The effect of fascin 1 inhibition on head and neck squamous cell carcinoma cells
Awais Wahab and Aini Hyytiäinen contributed equally to this study.
Abstract
Fascin 1 plays important pro-metastatic roles in head and neck carcinoma (HNSCC) migration, invasion, and metastasis. However, limited advancement in targeting metastasis remains a major obstacle in improving HNSCC patients’ survival. Therefore, we assessed the therapeutic potential of fascin 1 targeted inhibition and its potential prognostic value in HNSCC patients. Using in vitro and in vivo approaches, we investigated the effect of compound G2, a novel fascin 1 inhibitor, on HNSCC cells migration, invasion, and metastasis. High-throughput screening (HTS) was used to assess cytotoxic activity of compound G2 alone or combined with irradiation. We also evaluated the prognostic potential of fascin 1 in HNSCC patients. Interestingly, compound G2 reduced carcinoma cells migration and invasion in vitro and inhibited metastasis in vivo. Moreover, HTS revealed a modest cytotoxic activity of the compound G2 on HNSCC cell lines. Irradiation did not synergistically enhance the compound G2-mediated cytotoxic activity. Survival analyses showed that high fascin 1 immunoexpression, at the tumor invasive front, was associated with cancer-specific mortality in the advanced stages of HNSCC. Collectively, our findings suggest that fascin 1 represents a promising anti-metastatic therapeutic target and a useful prognostic marker in patients with HNSCC. Novel anti-metastatic agents could provide a valuable addition to cancer therapy.
INTRODUCTION
Head and neck squamous cell carcinomas (HNSCC) are the eighth most common cancer worldwide [1]. Despite progress in cancer research and treatment, the 5-year overall survival rate of HNSCC is only around 50% [2]. More than 60% of HNSCC patients are diagnosed with an advanced stage of the disease (III or IV), which is characterized by a poor prognosis [3]. In 2018, more than 600,000 new cases of HNSCC and 350,000 deaths were reported [4]. Treatment approaches for HNSCC include surgery, radio-, chemo-, targeted- and/or immuno-therapies. Current treatment protocols are associated with significant toxicity and many patients develop treatment resistance and cancer recurrence [5]. There is also a little advancement in targeting metastasis, which remains a leading cause of cancer related mortality. Therefore, there is an urgent need to develop new anti-metastatic therapeutics to improve the survival outcomes of HNSCC patients.
The fascin protein family is the main class of the actin filament (F-actin) bundling proteins and it consists of three members: Fascin 1–3 [6, 7]. Fascin 1 expression is weak or moderate in the healthy epithelium and its expression is restricted to the basal layer of epithelium [8]. However, fascin 1 is involved in the formation of protrusive structures that result in invasion, progression, and metastasis of many carcinomas including prostate, esophageal, breast, lung, pituitary adenomas and HNSCC [9-15]. Importantly, fascin 1 overexpression is associated with increased cancer cell invasion and metastasis in HNSCC [16]. Furthermore, fascin 1 expression correlates with disease progression and poor survival in oral SCC (OSCC), where it is regulated by cancer-associated fibroblasts and stromal growth factors [8, 17]. Given its evident prometastatic role, inhibiting fascin 1 activity may represent an attractive therapeutic target in cancer [18].
Compound G2, a small-molecule derived from indazol-furan-carboxamides, is a novel fascin 1 inhibitor [19]. Recently, compound G2 was shown to inhibit fascin 1 driven activities including F-actin bundling, cytoskeleton formation, lamellipodium protrusion, and migration and invasion of several colorectal cancer cell lines [18]. Moreover, this molecule led to a significant reduction in the metastatic capacity of the HCT-116 cancer cells in vivo [18].
In this study, we investigated the therapeutic potential of compound G2 in HNSCC, alone or with concurrent irradiation, using in vitro and in vivo approaches. This was further supported by evaluating the prognostic value of fascin 1 immunoexpression in patients diagnosed with HNSCC.
