Therapeutic choices and disease activity after 2 years of treatment with cladribine: An Italian multicenter study (CladStop)
Gianmarco Abbadessa, Angela Albanese, Simona Bonavita, Martina Cardi, Emanuele Cassano, Diego Centonze, Eleonora Cocco, Antonella Conte, Cinzia Cordioli, Massimiliano Di Filippo, Sonia Di Lemme, Elena Di Sabatino, Roberta Fantozzi, Diana Ferraro, Maria Teresa Ferrò, Jessica Frau, Carolina Gabri Nicoletti, Claudio Gobbi, Flora Govone, Luigi Grimaldi, Paolo Immovilli, Rosa Iodice, Doriana Landi, Luigi Lavorgna, Lorena Lorefice, Giacomo Lus, Leonardo Malimpensa, Girolama Marfia, Giuseppina Miele, Francesca Napoli, Livia Pasquali, Anna Repice, Francesca Ruscica, Irene Schiavetti, Alessio Signori, Elisabetta Signoriello, Carmine Siniscalchi, Maria Pia Sormani, Stefano Tozza, Francesca Vitetta, Chiara Zecca
Abstract
Background and purpose
Cladribine tablets, a purine analogue antimetabolite, offer a unique treatment regimen, involving short courses at the start of the first and second year, with no further treatment needed in years 3 and 4. However, comprehensive evidence regarding patient outcomes beyond the initial 24 months of cladribine treatment is limited.
Methods
This retrospective, multicenter study enrolled 204 patients with multiple sclerosis who had completed the 2-year course of cladribine treatment. The primary outcomes were therapeutic choices and clinical disease activity assessed by annualized relapse rate after the 2-year treatment course.
Results
A total of 204 patients were enrolled; most patients (75.4%) did not initiate new treatments in the 12 months postcladribine. The study found a significant reduction in annualized relapse rate at the 12-month follow-up after cladribine completion compared to the year prior to starting therapy (0.07 ± 0.25 vs. 0.82 ± 0.80, p < 0.001). Furthermore, patients with relapses during cladribine treatment were more likely to start new therapies, whereas older patients were less likely. The safety profile of cladribine was favorable, with lymphopenia being the primary registered adverse event.
Conclusions
This study provides insights into therapeutic choices and disease activity following cladribine treatment. It highlights cladribine's effectiveness in reducing relapse rates and disability progression, reaffirming its favorable safety profile. Real-world data, aligned with previous reports, draw attention to ocrelizumab and natalizumab as common choices after cladribine. However, larger, prospective studies for validation and a more comprehensive understanding of cladribine's long-term impact are necessary.
INTRODUCTION
In recent years, there has been a radical change in the therapeutic scenario of multiple sclerosis (MS). The new knowledge of the disease has enabled the development of new disease-modifying therapies (DMTs), with different mechanisms of action, greater efficacy, and different safety and tolerability conditions. The current generation of DMTs include a wide range of immune modulators, immune depletion, and repopulating agents [1].
Such treatment comprises cladribine tablets, a small purine analogue antimetabolite that mimics adenosine, inhibits the action of the adenosine deaminase enzyme, and causes an interim reduction of lymphocytes with predominance in B cell and T cell counts followed by reconstitution of adaptive functions [2].
In 2017, cladribine was licensed by the European Medicines Agency as the first oral pulsed therapy for the treatment of adult patients with highly active relapsing MS [3].
Each tablet contains 10 mg cladribine, and the recommended total dose is 3.5 mg/kg body weight administered through two short courses at the beginning of the first and second year. Following completion of the two courses, no further cladribine treatment is required in years 3 and 4, but it is not contraindicated (summaries of product characteristics (SmPCs) Mavenclad April 2022). During the clinical trials, repeating the dose routinely beyond year 2 was not associated with significantly improved disease control (SmPCs Mavenclad April 2022) [4].
This treatment-free window offers different opportunities compared to continuous therapy regimens, such as patients' flexibility in family planning and attenuating or active vaccine administration [5], albeit real-world data confirm a normal humoral and cell-mediated immune response to vaccination following cladribine treatment regardless of the time of application and the lymphocyte count [6, 7].
Different phase IV studies are ongoing aimed at collecting new evidence for further characterizing cladribine tablets' benefit/risk ratio.
