Long-term safety and efficacy of add-on cannabidiol in patients with Lennox–Gastaut syndrome: Results of a long-term open-label extension trial
[Corrections added on 24 September 2021, after first online publication the copyright line has been updated.]
Summary
Objective
Lennox–Gastaut syndrome (LGS) is an epileptic encephalopathy that is often treatment resistant. Efficacy and safety of add-on cannabidiol (CBD) to treat seizures associated with LGS was demonstrated in two randomized controlled trials (RCTs). Patients who completed the RCTs were invited to enroll in this long-term open-label extension (OLE) trial, GWPCARE5 (NCT02224573). We present the final analysis of safety and efficacy outcomes from GWPCARE5.
Methods
Patients received plant-derived highly purified CBD (Epidiolex in the United States; Epidyolex in the European Union; 100 mg/ml oral solution), titrated to a target maintenance dose of 20 mg/kg/day over 2 weeks. Based on response and tolerability, CBD could then be reduced or increased up to 30 mg/kg/day.
Results
Of 368 patients with LGS who completed the RCTs, 366 (99.5%) enrolled in this OLE. Median and mean treatment duration were 1090 and 826 days (range = 3–1421), respectively, with a mean modal dose of 24 mg/kg/day. Adverse events (AEs) occurred in 96% of patients, serious AEs in 42%, and AE-related discontinuations in 12%. Common AEs were convulsion (39%), diarrhea (38%), pyrexia (34%), and somnolence (29%). Fifty-five (15%) patients experienced liver transaminase elevations more than three times the upper limit of normal; 40 (73%) were taking concomitant valproic acid. Median percent reductions from baseline ranged 48%–71% for drop seizures and 48%–68% for total seizures through 156 weeks. Across all 12-week visit windows, 87% or more of patients/caregivers reported improvement in the patient's overall condition on the Subject/Caregiver Global Impression of Change scale.
Significance
Long-term add-on CBD treatment had a similar safety profile as in the original RCTs. Sustained reductions in drop and total seizure frequency were observed for up to 156 weeks, demonstrating long-term benefits of CBD treatment for patients with LGS.
Key Points
- A total of 366 patients with LGS were treated with long-term CBD, with results shown in this article
- Median and mean treatment duration were 1090 and 826 days, respectively, with a mean modal dose of 24 mg/kg/day administered
- The most common AEs were convulsion, diarrhea, pyrexia, and somnolence; most AEs were mild (20%) or moderate (48%) in severity
- Sustained reductions in drop and total seizures were observed through 156 weeks
- Eighty-seven percent or more of patients or caregivers reported improvement in overall condition across all visit windows up to 156 weeks of treatment
1 INTRODUCTION
Lennox–Gastaut syndrome (LGS) is a severe, chronic epileptic encephalopathy characterized by multiple seizure types, abnormal electroencephalographic (EEG) features, and intellectual disability, with onset typically before age 7 years.1, 2 LGS diagnostic criteria are as follows: (1) more than one seizure type, mainly generalized, including tonic, atonic, and atypical absence seizures, with seizure types evolving over time; (2) EEG studies typically showing interictal diffuse slow spike-and-wave complexes (1.5–2.5 Hz) and generalized paroxysmal fast activity; and (3) cognitive impairment/intellectual disability.1-3 Classic seizure types include tonic seizures specifically upon awakening and atonic generalized seizures that may result in drop attacks and can lead to serious harm. Approved medications for LGS include felbamate, lamotrigine, topiramate, rufinamide, clobazam, clonazepam, and most recently a purified plant-derived formulation of cannabidiol (CBD). Based on survey data, valproic acid is a frequently used first-line therapy but is not approved for LGS.2 The ketogenic diet is also frequently used to treat LGS.4 Long-term seizure control and cognitive outcomes are poor for patients with LGS, even with polypharmacological treatment.