MATERIAL AND METHODS
Cell lines
Two primary (UT-SCC-24A and UT-SCC-42A; from tongue and larynx, respectively) and their corresponding metastatic (UT-SCC-24B and UT-SCC-42B) cell lines were used. The clinical and pathological characteristics of these patient-derived HNSCC cell lines are provided in Table S1. These HNSCC cell lines were established at the Department of Otorhinolaryngology, Head and Neck Surgery, Turku University Hospital (Turku, Finland) and have been used in several studies related to anti-cancer compound testing [20-22]. In addition, the metastatic tongue-derived HSC-3 cell line (JCRB 0623; Osaka National Institute of Health Sciences) was used. Cells were cultured in 75 cm2 flasks with Dulbecco's modified Eagle's medium (DMEM)/F-12 (Gibco, Thermo Fisher Scientific) supplied with 100 U/mL penicillin, 100 µg/mL streptomycin, 250 ng/mL fungizone, 50 µg/mL ascorbic acid, 0.4 µg/mL hydrocortisone and 10% heat-inactivated fetal bovine serum (FBS).
Human tumor-derived matrix (Myogel)
The human leiomyoma-derived matrix Myogel was invented and prepared by our research group [23], and it has been recently proven to increase the reliability of anticancer drug testing in vitro [21]. The use of human leiomyoma tissue was approved by the Ethics Committee of Oulu University Hospital (35/2014).
Compound G2 source and optimization
The compound G2 was kindly gifted by Eurofins Villapharma Research. Compound G2 (N-(1-(4-(trifluoromethyl) benzyl)−1H-indazol-3-yl) furan-2-carboxamide; C20H15F3N3O2) is a small-molecule inhibitor of fascin 1, which was synthesized and characterized as previously described [19]. The concentrations of compound G2 have been optimized for each respective assay.
Scratch wound cell migration and invasion assays
Wells of a 96-well plate were coated with 50 µL of Myogel (0.5 g/mL) and left overnight in the cell culture incubator. On the next day, the leftover Myogel was removed and HSC-3 cells were seeded at the density of 25,000 cells/well. Cells were allowed to adhere overnight, followed by wound creation using a WoundMaker tool (IncuCyte, Essen Bioscience). For the migration wells, media were changed to fresh media with or without 10 µM of compound G2. For the invasion wells, medium was removed, and the wounds were filled with 50 µL of Myogel (2.4 mg/mL)/type 1 rat tail collagen (0.8 mg/mL; Corning) with or without 10 µM compound G2. NaOH was used to control the pH of the Myogel/collagen gel. The gel was allowed to solidify for 30 min before adding 100 µL of fresh media with or without 10 µM compound G2. The IncuCyte Live-Cell Imaging System (Essen Bioscience) was used to monitor the wound width for 2 days. All experiments were conducted three times independently, each performed at least in five replicates.
High-throughput screening assays
We performed high-throughput screening assays to test the therapeutic effect of the compound G2 alone and when combined with irradiation. To quantitatively profile alternate drug effects, we calculated the drug-sensitivity score (DSS) designed by the High Throughput Biomedicine Unit at the Institute for Molecular Medicine Finland (FIMM). The DSS score is used to determine dose-response relationships, which has been reported in several studies as an informative way to assess quantitative drug sensitivities [20, 24-26]. Drug Sensitivity and Resistance Testing (DSRT) protocol, adapted for leukemia cells, was used [25], with the DSRT plates were custom designed at the High Throughput Biomedicine Unit. Compound G2 was tested for a 10,000-fold concentration range (10-100,000 nM) with five different 10-fold concentrations. As a reference, we used docetaxel at 0.1-1,000 mM (LC Laboratories) -a clinically approved drug to treat HNSCC patients. Dose-response curves were calculated separately for each cell line and condition (control and irradiation). The response readout was normalized to negative control (DMSO, 0.1%) and positive control (100 µM benzethonium chloride, BzCl). To reduce cancer cell viability by 20%, half of the plates were subjected to optimized irradiation doses (IC20) (Table S2).