Several postapproval data are arising from international real-world cohorts and have well characterized the efficacy and safety of the first 2 years of treatment with cladribine tablets [8-10].
Furthermore a post hoc analysis of the CLARITY study already supported few years ago the continuation of treatment in year 2 even if a patient reported clinical disease activity in year 1, as most of them experienced benefit from treatment dosing completion [11].
However, the evidence on the follow-up at 2 years of treatment with cladribine tablets meant as the end of 24 months and beyond is limited.
This analysis aims to investigate the therapeutic choices and clinical disease activity of patients who completed a 2-year course with cladribine.
METHODS
Study design and data collection
This is a multicenter, retrospective observational study that enrolled patients with MS who had completed 2 years of treatment with cladribine, with an observational follow-up period of at least 6 months. The baseline for this study is defined as the completion of 2 years of treatment with cladribine, which corresponds to 24 months from the therapy start and is referred to as “CladStop” in this study.
Demographic characteristics, information about comorbidities and prior infections, and data related to the patients' MS history were collected. Additionally, clinical, laboratory, and magnetic resonance imaging (MRI) variables (in terms of gadolinium enhancing lesions and new T2 lesions) obtained during the 2 years of treatment with cladribine were retrieved from medical charts. These variables include lymphocyte count, Expanded Disability Status Scale (EDSS) scores, MRI lesion counts, and relapse information. Data on the therapies initiated after the 2-year course of cladribine treatment were also collected.
MRI data, relapse occurrences, and changes in EDSS scores were collected at 6 and 12 months after CladStop. The evaluation of disability progression was considered in terms of an increase of at least 1 point on the EDSS if the baseline EDSS was equal to or less than 5.5, or 0.5 points if the EDSS was greater than 5.5.
Any relevant adverse events that occurred from the start of treatment with cladribine were documented in the electronic case report form.
Study data were collected and managed using REDCap electronic data capture tools hosted at University of Genoa [12]. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing (i) an intuitive interface for validated data capture, (ii) audit trails for tracking data manipulation and export procedures, (iii) automated export procedures for seamless data downloads to common statistical packages, and (iv) procedures for data integration and interoperability with external sources [13].
Outcomes
The primary outcomes of this study are the therapeutic choices and clinical disease activity assessed by the annualized relapse rate (ARR) following the completion of the 2-year treatment course with cladribine.
The choice of ARR as the primary outcome is supported by its widespread use as an important measure in clinical trials evaluating the efficacy of treatments for MS [14, 15], such as cladribine. These trials have shown promising results, with a significant decline in relapse rates observed after the initiation of therapy [16, 17].
Statistical analysis
Continuous variables were described using mean and SD or median and range or interquartile range; categorical variables were reported as counts and percentages.
The time to initiate a new treatment after CladStop was assessed using a Kaplan–Meier curve and analyzed using a multivariable Cox regression model, which was adjusted for baseline characteristics.
Differences in ARR over time were determined with Friedman test and a post hoc pairwise comparison using the Bonferroni correction.
Significance level was set at 5% for all analyses, and all tests were two-tailed.
All analyses were performed with SPSS (IBM SPSS Statistics, version 24.0; IBM, Armonk, NY, USA).
Ethics statement
The study was conducted in compliance with the principles of the Declaration of Helsinki. The protocol was approved by the regional ethics committee (CER Liguria: 273/2022—DB id 12,395, 20 June 2022). Written informed consent was obtained from all participants before starting any study procedures.
RESULTS
A total of 204 people with MS (pwMS) from 17 Italian and one Swiss center were enrolled between June 2022 and May 2023. Demographic and clinical characteristics are shown in Table 1.