Highly purified CBD is approved in the United States as Epidiolex (Greenwich Biosciences) for the treatment of seizures associated with LGS, Dravet syndrome (DS), or Tuberous sclerosis complex (TSC) in patients 1 year or older; it is approved in the UK and European Union as Epidyolex (GW Pharma [International] B.V.) for LGS or DS in conjunction with clobazam in patients 2 years or older; it is additionally approved in Northern Ireland and the European Union for TSC in patients 2 years or older.5-9 CBD is the second most abundant phytocannabinoid derived from the Cannabis sativa L. plant.10 Compared to approved antiseizure medications (ASMs), CBD is structurally unique and has potentially novel multimodal mechanisms of action. CBD is thought to reduce neuronal hyperexcitability through the transient receptor potential vanilloid 1, antagonism of G-protein coupled receptor 55, and modulation of adenosine reuptake.10-12 CBD neither directly binds to nor activates CB1 or CB2 receptors at physiologically achievable concentrations.13-15 CBD has demonstrated antiseizure activity in vitro11, 16, 17 and in clinical trials in patients with DS, LGS, and TSC.5, 7-9, 18-24
In patients with LGS, in two randomized, double-blind, placebo-controlled trials (GWPCARE3 and GWPCARE4), add-on CBD significantly reduced drop and total seizure frequency versus placebo and had an acceptable safety profile.6, 7 Patients who completed GWPCARE3 or GWPCARE4 were invited to enroll in the ongoing open-label extension (OLE) trial (GWPCARE5), in which all patients received CBD. Interim data from patients with LGS enrolled in GWPCARE5 were published and included data through November 2016 (median treatment duration was 263 days [range = 3–430]), with efficacy data up to 48 weeks and safety data up to 61 weeks.19 Here, we present longer term safety and efficacy results for patients with LGS from the final analysis of GWPCARE5 as of December 3, 2019, with safety data over the full duration of follow-up (up to 203 weeks) and efficacy data up to 156 weeks. The OLE trial also included patients with DS who completed treatment in one of two Phase 3 trials (GWPCARE15 or GWPCARE28). These data will be published separately.
2 MATERIALS AND METHODS
2.1 Compliance with ethical standards
This trial was conducted in accordance with International Council for Harmonization Good Clinical Practice guidelines and ethical principles that have their origin in the Declaration of Helsinki. Prior to any trial procedures, written consent was obtained from patients or their parent, caregiver, or legal representative, and, when possible, written assent was obtained from the patient. The informed consent form, protocol, and amendments were approved by the institutional review board or independent ethics committee at each trial site. The trial protocol is registered on the clinicaltrials.gov website (NCT02224573).
2.2 Patients
Patients who completed the treatment period in trials GWPCARE3 (NCT02224560) or GWPCARE4 (NCT02224690) were eligible for enrollment in this OLE trial. All patients were 2–55 years of age and had a clinical diagnosis of LGS that was inadequately controlled by one or more current ASMs, had a history of slow (<3 Hz) spike-and-wave pattern EEG recordings, and had experienced two or more drop seizures per week during the 4-week baseline period of the parent study. Drop seizures in these trials were defined as atonic, tonic, or tonic–clonic seizures involving the entire body, trunk, or head that led (or could have led) to a fall, injury, slumping in a chair, or the patient's head hitting a surface.
2.3 Trial Design
Patients received plant-derived highly purified CBD (Epidiolex in the United States; Epidyolex in Europe; 100 mg/ml oral solution), titrated from 2.5 to 20 mg/kg/day and administered in two divided doses over a 2-week period. Patients continued to receive this dose during the maintenance period. Patients received CBD in addition to their existing ASMs. After obtaining approval by the study sponsor, investigators were permitted to decrease the dose of CBD and/or concomitant ASMs if a patient experienced intolerable adverse effects and could increase the dose to a maximum of 30 mg/kg/day if they considered it may be of benefit.
At data cutoff, patients could receive treatment for up to 1 year (UK, Spain, and The Netherlands), or up to 4 years (United States, France, and Poland). Upon completion of the OLE treatment period, either patients continued CBD if market authorization was granted or the CBD dose was tapered by 10% per day for 10 days for patients not continuing treatment. Patients who withdrew early could also begin the taper period following the withdrawal visit (unless continued dosing was not possible due to an adverse event [AE]). A follow-up visit was performed 4 weeks (+3 days) after the last dose of CBD (including the last tapered dose, where applicable).
The OLE trial was conducted at 53 sites (three in the UK, five in Spain, one in The Netherlands, 37 in the US, one in France, and six in Poland). The first patient entered the OLE trial on June 11, 2015, and data are included up to December 3, 2019, at which point the trial was still ongoing. The OLE trial was conducted with Epidiolex or Epidyolex, and results do not apply to other CBD-containing products.
2.4 Trial procedures
Patients or their caregivers completed a daily paper diary to record AEs and daily use of CBD, concomitant ASMs, and rescue medications. Information on seizure number and type was collected through an interactive voice recording system telephone diary, completed weekly until the end of treatment/withdrawal visit. Blood and urine sampling for clinical laboratory assessments was carried out at all clinic visits through end of taper. The 7-point Subject/Caregiver Global Impression of Change (S/CGIC) scale was assessed at Weeks 24, 38, 48, 76, 104, 132, and 156; the combined caregiver and subject score was used (see Supplemental Materials). The percentage of patients reporting improvement on the S/CGIC scale was assessed using the number of patients who completed the questionnaire as the denominator at all time points.