The 384-well plates (Corning) were coated with 5µL of Myogel (0.5 mg/mL) using an automated system and reagent dispenser (MultiFlo FX, BioTEK). The plates were then centrifuged for 2 min at 300 rpm at 4°C and left overnight in an incubator. On the following day, the cells were counted using the Scepter 2.0 Cell Counter (Merck Millipore) and suspended to 500 cells/well. Cells were seeded using the automated dispenser (20 µL/well) and left to adhere overnight in an incubator. On the following day, one set of the plates was irradiated with gamma-ray irradiator OB29/4 (STS Vertriebs). Cell viability was determined after 72 h using the CellTiter-Glo (CTG) 2.0 Luminescent Cell Viability Assay (Promega). Following a 15-min incubation at room temperature, the CTG reagent (25 µL/well) was pipetted to the plates using MultiFlo FX automated dispenser. The plates were shaken for 10 min (450 rpm) and subsequently centrifuged for 5 min (1000 rpm). The CTG signal was detected using the PheraStar plate reader (BMG Labtech). Experiments were performed in three replicates.
Zebrafish larvae microinjection
Two days post-fertilization (dpf) wild-type zebrafish (Danio rerio) larvae (from AB strain) were used for the in vivo assays. Fish were dechorionated, anesthetized with 0.04% Tricaine, and microinjected into the perivitelline space with 4 nl of HSC-3 cell suspension (500 cells per fish) stained with CellTrace Far Red dye (Thermo Fisher Scientific). Fish were then transferred into a 24-well plate (5 fish per well) containing 1 mL fresh embryonic medium and randomly distributed into 4 groups as follows: i) no treatment, control group; ii) compound G2 group (10 µM; diluted in embryonic medium); iii) irradiation group (2 Gy/day for 3 days); iv) combination group (compound G2 plus irradiation 2 Gy/day for 3 days). The concentration of the G2 for the zebrafish experiments were optimized and published before [18]. All larvae were kept for 3 days at 34°C. For irradiation, fish were placed in Gamma irradiator OB29/4 (STS) for 85 s/day for 3 days. The source of the radiation was Caesium, isotope 137. The irradiation dose was optimized before the experiments.
On the fourth day of the experiment, larvae were collected and fixed with 4% paraformaldehyde and mounted in 1% low melting agarose for imaging. Zebrafish were imaged with a Nikon Eclipse Ti-E Camera (Nikon), and the images were analyzed using ImageJ (National Institutes of Health). Tumor area and the number of metastatic clumps in each fish were measured. Zebrafish experiments were performed at the Zebrafish Unit (University of Helsinki) under the ethical permission (ESAVI/13139/04.10.05/2017) given by the regional state administrative agency. The method is compliant with the ARRIVE guidelines (https://arriveguidelines.org). Zebrafish larvae experiments were conducted three times independently. Ten to twenty-five fish (males and females) were used in each group in each independent experiment. No inclusion and exclusion criteria were set.
Tissue samples
A total of 156 patients diagnosed with HNSCC (between 2004 and 2013) and treated at Tampere University Hospital were included in this study. The patients were staged according to the seventh edition of American Joint Committee on Cancer (AJCC) [27]. The use of patients’ samples and follow-up data was approved by the ethical committee of Tampere University Hospital (no. R07039). The clinical and demographic parameters of the patients are presented in Table S3.