| Sex, females, n (%) | 144 (70.6%) |
| Age, years, mean (SD) | 36.6 (10.45) |
| BMI, kg/m2, mean (SD) | 25.1 (4.81) |
| Ethnicity, Caucasian, n (%) | 204 (100.0%) |
| MS duration, years, mean (SD) | 8.2 (7.45) |
| Last EDSS assessed prior to initiation of therapy, median [interquartile range] | 2.0 [1.0–3.0] |
| Total number of previous DMTs, median [range] | 1.0 [1.0–6.0] |
| Last previous DMT, n (%) | |
| Dimethyl fumarate | 54 (29.2%) |
| No previous DMT | 28 (15.1%) |
| Interferon | 26 (14.1%) |
| Glatiramer acetate | 24 (13.0%) |
| Fingolimod | 22 (11.9%) |
| Natalizumab | 10 (5.4%) |
| Teriflunomide | 10 (5.4%) |
| Rituximab | 6 (3.2%) |
| Ocrelizumab | 3 (1.6%) |
| Alemtuzumab | 1 (0.5%) |
| Daclizumab | 1 (0.5%) |
| At least one relapse in past 12 months, n (%) | 128 (62.7%) |
| Number of relapses in past 12 months, median [range] | 1.0 [0.0–4.0] |
| Presence of MRI activity within the past 12 months, n (%) | 136/164 (82.9%) |
| Presence of at least one GEL within the past 12 months, n (%) | 97/162 (59.9%) |
| Number of GELs within the past 12 months, median [range] | 1.0 [0.0–2.0] |
- Abbreviations: BMI, body mass index; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; GEL, gadolinium enhancing lesion; MRI, magnetic resonance imaging; MS, multiple sclerosis.
The mean age at CladStop was 36.6 (SD = 10.45) years with a range of 18 to 68 years and a predominance of female patients (70.6%). Approximately three quarters of the patients (76.0%) had no comorbidities (Table 2).
| Presence of at least one comorbidity | 49 (24.0%) |
| Major depressive disorder | 10 (4.9%) |
| Hypertension | 3 (1.5%) |
| Coronary heart disease | 3 (1.5%) |
| Malignant tumor | 3 (1.5%) |
| Cerebrovascular disease | 2 (1.0%) |
| Chronic liver disease | 2 (1.0%) |
| Diabetes | 2 (1.0%) |
| Chronic kidney disease | 0 (0.0%) |
| HIV | 0 (0.0%) |
| HBV | 0 (0.0%) |
| Hematological disease | 0 (0.0%) |
| Other | 32 (15.7%) |
| Presence of tuberculosis assessed prior to initiation of therapy | 2 (1.0%) |
| Presence of hepatitis C assessed prior to initiation of therapy | 1 (0.5%) |
| Presence of hepatitis B assessed prior to initiation of therapy | 0 (0.0%) |
- Note: Data are presented as n (%).
- Abbreviations: HBV, hepatitis B virus; HIV, human immunodeficiency virus.
Before starting cladribine treatment, patients had a median EDSS score of 2.0 (with an interquartile range of 1.0–3.0), and on average, they had undergone one (range = 0–6) previous DMT (the most common was dimethyl fumarate [DMF], 29.2%). Most patients (n = 128, 62.7%) experienced at least one relapse in the 12 months before starting cladribine, and 136 individuals presented MRI activity (data available on 164 pwMS).
The median observation period after CladStop was 17.5 months, ranging from 6 (minimum allowed) to 36 months. At 12 months, the probability of starting a new treatment was 12.1%, which increased to 24.6% at 24 months.
Among all participants, 37 individuals (18.1%) started a new treatment following cladribine therapy. Table 3 presents the characteristics of these patients. The most common treatments were ocrelizumab (n = 15, 7.4%) and natalizumab (n = 8, 3.9%). No patients received an additional cycle of cladribine during the entire follow-up as per Italian rules (Figure 1).