2.5 Randomization and blinding
All patients who completed the treatment period of the original randomized controlled trials (RCTs) and wished to continue were eligible for inclusion. All patients received CBD in the OLE.
2.6 Outcome measures
The primary objective of this OLE trial was to evaluate the long-term safety and tolerability of add-on CBD in children and adults with inadequately controlled LGS. The operational definition of the term “safety” in this paper includes both safety and tolerability. Safety variables included AEs, vital signs, 12-lead electrocardiograms, clinical laboratory parameters, and physical examination parameters including serum levels of hepatic enzymes; drug-induced liver injury was assessed as per Hy's law.
Secondary objectives evaluated the efficacy of CBD as determined by changes in drop seizures and total seizure frequency, seizure reduction responder rates (proportion of patients with ≥25%, ≥50%, ≥75%, and 100% reductions in drop and total seizure frequency), episodes of status epilepticus, and changes in the S/CGIC scale.
Changes to trial outcomes after the trial started included the addition of the assessment of total seizures in addition to seizure subtypes. Secondary efficacy assessments included total seizures in the two DS and two LGS RCTs that led into this trial.
2.7 Statistical analysis
2.7.1 Sample size
All patients who completed treatment in two previous RCTs were eligible for inclusion. Safety analyses included all enrolled patients (n = 366).
2.7.2 Statistical methods
All data collected during this trial were summarized across time, using appropriate descriptive statistical methods. Seizure frequencies (per 28 days) were determined for each 12-week period of treatment. For defined periods, total seizures in 12 weeks were counted, then divided by the number of days over which data were captured and multiplied by 28 to give "per 28 days" value. Percentage change in seizure frequency for each 12-week visit window through the 156 weeks was calculated relative to the prerandomization baseline period from the parent placebo-controlled trial. For weekly reporting of seizure frequency, if a weekly call was missed, then the seizure frequency for the 12-week period that included that week would be averaged using only the available data, that is, the denominator, number of days in the 12-week period, would be lower. This is equivalent to assuming the missed period would have a frequency similar to nonmissing weeks. Analyses of seizure frequency and seizure reduction responder rates were repeated using inclusion of a last observation carried forward (LOCF) step, described in detail in the Supplemental Materials. All analyses were descriptive, and no formal hypothesis testing was conducted.
3 RESULTS
3.1 Disposition of patients
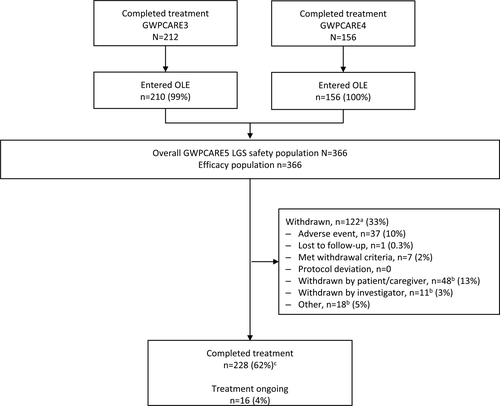
Of the 368 patients with LGS who completed the GWPCARE3 and GWPCARE4 RCTs, 366 (99%) enrolled in this OLE trial across 53 sites in the United States and Europe. Overall, 122 patients (33%) withdrew from treatment; the most common primary reason was patient or parent/guardian decision (n = 48, 13%) or AEs (n = 37, 10%; Figure 1). Although lack of efficacy was not a prespecified option, of the 77 patients with primary reasons for withdrawal reported as withdrawn by patient/caregiver, withdrawn by investigator, or other, 55 patients had additional free-text comments from the investigator suggesting withdrawal due to lack of efficacy. Time to withdrawal for any reason is presented in Figure S1. At the time of analysis, 228 patients (62%) had completed treatment (per country-specific protocols that limited treatment to 1 year) and 16 patients (4%) had ongoing treatment and had not yet reached later treatment windows.
3.2 Demographics
Patient demographics are outlined in Table 1. The mean age of the patients was 16 years, and 54% were male. Patients were taking a median of three concurrent ASMs at baseline; approximately 74% of patients were receiving three or more concurrent ASMs. During the OLE, 54% of patients were taking clobazam and 40% were taking valproate. At baseline of the RCTs, patients had a median of 80 drop and 168 total seizures per 28 days.