Immunohistochemical staining and microscopic evaluation
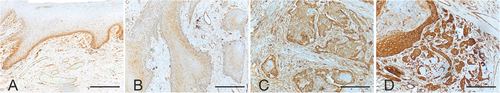
Paraffin sections, one block per patient from the most representative tumor area, of 4 µm thickness were stained using Ventana BenchMark LT Automated IHC Stainer and Ultraview Universal DAB detection kit (Ventana Medical System). Deparaffinization was performed with Ventana EZ Prep solution (Ventana Medical System). Cell Conditioning 1: Tris-EDTA buffer pH 8.0 (Ventana Medical System) was employed for epitope retrieval at 95°C to 100°C for 30 min. UV-Inhibitor 3% H202 (Ventana Medical System) was used to block endogenous peroxide for 4 min at 37°C. We used Ventana Tris-based Reaction buffer (Ventana Medical System) to rinse the tissue slides between steps and then we incubated the slides with mouse anti-human fascin 1 antibody (1:400, Agilent Technologies) at 37°C for 32 min. Following this, Ventana Ultraview HRP Universal Multimer was applied for 4 min at 37°C. Chromogen and hematoxylin were substituted with 3,3´-diaminobenzidine (DAB) and nuclear stain, respectively. Esophageal adenocarcinoma tissue was used as a positive control to confirm the immunostaining reliability. For negative control, the primary antibody was omitted or replaced with irrelevant antisera to control the specificity of immunohistochemistry. Leica DM 2000 light microscopy (Leica Microsystems) was used to evaluate the immunoexpression status of fascin 1 in the patient samples. Scoring was conducted semi-quantitatively by four independent researchers (STS, LM, LJ, and AW), who were blinded to patients’ clinicopathologic characteristics and outcomes. Cytoplasmic staining intensity of the fascin 1 positive cells was evaluated at the invasive front areas. The staining intensity was scored as mild, moderate or strong (Figure 1).
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 25 (IBM SPSS Statistics). For the migration and invasion experiments, a repeated measures mixed model analysis of variance was used with time, group and time x group as fixed effects and well as a random effect with a two-sided 5% level of significance. For the fish experiments, Mann-Whitney U test was used to compare the tumor area between the control and the G2 treated group, and the Kruskal-Wallis test was used to compare medians of the metastatic clumps between groups.
Cross-tabulation and Chi-square test were conducted to evaluate the associations between fascin 1 expression and the clinicopathologic characteristics: age, sex, WHO grade, TNM stage, recurrence, smoking, alcohol consumption, adjuvant chemo- or radiotherapy, and the performed surgery. Kaplan-Meier curves were constructed for cancer-related mortality. The log-rank test was used to assess differences in survival between the groups. The Cox proportional hazards model was used to estimate the effect of covariates on the survival. Univariable and multivariable analysis were conducted for all cases, and then for early and advanced stage cases, separately.
RESULTS
Compound G2 effects on HNSCC cell migration and invasion
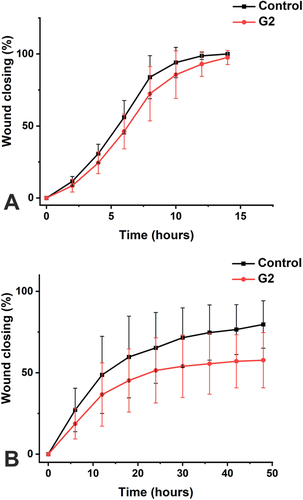
We have previously shown that fascin 1 is overexpressed in OSCC tissues and cells including the highly metastatic HSC-3 cell line (16). Thus, to explore the potential anti-invasive effects of the novel fascin 1 inhibitor (compound G2) in HNSCC, we performed the scratch wound migration and invasion assays using the highly metastatic HSC-3 cell line (Figure S1). Interestingly, compound G2 treatment hampered wound closure (up to 18 h), revealing around 15% reduction in HSC-3 cell migration (P = 0.03; Figure 2A). Likewise, the invasion assay showed that compound G2-treated HSC-3 cells had around 25% less invasion capacity (up to 48 h) compared with the untreated cells (P = 0.001; Figure 2B).