| Sex | Time to start, months | Age, years | Comorbidities | Previous DMT | Disease duration, yrs | EDSS prior to cladribine | EDSS during first year of treatment | EDSS during second year of treatment | Relapses in past 12 months | Relapses during cladribine treatment | Previous MRI activity | MRI activity during treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ocrelizumab | ||||||||||||
| Female | 12 | 27 | Yes | No previous DMT | 2 | 2.0 | 2.0 | 2.0 | Yes | Yes | ||
| Female | 10 | 43 | Fingolimod | 17 | 1.0 | 2.0 | 2.0 | Yes | ||||
| Female | 11 | 24 | Yes | Glatiramer acetate | 4 | 1.0 | 1.0 | 2.0 | Yes | Yes | Yes | |
| Female | 11 | 46 | Dimethyl fumarate | 9 | 5.0 | 3.5 | 6.0 | Yes | Yes | Yes | Yes | |
| Male | 14 | 35 | Natalizumab | 9 | 2.5 | 2.5 | 2.5 | Yes | ||||
| Male | 2 | 31 | Dimethyl fumarate | 3 | 2.0 | 2.0 | 1.5 | Yes | Yes | Yes | ||
| Female | 11 | 31 | Yes | Dimethyl fumarate | 13 | 2.0 | 1.5 | 1.5 | Yes | Yes | Yes | Yes |
| Female | 14 | 28 | Yes | No previous DMT | 2 | 1.0 | 1.0 | 1.0 | Yes | Yes | ||
| Female | 10 | 23 | Dimethyl fumarate | 5 | 1.0 | 1.0 | 1.0 | Yes | Yes | Yes | Yes | |
| Female | 11 | 22 | Fingolimod | 7 | 1.0 | 1.0 | 1.0 | Yes | Yes | Yes | ||
| Female | 18 | 25 | Dimethyl fumarate | 6 | 3.0 | 2.0 | 2.0 | Yes | Yes | |||
| Female | 0 | 30 | No previous DMT | 2 | 1.5 | 1.5 | 0.0 | Yes | Yes | Yes | ||
| Female | 0 | 62 | Interferon | 7 | 3.0 | 3.0 | 2.5 | Yes | Yes | Yes | Yes | |
| Male | 0 | 28 | Dimethyl fumarate | 4 | 1.0 | 1.0 | 1.0 | Yes | Yes | Yes | ||
| Female | 0 | 27 | Fingolimod | 5 | 0.0 | 0.0 | 1.0 | Yes | Yes | Yes | ||
| Natalizumab | ||||||||||||
| Female | 2 | 24 | Interferon | 5 | 0.0 | 0.0 | 0.0 | |||||
| Female | 14 | 33 | Interferon | 2 | 1.0 | 1.0 | 1.0 | Yes | Yes | |||
| Female | 19 | 17 | No previous DMT | 2 | 0.0 | 0.0 | 2.0 | Yes | Yes | Yes | ||
| Female | 4 | 36 | Dimethyl fumarate | 10 | 1.0 | 1.0 | 1.0 | Yes | Yes | Yes | Yes | |
| Female | 0 | 30 | Fingolimod | 8 | 1.0 | 0.0 | 0.0 | Yes | Yes | Yes | ||
| Female | 12 | 26 | Yes | Fingolimod | 5 | 2.0 | 2.0 | 2.0 | Yes | Yes | ||
| Female | 0 | 33 | Fingolimod | 18 | 1.0 | 1.0 | 1.0 | Yes | Yes | Yes | ||
| Female | 0 | 28 | Yes | Glatiramer acetate | 6 | 1.0 | 2.0 | 2.0 | Yes | Yes | Yes | |
| Dimethyl fumarate | ||||||||||||
| Male | 7 | 47 | No previous DMT | 2 | 2.0 | 1.0 | 2.0 | Yes | Yes | Yes | ||
| Female | 10 | 29 | No previous DMT | 2 | 1.0 | 1.0 | 1.0 | Yes | Yes | |||
| Female | 12 | 41 | Teriflunomide | 11 | 2.5 | 1.5 | 1.0 | Yes | Yes | Yes | Yes | |
| Male | 13 | 36 | Dimethyl fumarate | 10 | 2.0 | 1.0 | 1.0 | Yes | Yes | |||
| Female | 17 | 35 | No previous DMT | 2 | 2.0 | 1.0 | 1.0 | Yes | Yes | |||
| Ofatumumab | ||||||||||||
| Female | 13 | 24 | Dimethyl fumarate | 3 | 1.0 | 1.5 | 2.0 | Yes | Yes | Yes | Yes | |
| Female | 17 | 48 | Dimethyl fumarate | 8 | 5.5 | 5.5 | 6.0 | Yes | Yes | |||
| Female | 2 | 52 | Yes | Glatiramer acetate | 21 | 3.0 | 4.0 | 4.0 | ||||
| Teriflunomide | ||||||||||||
| Female | 15 | 45 | Yes | Interferon | 4 | 2.0 | 2.0 | 3.0 | Yes | Yes | Yes | |
| Female | 9 | 24 | Interferon | 12 | 1.0 | 0.0 | 0.0 | Yes | Yes | |||
| Alemtuzumab | ||||||||||||
| Female | 13 | 23 | Teriflunomide | 6 | 1.0 | 1.0 | 1.0 | |||||
| Female | 16 | 24 | Yes | Natalizumab | 6 | 1.0 | 1.0 | 1.0 | Yes | |||
| Siponimod | ||||||||||||
| Female | 11 | 27 | Glatiramer acetate | 6 | 6.0 | 6.0 | 6.0 | Yes | ||||
| Interferon | ||||||||||||
| Female | 7 | 20 | Natalizumab | 2 | 1.5 | 2.0 | 1.5 | Yes | Yes | Yes | ||
| Overall | 9.1 ± 6.04 | 32.0 ± 9.97 | 9/37 (24.3%) | 6.6 ± 4.78 | 1.0 [1.0–2.0] | 1. 0 [1.0–2.0] | 1.5 [1.0–2.0] | 24/37 (64.9%) | 14/37 (37.8%) | 33/37 (89.2%) | 24/37 (64.9%) | |
- Abbreviations: DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging.