| Parameter | CBD, N = 366 |
|---|---|
| Age at entry to OLE, years | |
| Mean (SD) | 15.9 (9.5) |
| Median (range) | 13.7 (3.0–48.3) |
| Age group, years, n (%) | |
| 2–5 | 36 (9.8) |
| 6–11 | 121 (33.1) |
| 12–17 | 89 (24.3) |
| 18–55 | 120 (32.8) |
| Gender, n (%) | |
| Male | 198 (54.1) |
| Geographical region, n (%) | |
| United States | 284 (77.6) |
| Rest of world | 82 (22.4) |
| Body mass index at entry into OLE, mean (SD) | 20.2 (6.3) |
| Baseline seizure frequency per 28 days, median (lower quartile, upper quartile) | |
| Drop | 80.0 (39.0, 154.0) |
| Total | 167.6 (79.2, 385.7) |
| Number of concomitant ASMs, median (range) | 3 (1–13) |
| Concomitant ASMs at baseline, >20%, n (%) | |
| Clobazam | 199 (54.4) |
| Valproic acid | 148 (40.4) |
| Lamotrigine | 134 (36.6) |
| Levetiracetam | 127 (34.7) |
| Rufinamide | 109 (29.8) |
| Concomitant antiepileptic therapies, n (%) | |
| Ketogenic diet | 29 (7.9) |
| Vagus nerve stimulation | 105 (28.8) |
| Time on CBD treatment, days, median (range) | 1090 (3–1421) |
| Modal CBD dose, mg/kg/day, mean (SD) | 24 (5.6) |
- Abbreviations: ASM, antiseizure medication; CBD, cannabidiol; N, number of patients in analysis set; n, number of patients with data/characteristic; OLE, open-label extension.
3.3 Drug exposure
The mean duration of CBD dosing was 826 days, equating to 825.5 patient-years of exposure, and the mean modal dose, the average of doses each patient was on the most, was 24 (range = 2.5–30) mg/kg/day over the treatment period for all patients. Over each 12-week reporting interval, and for the duration of the trial period to the data cutoff, the daily dose remained stable; the mean modal dose per 12-week reporting interval ranged from 21 to 25 mg/kg/day over 156 weeks of treatment, and during the last 12 weeks of treatment, the mean modal dose was 24 mg/kg/day (n = 364). The median CBD treatment duration was 1090 days (156 weeks, range = 3–1421 days); these data included patients for whom national regulations limited the period of treatment to 1 year.
3.4 Safety
Treatment-emergent AEs were reported by 353 of 366 (96%) patients overall at any time during OLE treatment (Table 2), by 137 of 145 (94%) patients with modal dose of 20 mg/kg/day or less, 85 (100%) patients with modal dose of more than 20 up to 25 mg/kg/day, and 131 of 136 (96%) patients with modal dose of more than 25 mg/kg/day. Most treatment-emergent AEs were moderate in severity (48%), 20% were mild, and 28% were severe. Convulsion, diarrhea, pyrexia, somnolence, and vomiting were the most common AEs; somnolence was reported in 74 of 199 (37%) patients who received concomitant clobazam, and 33 of 167 (20%) who did not receive clobazam. AEs of somnolence, sedation, or lethargy were reported in 104 of 199 (52%) patients who received concomitant clobazam, and 45 of 167 (27%) who did not receive clobazam.
| CBD modal dose | CBD, N = 366 | |||
|---|---|---|---|---|
| ≤20 mg/kg/day, n = 145 | >20–25 mg/kg/day, n = 85 | >25 mg/kg/day, n = 136 | ||
| All-causality AEs, n (%) | 137 (94.5) | 85 (100) | 131 (96.3) | 353 (96.4) |
| AEs leading to withdrawal, n (%)a | 31 (21.4) | 9 (10.6) | 3 (2.2) | 43 (11.7) |
