizures, thus targeting MyD88-mediated signaling may present a novel strategy to mitigate seizures.19 Recent studies demonstrated that intracerebroventricular administration of the small synthetic inhibitor of MyD88 protein prevented pilocarpine-induced seizures in rats.19 Furthermore, patients with refractory temporal lobe epilepsy showed enhanced expression of MyD88 in the resected pathology specimens.29 We found that MyD88-mediated signaling also supports seizure responses induced by anti-NMDAR antibodies in that their removal protects against seizures. The inability of MyD88 knockout mice to develop seizures in response to antibodies could be due to interruption of their IL-1 receptor-mediated signaling as shown by us with administration of anakinra in mice.9 The effects of MyD88 removal on seizures in our model could be caused by interruption of the IRAK-Traf 6 or NF-κB signaling downstream of MyD88. Aberrant IRAK 1-4/Traf 6 signaling is the key regulator of inflammation in several autoimmune disorders such as rheumatoid arthritis, psoriasis, and lupus; however, data on the involvement of these proteins in autoimmune central nervous system disorders are just beginning to emerge.29 Thus, genetic deficiency of IRAK 4 was protective against the induction and development of experimental encephalomyelitis in mice,30 whereas Traf 6 was involved in the inflammatory responses relevant to traumatic brain injury, Alzheimer's disease (AD), and seizures.18, 31-33 We did not assess the expression of these proteins; however, these signaling molecules deserve further attention as potential therapeutic targets in encephalitis. The agents targeting the NF-κB complex are available and have already been effective in Parkinsons disease and other conditions.34
An alternative role of MyD88 in seizure generation in encephalitis may involve a non-transcription-dependent activation of the ceramide pathway.17 Recently, proinflammatory and epileptogenic roles of ceramide have been highlighted in epilepsy and other neurodegenerative conditions.17, 35-37 In seizures, this pathway starts from IL-1β-mediated activation of MyD88 and ceramide-producing enzyme neuronal sphingomyelinases (N-Smase), which leads to the subsequent phosphorylation of the Src family of tyrosine kinases and target receptor protein NR2B subunit.38 This leads to an increase of NMDAR calcium conductance and increased neuronal excitability in the hippocampus within minutes, which in turn was thought to exacerbate the convulsive effects of IL-1.35 Pharmacological blockage of the N-Smase resulted in attenuation of seizures and neuronal damage,35, 39 whereas blockage of ceramide receptors with fingolimod was antiepileptic in the lithium–pilocarpine seizure model40 and genetic absence seizure model.41 Thus, the protection from seizures in MyD88−/− mice could be rendered by their inability to support the downstream effects of ceramide pathway activation; if so, the former can serve as a target for novel treatment approaches in resistant seizures in encephalitis.
Building upon our previous findings of CCL2 induction in WT mice with anti-NMDAR antibody-induced seizures,23 we showed in the present study that MyD88−/− mice do not undergo CCL2 induction during the exposure to antibodies. CCL2 is released by activated microglia and acts on the CCR2 receptor on monocytes to facilitate their entry across the capillaries in traumatic brain injury, epilepsy, and AD models.42-44 In human monocyte cells, TLR4-dependent activation of CCL2 gene expression by lipopolysaccharide requires MyD88 recruitment and activation of NF-κB1 transcription factors.45 CCL2 also has a proconvulsant effect in the settings of systemic inflammation induced by lipopolysaccharide in kainate-treated mice.46 In a recent study, serum and CSF levels of CCL2 were found to be significantly higher in patients with NORSE compared to those with other forms of drug-resistant status epilepticus; higher levels were correlated with worse patient outcomes.28 Collectively, these data provide evidence that CCL2 could serve as an important mediator between peripheral immune activation and neuronal hyperexcitability, and its role in recruiting brain-invading monocytes in seizures has been recently underscored in pharmacologically induced epilepsy models.12, 44 We did not find any evidence of microglial or astrocytic activation in the hippocampus on day 17 after the start of exposure to antibodies, which was consistent with our previous studies using monoclonal anti-NMDAR antibodies in our model.9 It is possible that the histopathological changes take a longer time to develop, or they precede the changes in the expression of proinflammatory mediators demonstrated with polymerase chain reaction. Alternatively, astrogliosis and microgliosis may be developing in regions outside of the hippocampal CA1, and the latter were not assessed in the present study. We have previously reported that there was no evidence of neuronal death in the WT mice with seizures induced by anti-NMDAR antibodies.8 Given that the MyD88 knockout mice did not have any frank behavioral or pathohistological features of encephalitis, it is even less likely that they would have any neuronal death.