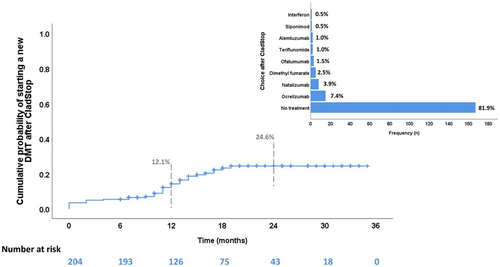
Table 4 shows the analysis of time to starting a new treatment after CladStop. Patients who experienced relapses during the 2 years of cladribine treatment had a higher probability of starting a new therapy after CladStop (hazard ratio [HR] = 3.78, 95% confidence interval [CI] = 1.82–7.85, p < 0.001) compared to patients without relapses. Furthermore, the probability of starting a new therapy decreased with the age of the patients (HR = 0.68, 95% CI = 0.47–0.98, p = 0.039). Patients who had higher disease activity before cladribine, as indicated by MRI activity or previous relapses, did not exhibit a significantly higher probability of initiating new therapy compared to other patients.
| Parameter | Univariate HR (95% CI); p | Multivariate HR (95% CI); p |
|---|---|---|
| Sex, males vs. females | 0.32 (0.13–0.83); 0.019 | 0.44 (0.17–1.16); 0.10 |
| Age, 10 years | 0.60 (0.42–0.88); 0.008 | 0.68 (0.47–0.98); 0.039* |
| MRI activity in the previous 12 months | 1.65 (0.58–4.70); 0.35 | – |
| Presence of relapses in the previous 12 months | 1.12 (0.57–2.21); 0.74 | – |
| MS duration, years | 0.97 (0.92–1.02); 0.23 | – |
| Presence of relapses during cladribine treatment | 5.23 (2.68–10.22); <0.001 | 3.78 (1.82–7.85); <0.001* |
| Last EDSS during cladribine treatment | 0.89 (0.73–1.10); 0.28 | – |
| Presence of MRI activity during cladribine treatment | 1.99 (1.01–3.91); 0.046 | 1.29 (0.63–2.67); 0.49 |
- Note: Cox regression model. * indicates statistically significant.
- Abbreviations: CI, confidence interval; EDSS, Expanded Disability Status Scale; HR, hazard ratio; MRI, magnetic resonance imaging; MS, multiple sclerosis.
At 12 months after CladStop, 40 patients (28.0% among those with this minimum follow-up) showed an improvement of at least 0.5 points on the EDSS, whereas 18.8% reported a disability progression. Of these, 70.4%, still at 12 months, had neither MRI activity nor relapses.
Nineteen patients showed MRI activity (data not shown).
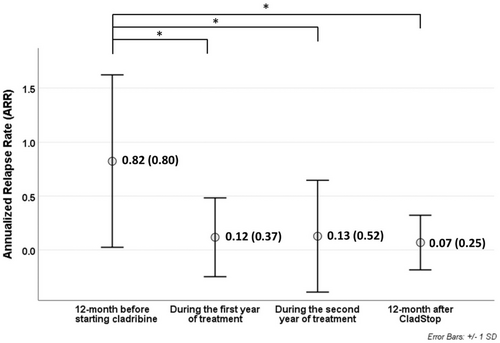
The ARR improved significantly as early as the first year of treatment (0.12 ± 0.37, p < 0.001), confirmed after the second year (0.13 ± 0.52, p < 0.001); the ARR further decreased after 12 months of follow-up after CladStop (0.07 ± 0.25, p < 0.001), again compared with the ARR in the year prior to cladribine start (0.82 ± 0.80; Figure 2).