| Serious AEs, n (%) | 58 (40.0) | 34 (40.0) | 63 (46.3) | 155 (42.3) |
| AEs reported in >10% of patients by MedDRA preferred term, n (%) | ||||
| Convulsion | 52 (35.9) | 37 (43.5) | 52 (38.2) | 141 (38.5) |
| Diarrhea | 50 (34.5) | 33 (38.8) | 57 (41.9) | 140 (38.3) |
| Pyrexia | 36 (24.8) | 31 (36.5) | 59 (43.4) | 126 (34.4) |
| Somnolence | 40 (27.6) | 28 (32.9) | 39 (28.7) | 107 (29.2) |
| Vomiting | 36 (24.8) | 21 (24.7) | 50 (36.8) | 107 (29.2) |
| Upper respiratory tract infection | 38 (26.2) | 25 (29.4) | 39 (28.7) | 102 (27.9) |
| Decreased appetite | 43 (29.7) | 20 (23.5) | 30 (22.1) | 93 (25.4) |
| Cough | 16 (11.0) | 20 (23.5) | 27 (19.9) | 63 (17.2) |
| Weight decreased | 21 (14.5) | 13 (15.3) | 27 (19.9) | 61 (16.7) |
| Nasopharyngitis | 16 (11.0) | 17 (20.0) | 24 (17.6) | 57 (15.6) |
| Pneumonia | 12 (8.3) | 13 (15.3) | 32 (23.5) | 57 (15.6) |
| Urinary tract infection | 12 (8.3) | 12 (14.1) | 27 (19.9) | 51 (13.9) |
| Ear infection | 15 (10.3) | 16 (18.8) | 19 (14.0) | 50 (13.7) |
| Sinusitis | 8 (5.5) | 13 (15.3) | 28 (20.6) | 49 (13.4) |
| Nasal congestion | 11 (7.6) | 13 (15.3) | 22 (16.2) | 46 (12.6) |
| Influenza | 11 (7.6) | 13 (15.3) | 21 (15.4) | 45 (12.3) |
| Constipation | 15 (10.3) | 7 (8.2) | 21 (15.4) | 43 (11.7) |
| Status epilepticus | 16 (11.0) | 7 (8.2) | 19 (14.0) | 42 (11.5) |
| Insomnia | 12 (8.3) | 13 (15.3) | 15 (11.0) | 40 (10.9) |
| Fatigue | 13 (9.0) | 11 (12.9) | 14 (10.3) | 38 (10.4) |
| Serious AEs reported in >1% of patients by MedDRA preferred term, n (%) | ||||
| Convulsion | 14 (9.7) | 10 (11.8) | 19 (14.0) | 43 (11.7) |
| Status epilepticus | 16 (11.0) | 7 (8.2) | 19 (14.0) | 42 (11.5) |
| Pneumonia | 5 (3.4) | 8 (9.4) | 17 (12.5) | 30 (8.2) |
| Pneumonia aspiration | 9 (6.2) | 3 (3.5) | 4 (2.9) | 16 (4.4) |
| Vomiting | 8 (5.5) | 1 (1.2) | 4 (2.9) | 13 (3.6) |
| Pyrexia | 3 (2.1) | 2 (2.4) | 6 (4.4) | 11 (3.0) |
| Acute respiratory failure | 3 (2.1) | 2 (2.4) | 5 (3.7) | 10 (2.7) |
| Urinary tract infection | 3 (2.1) | 2 (2.4) | 4 (2.9) | 9 (2.5) |
| Hypoxia | 2 (1.4) | 0 | 7 (5.1) | 9 (2.5) |
| Respiratory failure | 4 (2.8) | 1 (1.2) | 3 (2.2) | 8 (2.2) |
| ALT increased | 5 (3.4) | 1 (1.2) | 1 (.7) | 7 (1.9) |
| Sepsis | 2 (1.4) | 0 | 4 (2.9) | 6 (1.6) |
| AST increased | 4 (2.8) | 0 | 2 (1.5) | 6 (1.6) |
| Hepatic enzyme increased | 4 (2.8) | 0 | 2 (1.5) | 6 (1.6) |
| Mental status changes | 0 | 1 (1.2) | 5 (3.7) | 6 (1.6) |
| Respiratory distress | 4 (2.8) | 1 (1.2) | 1 (.7) | 6 (1.6) |
| Diarrhea | 2 (1.4) | 1 (1.2) | 2 (1.5) | 5 (1.4) |
| Transaminases increased | 2 (1.4) | 1 (1.2) | 2 (1.5) | 5 (1.4) |
| Dehydration | 2 (1.4) | 0 | 3 (2.2) | 5 (1.4) |
| Acute kidney injury | 2 (1.4) | 1 (1.2) | 2 (1.5) | 5 (1.4) |
| Ileus | 2 (1.4) | 0 | 2 (1.5) | 4 (1.1) |
| Hypotension | 2 (1.4) | 0 | 2 (1.5) | 4 (1.1) |
| Weight decreased | 1 (.7) | 2 (2.4) | 1 (.7) | 4 (1.1) |
- Abbreviations: AE, treatment-emergent adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBD, cannabidiol; MedDRA, Medical Dictionary for Regulatory Activities; N, number of patients in analysis set; n, number of patients with data/characteristic.
- a Includes all patients with an AE listed as one of the reasons for withdrawal.