Effects of compound G2 on HNSCC cell metastasis in vivo
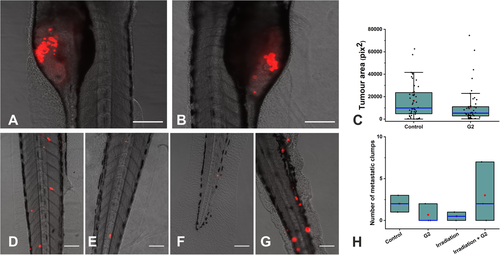
After confirming the anti-invasive effect of compound G2 in vitro, we further investigated its effects in vivo. Initially, we determined the effect of compound G2 on the tumor size (i.e. tumor area) in zebrafish xenograft model of HNSCC. Of note, compound G2 did not have a significant effect on HSC-3 cell proliferation as represented by the tumor area (Figure 3A). Next, we evaluated the anti-metastatic effects of compound G2 in vivo alone and combined with irradiation. Importantly, compound G2 reduced the number of metastatic clumps of HSC-3 by 57%, which was closely comparable to the irradiation effect (61%), the effect was clinically but not statistically significant (Figure 3B). Nevertheless, such compound G2-mediated anti-metastatic effect was detected in each of the three in vivo experiments (42%, 41%, and 88%). However, no effect on metastasis formation was observed when combining compound G2 with concurrent irradiation (Figure 3B).
Cytotoxic activity of compound G2 on primary and metastatic HNSCC
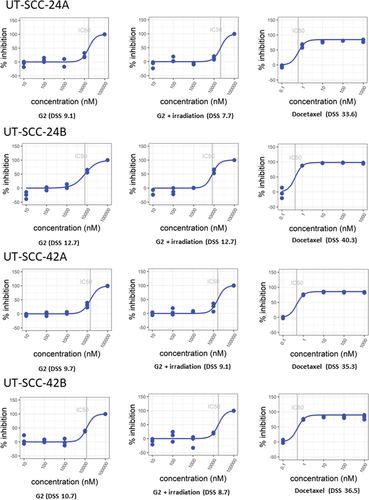
To explore the therapeutic activity profile of compound G2-mediated fascin 1 inhibition on patient-derived primary and metastatic HNSCC cell lines, high-throughput screening was conducted with an FDA-approved drug (docetaxel) as a reference. These assays were performed on Myogel-coated wells because of its superior reliability [21]. The DSS value was categorized into the following four classes describing the drug effect: inactive DSS < 5; low 5 ≥ DSS < 10; moderate 10 ≥ DSS < 15; and high DSS ≥ 15 [28]. Interestingly, the high-throughput screening results disclosed a moderate cytotoxic activity of compound G2 on the metastatic tumor cell lines (24B, 42B; DSS 12.7 and 10.7, respectively) compared with a low activity on the primary ones (24A, 42A; DSS 9.1 and 9.7, respectively) (Figure 4). However, compound G2 concurrent with irradiation treatment did not enhance these DSS values, suggesting no synergistic antitumor effect of this combined therapy in HNSCC. As expected, treatment with docetaxel showed a much higher response (DSS > 30) than compound G2 alone or with irradiation.
Fascin 1 expression in HNSCC samples
Based on the observed therapeutic potential of targeting fascin 1 in vitro and in vivo, we assessed its association with the clinicopathologic parameters of HNSCC patients. Thus, we first evaluated fascin 1 immunoexpression in a cohort of 156 patients. Fascin 1 was moderately expressed in the basal cell layer of the healthy epithelium (Figure 1). Interestingly, fascin 1 was induced (moderate to high) in the majority of HNSCC tissue samples as follows: mild (n = 9; 5.8%), moderate (n = 77; 49.4%), and strong expression (n = 70; 44.8%). Furthermore, a stronger expression of fascin 1 was associated with larger T3-T4 tumors (57.4%) compared with those small T1-T2 tumors (38.2%). No significant difference in the expression of fascin 1 was observed between oral and oropharyngeal cancers or with other clinicopathologic parameters.