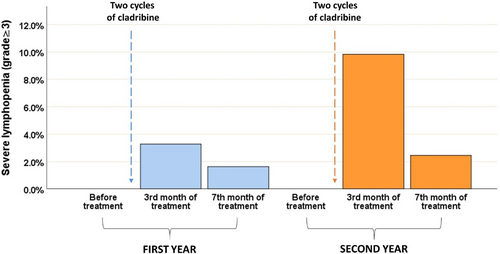
In terms of lymphocyte counts, the trends over the 2 years mirror each other, with a marked increase in lymphopenia (grade III or higher) immediately after the treatment cycle (3.6% in the first year and 8.8% in the second year), followed by a recovery at month 7 (1.8% in the first year and 2.6% in the second year). This trend is particularly noticeable in the second year (Figure 3). The presence of high grade of lymphopenia was consistent among all patients throughout the entire study period, regardless of their age, except during the third month of treatment in the second cycle, when a higher frequency of events was observed in the elderly group (>50 vs. ≤50 years old: 26.3% vs. 6.2%, p = 0.004).
Eighteen (8.8%) patients presented at least one adverse event (AE) for a total of 23 cases. Twenty AEs occurred in 15 patients during the 2 years of treatment and three in three patients in the 12 months following CladStop. One serious AE, prostate cancer, occurred in one pwMS during the 12-month following CladStop; this AE was considered not related to the treatment by the treating neurologist (Tables 5 and 6). Three cases of oral herpes, indicated by physician as probably or certainly related to cladribine use, were reported during 2 years of treatment. They were mild and resolved within a few days; these patients did not have lymphopenia. However, it is worth mentioning that prophylaxis for herpes zoster is recommended for patients with grade 4, for grade 3 lymphopenia, or for immunocompromised individuals [18].
| Total | During treatment with cladribine | During 12-month following CladStop | ||
|---|---|---|---|---|
| Patients with at least one AE | 18 | 15 | 3 | |
| Total number of AEs | 23 | 20 | 3 | |
| Severity | Mild | 11 (47.8%) | 10 (50.0%) | 1 (33.3%) |
| Moderate | 11 (47.8%) | 9 (45.0%) | 2 (66.7%) | |
| Severe | 1 (4.3%) | 1 (5.0%) | 0 (0.0%) | |
| Relationship with cladribine | Certainly related | 4 (17.4%) | 4 (20.0%) | 0 (0.0%) |
| Definitely related | 1 (4.3%) | 1 (5.0%) | 0 (0.0%) | |
| Probably related | 6 (26.1%) | 6 (30.0%) | 0 (0.0%) | |
| Possibly related | 6 (26.1%) | 5 (25.0%) | 1 (33.3%) | |
| Likely related | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Not related | 6 (26.1%) | 4 (20.0%) | 2 (66.7%) | |
| SAEs | 1 (4.3%) | 0 (0.0%) | 1 (33.3%) |
- Note: Data are presented as n (%).
- Abbreviations: AE, adverse event; SAE, serious AE.
| System organ class (SOC) | Preferred terms (PT) | Severity | Relationship with cladribine |
|---|---|---|---|
| During treatment with cladribine | |||
| General disorders and administration site co | Pyrexia | Mild | Probably related |
| Infections and infestations | Influenza | Mild | Not related |
| Infections and infestations | Nasopharyngitis | Mild | Probably related |
| Infections and infestations | Fungal infection | Mild | Probably related |
| Infections and infestations | Oral herpes | Mild | Probably related |
| Infections and infestations | Oral herpes | Mild | Certainly related |
| Infections and infestations | Oral herpes | Mild | Certainly related |
| Skin and subcutaneous tissue disorders | Neurodermatitis | Mild | Not related |
| Skin and subcutaneous tissue disorders | Eczema herpeticum | Mild | Possibly related |
| Skin and subcutaneous tissue disorders | Pityriasis | Mild | Possibly related |
| Skin and subcutaneous tissue disorders | Psoriasis | Moderate | Probably related |
| Investigations | Platelet count decreased | Moderate | Not related |
| Gastrointestinal disorders | Abdominal pain upper | Moderate | Not related |
| Gastrointestinal disorders | Pharyngitis | Moderate | Possibly related |
| Nervous system disorders | Writer's cramp | Moderate | Possibly related |
| Nervous system disorders | Coordination abnormal | Moderate | Possibly related |
| Immune system disorders | Urticaria | Moderate | Probably related |
| Vascular disorders | Hypotension | Moderate | Certainly related |
| Blood and lymphatic system disorders | Lymphopenia | Severe | Certainly related |
| Infections and infestations | Vulvovaginal candidiasis | Moderate | Definitely related |
| During 12 months following CladStop | |||
| Pregnancy, puerperium, and perinatal conditions | Pregnancy | Mild | Not related |
| Infections and infestations | Gingivitis | Moderate | Possibly related |
| Neoplasms benign, malignant, and unspecified | Prostate cancer | Moderate | Not related |
DISCUSSION
This study reports on the therapeutic choice at the end of 2 years of treatment, the evidence of cladribine's effectiveness in reducing relapse rates and disability progression, and the drug's safety profile.