Serious AEs were reported in 155 (42%) patients; the most common were convulsion (12%), status epilepticus (12%), and pneumonia (8%; Table 2). Incidence of serious AEs was 43%–48% for pediatric age groups (age <2, 2–5, 6–11, or 12–17 years) and 34% for adult patients (age 18 years or older 18 years). Forty-three patients discontinued due to AEs (12%), most commonly (>1%) due to convulsion (n = 7, 2%) and diarrhea (n = 6, 2%); some patients discontinued due to multiple AEs. Incidence of AEs leading to discontinuation was 9%–16% for pediatric age groups and 12% for adult patients. There were 11 deaths during the OLE trial period reported here, of which three were due to sudden unexpected death in epilepsy (SUDEP) and one was due to convulsion (the death occurred during sleep, but the patient had fever, low oxygen saturation, and reduced urine output for 4 days and was seen in the emergency department the day before death); no death was considered related to CBD by the investigator. The incidence of SUDEP in this OLE trial is ≈3.6 deaths/1000 person-years. Although the rate of deaths/1000 person-years among patients with LGS has not been reported to date, the incidence rate reported in patients with chronic epilepsy is 1–2 deaths/1000 person-years, and is higher with severe, refractory seizures at 3–9 deaths/1000 person-years.25
Increases in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) more than three times the upper limit of normal (ULN) occurred in 55 patients (15%); of these patients, 40 (73%) were on concomitant valproic acid. No patient met the standard criteria for severe drug-induced liver injury (Hy's law) with concurrent elevated bilirubin more than two times ULN. Fifteen (27%) patients withdrew from treatment due to elevated ALT or AST levels. After initiating CBD treatment in the OLE, of the 55 patients with ALT or AST elevations, 12 (22%) patients first had an ALT or AST elevation within 1 month (30 days); for 26 (47%) patients, this was between 1 and 3 months, and for 17 (31%) patients, this was after 3 months (100 days). At the time of this analysis, 52 (95%) patients had resolved ALT or AST levels, either spontaneously (n = 25, 48%, of whom 17 were on valproic acid), following discontinuation from trial (n = 14, 27%, of whom nine were on valproic acid), or after CBD/ASM dose reduction (n = 13, 25%, of whom 12 were on valproic acid), with three elevations still ongoing. Of the 13 patients who had their CBD/ASM dose reduced, seven patients had their valproic acid dose reduced.
3.5 Efficacy
Median drop seizure frequency reduction from baseline was 48% at Weeks 1–12 (a decrease from a median of 80 seizures per month at baseline to 38 per month) and was sustained through 156 weeks (Figure 2A). In the LOCF analysis, median percentage reductions ranged from 48% to 59% during each 12-week visit window (Figure S2A). For the 122 patients who discontinued the study, median (Q1, Q3) drop seizure reduction during their last 12 weeks of treatment was 37% (70%, −17%). Almost half of patients had drop seizure reductions of 50% or more at each visit window (49%–68%); 25% or more, 50% or more, 75% or more, and 100% responder rates are shown in Figure 3A. Responder rates were generally similar in the LOCF analysis; 49%–58% of patients had drop seizure reductions of 50% or more at each visit window (Figure S3A).
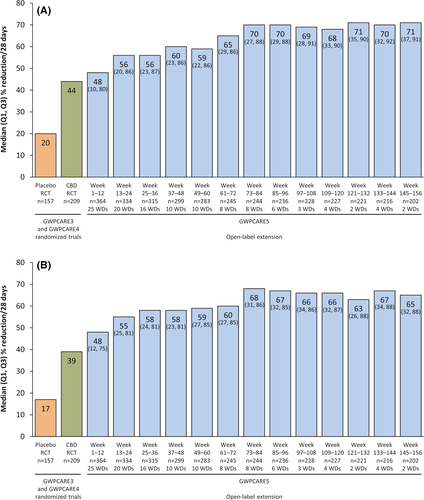
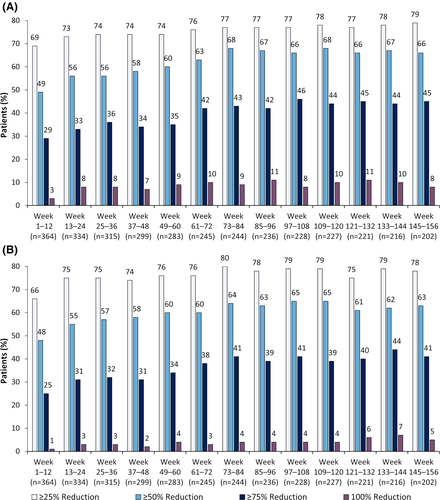
Median total seizure frequency reduction from baseline was 48% at Weeks 1–12 (a decrease from a median of 168 seizures per month at baseline to 79 per month); in the subsequent 12-week visit windows, reductions ranged from 55% to 68% (Figure 2B). In the LOCF analysis, median reductions ranged from 48% to 56% during each 12-week visit window (Figure S2B). Seventeen patients (5%) were seizure-free during their last 12 weeks of treatment. Almost half of patients showed total seizure frequency reductions of 50% or more after the first 12-week period (48%–65%); responder rates at 25% or more, 50% or more, 75% or more, and 100% total seizure reduction thresholds are shown in Figure 3B. Responder rates were generally similar in the LOCF analysis (Figure S3B), with 50% or more responder rates ranging from 48% to 54%.