Fascin 1 association with HNSCC patients’ survival
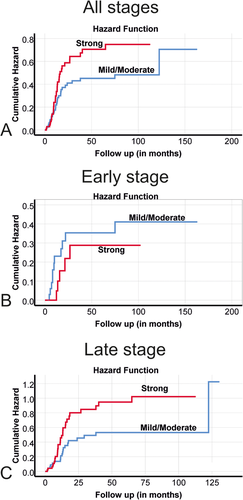
Finally, we assessed the prognostication utility of fascin 1 in HNSCC patients. Noteworthy, tumors with a strong expression of fascin 1 were associated with the highest mortalities compared with those with mild or moderate expression. Therefore, when analyzing stage-specific survival, tumors with mild/moderate fascin 1 expression were categorized together versus the strong fascin 1-expressing tumors. When all cases were combined (n = 156), a strong expression of fascin 1 showed a correlation trend with cancer-related mortality hazard ratio (HR) 1.53, [95% confidence interval (CI) 0.94-2.48] although it did not reach a statistical significance (Table 1). In the early stages of HNSCC patients, no significant association between fascin 1 and survival was found (n = 57; HR 0.69, 95%CI 0.24-1.99, Table 1). Interestingly, in the advanced stages (n = 99), the univariate analysis showed a significant association between strong fascin 1 expression and higher cancer-related mortality (HR 1.86, 95%CI 1.05-3.29; Table 1). This association was further confirmed by the multivariate analysis (HR 1.88, 95%CI 1.04-3.41; Table 1). The association between fascin 1 expression and cancer-related mortality is also presented using Kaplan-Meier survival curves as depicted in Figure 5.
| Cancer-related mortality | |||||
|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis Model 1 | Multivariable Analysis Model 2 | Multivariable Analysis Model 3 | ||
| Variable | Values | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) |
| Age, years | <60 | Reference | Reference | Reference | Reference |
| ≥60 | 1.01 (0.62-1.65) | 1.12 (0.68-1.85) | 1.28 (0.43-3.82) | 1.06 (0.59-1.88) | |
| Sex | Male | Reference | Reference | Reference | Reference |
| Female | 0.84 (0.52-1.38) | 1.08 (0.65-1.79) | 0.96 (0.32-2.88) | 0.99 (0.55-1.80) | |
| WHO Grade (differentiation) | Well diff. | Reference | Reference | Reference | Reference |
| Moderately diff. | 2.43 (1.21-4.85) | 2.36 (1.17-4.76) | 2.78 (0.76-10.19) | 2.19 (0.95-5.02) | |
| Poorly diff. | 2.77 (1.24-6.17) | 2.35 (1.02-5.39) | 3.25 (0.28-38.44) | 2.28 (0.91-5.73) | |
| Stage | Early (I-II) | Reference | Reference | ||
| Advanced (III-IV) | 2.24 (1.28-3.94) | 2.08 (1.15-3.79) | n.a. | n.a. | |
| Fascin expression (All cases; n = 156) | Mild/Moderate | Reference | Reference | ||
| Strong | 1.53 (0.94-2.48) | 1.44 (0.87-2.37) | n.a. | n.a. | |
| Fascin expression (Early stage; n = 57) | Mild/Moderate | Reference | Reference | ||
| Strong | 0.69 (0.24-1.99) | n.a. | 0.65 (0.21-2.01) | ||
| Fascin expression (Advanced stage; n = 99) | Mild/Moderate | Reference | Reference | ||
| Strong | 1.86 (1.05-3.29) | n.a. | n.a. | 1.88 (1.04-3.41) | |
- n.a.: not applicable.
- Note: Model 1 including all 156 cases;.
- Model 2 including cases at early-stage only (n = 57);.