Consistent with our previous studies and other reports,9, 47 WT mice exposed to anti-NMDAR antibodies in this study demonstrated impaired memory function in a novel object paradigm, whereas MyD88 knockout mice did not demonstrate such deficits. The antibody-induced internalization of the surface NMDAR clusters has been shown to contribute to the behavioral phenotype of anti-NMDAR encephalitis.1 In our study, both WT and knockout mice demonstrated the characteristic pattern of immunostaining for NMDAR receptors in the hippocampus (Figure S2). Although unlikely, the possibility of failure of the NMDARs to internalize following the exposure to anti-NMDAR antibodies in MyD88 mice with the resultant protected memory and seizure phenotype cannot be ruled out. This could be addressed by the assessment of the synaptic and extrasynaptic NMDAR pools in the knockout mice in future studies. The potential mechanisms of memory loss in epilepsy include neuronal loss resulting from seizures, neuroinflammation, and aberrant adult neurogenesis in the hippocampus.48 Histopathological examination of biopsy and postmortem brain specimens of patients with anti-NMDAR encephalitis and animal models of encephalitis failed to identify neuronal loss in the hippocampus,49 and we did not previously find any evidence of hippocampal neuronal death in our mouse model.8 Independent of seizures, higher baseline CCL2 levels in CSF were associated with faster rates of cognitive decline during the early stages of AD, further supporting the premise that neuroinflammation may work synergistically with other factors to facilitate cognitive loss.50 The lack of CCL2 activation in MyD88 knockout mice could reflect reduced neuroinflammation; however, the degree of histological hippocampal inflammation is difficult to establish in the absence of astro- and microgliosis in the corresponding groups of WT mice. Finally, the TLR/MyD88-mediated signaling could affect neurogenesis51 and loss of MyD88 function could render neuroprotection.
The limitations of this study include the limited capacity of our model to represent the full spectrum of all anti-NMDAR encephalitis symptoms, including various seizure types.8 Despite that, the most disabling manifestations of encephalitis are well represented in this model. The other limitation is the possible physiological adaptations in MyD88 knockout mice that could contribute to protection against seizures and memory loss and limit the interpretation of findings. To further confirm the effects of MyD88 removal, the pharmacological inhibition of MyD88 with selective antagonists may be undertaken in future studies using the same experimental paradigm. This approach could further help to discern the contribution of the IL-1 receptor and TLR signaling. Another limitation includes a possible partial resistance of MyD88 knockout mice to the effects of antibodies on seizures rather than a complete resistance. These mice may require a higher dose of antibodies or longer exposure to antibodies to develop seizures. This was not possible to address without further developing our seizure model, including the assessment of WT mice under the extended timeline and higher IgG concentrations. Overall, the MyD88 knockout mice and WT have been shown to have a similar behavioral profile, although the former are known to exhibit higher baseline anxiety levels and slower learning but comparable working memory performance.24
In conclusion, the present study demonstrated that loss of MyD88 could suppress seizure activity, memory loss, and CCL2 activation during exposure to anti-NMDAR antibodies. These findings suggest that MyD88 is involved in the pathophysiology of anti-NMDAR encephalitis and targeting this adaptor protein might represent a novel therapeutic strategy to inhibit seizures and mitigate cognitive disability in this devastating disorder.
AUTHOR CONTRIBUTIONS
Olga Taraschenko, Howard S. Fox, and Raymond Dingledine conceptualized the study and drafted and edited the manuscript. Ember Eldridge, Priscilla Heliso, Kayley Anderson, and Wenyi Wang performed biological experiments and analyzed the data. Gregory Willcockson and George Casale performed an imaging analysis. Fetweh Al-Saleem and Scott Dessain generated and tested the monoclonal antibodies. All authors read and approved the manuscript.
ACKNOWLEDGMENTS
O.T. received support from the NIH P20GM130447 Cognitive Neuroscience and Development of Aging Award and the DHHS LB606 Nebraska Stem Cell Grant. R.D. received support from 1R01NS112308. R.D. is a named inventor on intellectual property owned by Emory University, is a co-founder and member of the Board of Directors of NeurOp, Inc., and is a co-founder and chair of the Board of Directors of Pyrefin, Inc. The authors would like to thank Robin Taylor for her excellent editorial support.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data are in the article.