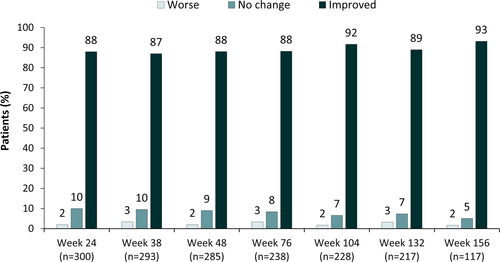
Of the 300 patients/caregivers who completed the S/CGIC at Week 24, 88% considered the patient's overall condition improved with CBD treatment, and this percentage was similar at Weeks 38–156 (87%–93%; Figure 4).
4 DISCUSSION
Our long-term OLE data extend previous findings, demonstrating that add-on CBD treatment in patients with LGS has sustained efficacy with an acceptable safety profile. CBD reduced the frequency of drop and total seizures up to 156 weeks of treatment. Doses above 20 mg/kg/day had acceptable tolerability, and no new safety issues emerged.
At the time of analysis, 33% patients had withdrawn, 62% had completed treatment (per country-specific protocols), and 4% had ongoing treatment and had not yet reached later treatment windows. During the OLE trial, several clinical trials began enrollment (TAK-935 [NCT03635073 and NCT03650452] and fenfluramine hydrochloride [NCT03355209]). These studies could have led to some patients discontinuing CBD to participate in other ASM trials, as patients are often excluded if they are receiving another investigational treatment.
The most common AEs were convulsion, diarrhea, pyrexia, somnolence, and vomiting; these were consistent with what was reported in previous studies.5-8 Most (73%) patients with liver enzyme elevations (ALT and/or AST more than three times ULN) were receiving concomitant valproic acid. An interaction between CBD and valproic acid leading to increased risk of liver enzyme elevations was reported in prior clinical trials and an expanded access program of GW CBD.7, 24, 26 Based on the Epidiolex pivotal trial program, patients taking valproic acid and CBD (especially 20 mg/kg/day vs. 10 mg/kg/day) are at greater risk of liver enzyme elevations, for reasons that are not yet understood.9 Although the transaminase elevations typically occurred within 3 months after initiation of CBD treatment, onset was sometimes later, typically in patients taking concomitant valproic acid. Factors that could have contributed to later onset include dose adjustments of CBD or concomitant ASMs, or the occurrence of other events such as infections or dehydration. The elevations seem to resolve spontaneously or with medication changes; therefore, discontinuation or reduction of CBD dose and/or concomitant valproic acid discontinuation or dose reduction could be considered.
In the original RCTs,6, 7 the 20 mg/kg/day group had a markedly higher incidence of liver enzyme elevations than the 10 mg/kg/day group. During the OLE phase, after obtaining sponsor approval, investigators could titrate CBD doses up and down. Patients who tolerated CBD were more likely to receive a higher dose of CBD than those who did not. Given this selection bias, it is difficult to draw conclusions regarding the relationship between AEs and modal dose of CBD during the OLE phase.
Eleven deaths occurred during follow-up, of which three were due to SUDEP and one was due to convulsion. No death was considered related to CBD by the investigator in this OLE trial or the prior trials.
Median and mean CBD treatment duration in this OLE trial was 1090 and 826 days, respectively, with patients treated for up to 1421 days. The median CBD exposure was approximately 11 times the duration of the original RCTs,6, 7 and more than double the duration of the previously reported data from this OLE.19, 24
The reductions in drop seizure frequency reported in GWPCARE3 and GWPCARE4 were maintained in the OLE trial.6, 7 The reduction in seizure frequency was evident in both observed case and LOCF analyses, which can better account for the impact of early discontinuations on estimates of treatment effect. This finding is notable, considering that patients received a median of three concomitant medications during the OLE and the number of therapies discontinued by patients before CBD treatment (median of six ASMs in GWPCARE3 and GWPCARE46, 7). The reductions in total seizure frequency observed in GWPCARE3 and GWPCARE4 were maintained in this OLE,19, 24 and were similar to reductions in drop seizure frequency. Slightly more than half (54%) of patients in the OLE were taking concomitant clobazam. In line with a recent study demonstrating that with or without concomitant clobazam, CBD can be effective in reducing seizure frequency, patients not taking clobazam in this OLE trial still experienced reduction in seizure frequency.27
The mean modal CBD dose was generally consistent across each 12-week period as well as in the last 12 weeks of data for each patient. That and the high retention rate in this OLE trial suggest tolerance to CBD did not occur for the duration reported in this trial. In addition, seizure frequency reductions were sustained without increased CBD dose, with individualized dosing adopted for optimal effect on seizure frequency. Given the selection bias noted above that is introduced with flexible dosing, we cannot evaluate the efficacy of different doses from this trial. The proportion of patients/caregivers reporting improvement was 87% or more at all time points assessed, suggesting that the reduced seizure frequencies were clinically meaningful for most patients/caregivers.