- Model 3 including cases at advanced stage only (n = 99).
DISCUSSION
Fascin 1 plays a key protumorigenic role in many cancers including HNSCC, in which its overexpression predicted a more aggressive clinical course of the disease [9-15, 28, 29]. To our knowledge, this is the first study showing that targeted inhibition of fascin 1, via its novel inhibitor (compound G2), reduced the invasive and metastatic potential of HNSCC cells in vitro and in vivo, regardless of irradiation and without a strong impact on cell proliferation. We also affirmed the prognosticator role of fascin 1 in the advanced cases of HNSCC patients.
Cancer cell ability to invade local tissues is a crucial feature in cancer that precedes the initiation of metastasis [30]. In this regard, fascin 1 promotes invasive filopodia formation and plays an important role in epithelial-to-mesenchymal transition (EMT), which facilitates the metastatic properties in cancer cells [6]. Moreover, fascin 1 induces morphological transformation in cell membrane protrusions, reduces intercellular adhesion, and promotes micro-invasion and metastasis [31-33]. In several metastatic carcinomas, it plays a critical role in cellular migration and cell to cell interactions that eventually lead to poor survival outcomes [13, 14, 34, 35]. Our group has shown that also oral carcinoma cells (HSC-3 and SCC-25) express high fascin 1 on mRNA and protein levels [8] and thus fascin 1inhibitors would be attractive therapeutical compounds to be studied also in treatment of HNSCC cancer patients.
The compound G2 and its derivatives were shown to block fascin 1-driven actin binding, bundling and cytoskeletal reorganization activities in breast cancer cells [19]. Moreover, compound G2 showed stronger inhibitory effects on fascin 1 compared with its typical inhibitor, migrastatin, in colon cancer cell lines [18]. In agreement with these reports, our in vitro data reveal an inhibitory effect of compound G2 on tumor cell migration and invasion in HNSCC. This inhibitory impact was further supported by in vivo assays using zebrafish larvae, where compound G2 impeded tumor cell metastasis in a comparable degree to irradiation (57% and 61%, respectively). However, such anti-metastatic effect was lost when exposing fish to compound G2 with concurrent irradiation, implying a potential acquisition of resistance to the combined therapy. Interestingly, compound G2 did not influence tumor cell proliferation in vivo, perhaps in part because of its specific inhibitory effects on fascin 1-mediated activities (i.e., metastasis and migration), rather than on cell proliferative processes.
Although metastasis is responsible for the majority of cancer-specific deaths with solid tumors, most anticancer drugs are designed to target the viability of tumor cells [36]. Thus, new anti-metastatic agents are needed to join the therapeutic arsenal against cancer. Importantly, while compound G2 had clear anti-metastatic effects, its cytotoxic activity on HNSCC cells lines remained limited as revealed by our high-throughput screening and zebrafish model assays. Such low-to-moderate cytotoxic activity of compound G2 was not synergistically enhanced by irradiation. In line with our findings, Huang et al. observed that targeted inhibition of fascin 1 had no significant effect on the growth of primary breast tumors [12]. Additionally, we previously showed that shRNA-knockdown of fascin 1 (shRNA-FSCN) in OSCC cells did not impact tumor cell proliferation in vitro [8]. In an orthotopic mouse model of OSCC, the frequency of lymph node metastases was less in the shRNA-FSCN tumors, which had slightly less volumes compared with the non-silenced controls. Furthermore, treatment with shRNA-FSCN induced E-cadherin and attenuated vimentin expression, which indicates a potential role of fascin 1 in mediating the EMT program [6, 8]. Collectively, these findings support the notion that targeting fascin 1 suppress metastatic-related events rather than significantly impacting tumor cell proliferation and growth of its volume.