In general, the results from this study overall demonstrate an overall significant improvement in the ARR at the 12-month follow-up after CladStop compared to the pretreatment period and the first 2 years of treatment. This observation suggests a concrete reduction in the occurrence of relapses in pwMS irrespective of their treatment choice after the completion of the second cycle of cladribine.
The analysis of time to starting a new treatment after CladStop reveals two significant associations (both p < 0.001) and provide insights into the factors influencing treatment decision following 2 years of treatment with cladribine. First, patients who experienced relapses during the 2 years of cladribine treatment demonstrated a threefold higher probability of initiating a new therapy after CladStop. Second, there is an inverse proportional relationship between patients' age and the likelihood of starting a new therapy after CladStop. However, most of the patients did not initiate new treatments during the 12 months following the completion of the treatment with cladribine, suggesting a stable clinical status that did not require subsequent additional therapies. Only a small proportion of patients (9%) showed MRI activity and/or worsening on the EDSS (18.8% in 143 patients with evaluation at 12-month follow-up).
Overall, cladribine showed a favorable safety profile consistent with its mechanism of action [19]; lymphopenia (grade ≥ 3) was reported in a subgroup of patients immediately after each cycle of cladribine, both during the first year (about 4%) and during the second year (about 9%). Lymphocyte counts returned to a normal range as we moved away from the last dose (at the seventh month of treatment), consistent with data from the literature [2].
Noticeably, two of three cases of oral herpes occurred in nonelderly adult patients coming from a previous DMF treatment, whereas one was not previously treated. This aligns with recent literature [20], which reported that herpes infections predominantly occurred in patients who had received DMF as prior therapy.
After CladStop, only three AEs were recorded in three patients. Among these, only one case of moderate severity gingivitis was reported as possibly related to cladribine and occurred 7 months after 2 years of treatment.
These results are consistent with other reports based on real-life data, confirming that ocrelizumab and natalizumab are the primary choices for switching after 2 years of treatment with cladribine [21]. Additionally, they indicate a reduction in ARR during and after treatment compared to the pretreatment period, as well as a predominance of mainly mild to moderate adverse events [16]. Finally, they demonstrate a similar rate of improvement in EDSS after the end of 2 years of treatment [22].
This study has limitations. To begin with, it is important to note that the study is observational and retrospective. There is a possibility that physicians might not have accurately reported specific details, and the lack of clinical evaluations at certain time points could affect the overall reliability of the findings.
Additionally, the chosen follow-up period after the completion of 2 years of cladribine treatment is relatively short (a minimum of 6 months and a maximum of 36 months). A longer term follow-up would provide a more comprehensive understanding of the sustained effectiveness and safety profile of cladribine beyond the immediate posttreatment period.
Finally, the study primarily focuses on clinical outcomes, such as relapse rates and disability progression, but does not extensively explore or collect information about patient preferences or factors influencing their subsequent therapeutic choices. A more comprehensive understanding of patient perspectives, for example, through patient-reported outcomes, could enhance the interpretation of treatment decisions.
CONCLUSIONS
Evidence from these real-world data suggests predictors for starting a new treatment after 2 years of cladribine treatment and confirms the efficacy of cladribine on disease activity and on disability worsening (with a consistent reduction on ARR and an improvement on the EDSS).
The incidence of AEs was relatively low, with no treatment-related serious AEs reported. However, the retrospective design of this study together with the selection of a limited Italian–Swiss cohort of patients could bias these results, and further studies with larger sample sizes and longer follow-up would be useful to validate these findings.