4.1 Limitations
There are several limitations of this trial that warrant caution in interpreting our findings. Common to OLE trials was the lack of a control arm. Changes to the number and/or doses of concomitant ASMs (as well as ketogenic diet and neuromodulation therapies such as vagus nerve stimulation) and changing CBD dose were allowed, and the analyses presented here do not investigate the potential impact of these changes on the trial outcomes. Similar limitations exist for all similar previously published studies. Efficacy data obtained at later timepoints may be subject to selection bias, with patients with lower efficacy or worse tolerability discontinuing the trial earlier. Efficacy and S/CGIC data were determined as percentage change from the pretreatment baseline from the original RCTs. This is a potential confounding factor due to the additional 14 weeks of exposure to CBD for patients randomized to CBD compared with those originally randomized to placebo; however, the longer duration of this OLE analysis would dilute this difference over time. The high proportion of subjects/caregivers reporting improvement in overall condition via the S/CGIC questionnaire may have been affected by the closer monitoring from participating in a clinical trial. Safety and tolerability data are reported for the OLE only. Due to this, there is the potential to underestimate AE burden, as patients who experienced AEs or dose changes due to AEs in the preceding RCTs are not reflected in this report due to withdrawal. Interpretation of safety and tolerability data should also consider the extended treatment duration, as spontaneously occurring conditions are more likely to be experienced when observations are extended over a prolonged multiyear period. For this interim analysis, patients had different durations of exposure to drug. Because this trial was long and required weekly seizure data entry, there is potential for “reporting fatigue” by parents that may impact the results.
5 Conclusions
Long-term, add-on CBD treatment had a similar safety profile to that observed in the original RCTs. Sustained reductions in drop and total seizures were observed up to 156 weeks, with 87% or more of patients/caregivers reporting an improvement in overall condition throughout. This OLE demonstrates the sustained long-term benefits of Epidiolex/Epidyolex, the regulated and highly purified formulation of plant-based CBD, as a treatment for patients with LGS.
ACKNOWLEDGMENTS
The authors are indebted to the patients who took part in the trial, as well as to the staff at the clinical research sites. The authors would like to thank Dr Lesley Taylor of Alchemy Medical Writing for medical writing and editorial support, funded by Greenwich Biosciences. The views expressed are those of the authors. The trial was sponsored by GW Research.
CONFLICT OF INTEREST
A.D.P. serves on the scientific advisory board for GW Research and Neurelis. He receives research funding from the Pediatric Epilepsy Research Foundation and National Institutes of Health. He receives institutional research support from Stoke. M.M.-B. has been a principal investigator for GW Research, Biogen, Roche, and Ovid/Takeda. R.F.C. has received consultancy fees from GW Pharmaceuticals companies, Zogenix, and Eisai, and has been a principal investigator for GW Research. A.G.-N. has received an unrestricted grant from Fundacion GMP for research in epilepsy and developmental disabilities, and has received speaker or advisory honoraria from Eisai, Esteve, Sanofi, and Zogenix, and speaker honoraria and research grants from BIAL and UCB Pharma. B.G. has received consultancy fees from GW Pharmaceuticals companies, Ovid/Takeda, and Zogenix, and has been a principal investigator for GW Research, Zogenix, Marinus Pharmaceuticals, and LivaNova. J.J.H. has received research funding from GW Pharmaceuticals and consults for SK Life Sciences, NCGS, and Takeda. W.M. has no conflicts of interest to declare. M.S.P. has received consultancy fees from Stoke Therapeutics and Encoded Therapeutics, has served on advisory boards for Zogenix, Greenwich Biosciences, BioMarin, Neurelis, and Biocodex, and has institutional research funding support from Ovid and Marinus. E.A.T. has served on advisory boards from Zogenix, Biocodex, Aquestive, Eisai, REGENXBIO, and Alphanobel, and institutional research funding has been received from GW Pharmaceuticals companies and Zogenix. A.W. has served on the speaker bureau for GW Pharmaceuticals companies. E.D. is employed by and holds share options in Greenwich Biosciences. D.C. is employed by and holds share options in GW Research. O.D. receives grant support from the National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, Multidisciplinary University Research Initiative, US Centers for Disease Control and Prevention, and National Science Foundation. He has equity in and/or has received compensation from the following companies: Privateer Holdings, Tilray, Receptor Life Sciences, Qstate Biosciences, Tevard, Empatica, Engage, Egg Rock/Papa & Barkley, Rettco, SilverSpike, and California Cannabis Enterprises. He has received research support from GW Pharmaceuticals and Zogenix. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.