Fascin 1 represents an attractive prognostic marker in metastatic carcinomas. Several studies reported an elevated fascin 1 protein expression at the tumor invasive borders in esophageal, gastric and oral carcinomas [31-33]. Overexpression of fascin 1 promotes an aggressive clinical course in patients with HNSCC [8, 15, 16, 28, 37, 38]. Here, we found a significant correlation between fascin 1 protein expression and HNSCC-related mortality in both univariate and multivariate analyses. Interestingly, fascin 1 overexpression was associated with increased cancer-related deaths in the advanced stages of HNSCC, which may highlight the dismal role of fascin-mediated metastasis in the clinical course of the disease. However, we acknowledge the limitations of our study including the lack of data regarding the extra-nodal extension in addition to the HPV status for some cases of oropharyngeal cancer. Thus, further studies are needed to validate this prognostic significance according to the eighth edition of the AJCC staging system [39].
Taken together, our in vitro and in vivo results suggest that inhibition of fascin 1, via compound G2, may represent an attractive anti-metastatic therapeutic approach in patients with HNSCC. This procancerous role of fascin 1 was further supported by our survival analyses which affirmed its prognostic utility in the advanced stages of HNSCC. Yet, additional preclinical and mechanistic studies evaluating the potential off-target effects of compound G2 are still needed. Finally, supplementing the current anti-growth drugs with anti-metastatic agents should be carefully evaluated, as it may represent a better strategy for managing metastatic HNSCC in future.
ACKNOWLEDGEMENTS
The members of the High Throughput Biomedicine Unit at the Institute for Molecular Medicine Finland (FIMM), especially Swapnil Potdar, Laura Turunen, Jani Saarela, and Sergey Kuznetsov are gratefully acknowledged for their assistance in designing the drug plates, data processing, and illustration. We thank Hannu Vähänikkilä for his professional help in the statistical analysis of the data.
CONFLICTS OF INTEREST
The authors declare that they have no competing or conflicts of interests.
AUTHOR CONTRIBUTIONS
Conceptualization: Awais Wahab, Aini Hyytiäinen, Pablo Conesa-Zamora, Timo Paavonen, Sanna Toppila-Salmi, Tuula Salo, Ahmed Al-Samadi. Formal analysis: Awais Wahab, Aini Hyytiäinen, Wafa Wahbi, Katja Tuomainen, Alhadi Almangush, Ahmed Al-Samadi. Investigation: Awais Wahab, Aini Hyytiäinen, Wafa Wahbi, Katja Tuomainen, Laura Jauhiainen, Laura K. Mäkinen, Abdelhakim Salem. Resources: Pablo Conesa-Zamora, Tuula Salo. Data Curation: Awais Wahab, Aini Hyytiäinen, Sanni Tervo. Writing – original draft preparation: Awais Wahab, Aini Hyytiäinen. Writing – review and editing: Wafa Wahbi, Katja Tuomainen, Sanni Tervo, Pablo Conesa-Zamora, Laura Jauhiainen, Laura K. Mäkinen, Timo Paavonen, Sanna Toppila-Salmi, Abdelhakim Salem, Alhadi Almangush, Tuula Salo, Ahmed Al-Samadi. Visualization: Awais Wahab, Sanni Tervo, Laura Jauhiainen, Laura K. Mäkinen. Supervision: Pablo Conesa-Zamora, Timo Paavonen, Sanna Toppila-Salmi, Alhadi Almangush, Tuula Salo, Ahmed Al-Samadi. Funding acquisition: Pablo Conesa-Zamora, Tuula Salo.
FUNDING
This research was funded by Sigrid Jusélius Foundation, the Cancer Society of Finland, Instituto de Salud Carlos III (Spanish Ministry of Health) and FEDER funds (ref: PI18/00144), the Oulu University Hospital MRC grant, the Helsinki University Central Hospital research funds, Research Fund of Finnish Otolaryngological Society, and Jane and Aatos Erkko Foundation. The funders took no part in the design or performance of the study. Funding consists of academic grants without any engagements considering the research project.