AUTHOR CONTRIBUTIONS
Irene Schiavetti: Conceptualization; writing – original draft; writing – review and editing; methodology; formal analysis; project administration; supervision. Alessio Signori: Methodology; writing – review and editing; supervision; conceptualization; project administration. Angela Albanese: Writing – review and editing; writing – original draft. Jessica Frau: Writing – review and editing; investigation; data curation. Eleonora Cocco: Data curation; investigation; writing – review and editing. Lorena Lorefice: Data curation; investigation; writing – review and editing. Sonia di Lemme: Data curation; investigation; writing – review and editing. Roberta Fantozzi: Data curation; investigation; writing – review and editing. Diego Centonze: Writing – review and editing; investigation; data curation. Doriana Landi: Data curation; investigation; writing – review and editing. Girolama Marfia: Data curation; investigation; writing – review and editing. Elisabetta Signoriello: Data curation; investigation; writing – review and editing. Giacomo Lus: Data curation; investigation; writing – review and editing. Chiara Zecca: Data curation; investigation; writing – review and editing. Claudio Gobbi: Data curation; investigation; writing – review and editing. Rosa Iodice: Data curation; investigation; writing – review and editing. Leonardo Malimpensa: Data curation; investigation; writing – review and editing. Cinzia Cordioli: Data curation; investigation; writing – review and editing. Diana Ferraro: Data curation; investigation; writing – review and editing. Francesca Ruscica: Data curation; investigation; writing – review and editing. Livia Pasquali: Data curation; investigation; writing – review and editing. Anna Repice: Data curation; investigation; writing – review and editing. Paolo Immovilli: Data curation; investigation; writing – review and editing. Maria Teresa Ferrò: Data curation; investigation; writing – review and editing. Simona Bonavita: Data curation; investigation; writing – review and editing. Massimiliano Di Filippo: Data curation; investigation; writing – review and editing. Gianmarco Abbadessa: Data curation; investigation; writing – review and editing. Flora Govone: Data curation; investigation; writing – review and editing. Maria Pia Sormani: Validation; supervision; writing – review and editing; methodology; conceptualization; project administration.
CONFLICT OF INTEREST STATEMENT
Abbadessa Gianmarco personal compensation from Janssen and Merck for traveling and/or advisory boards. Albanese Angela is an employee of Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA, Darmstadt, Germany. Bonavita Simona was Speaker and/or AB honoraria from Novartis, BMS, Merck Serono, Biogen, Janssen Cilag, Alezio, Horizon, Roche. Centonze Diego is an advisory board member of Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva and has received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva. He also is the principal investigator in clinical trials for Bayer Schering, Biogen, Merck KGaA (Darmstadt, Germany), Mitsubishi, Novartis, Roche, Sanofi-Genzyme and Teva. His preclinical and clinical research was supported by grants from Bayer Schering, Biogen Idec, Celgene, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva. Cocco Eleonora serves on scientific advisory boards and received honoraria for speaking from Alexion, Biogen, BMS, Janssen, Merck, Novartis, Roche, and Sanofi Genzyme. Cordioli Cinzia received Honoraria for travelling or speaking in advisory Board from Biogen, Novartis, Merck Serono, Roche, BMS. Di Filippo Massimiliano participated on advisory boards and steering committees for and received speaker or writing honoraria, research support and funding fortravelling from Alexion, BMS, Bayer, Biogen Idec, Genzyme, Horizon, Merck, Mylan, Novartis, Roche, Siemens Healthineers, Teva and Viatris. Fantozzi Roberta received honoraria or consultation fees from Roche, Novartis, Merck Serono, BMS. Ferraro Diana has received travel grants and/or fees for speaking and/or advisory boards from Alexion, Biogen, Bristol-Myers Squibb, Celgene, Merck, Novartis, Roche, Sanofi. Frau Jessica serves on scientific advisory boards for Biogen, Merck, Genzyme, Novartis, and has received honoraria as a speaker from Merck, Biogen, Novartis, Genzyme, TEVA, Alexion. Gobbi Claudio received consulting fees, or research grants from Almirall, Biogen Idec, Bristol Meyer Squibb, Lundbeck, Merck, Novartis, Sanofi, Teva Pharma, Roche. (Ente Ospedaliero Cantonale (employer) received compensation for Gobbi Claudio's speaking activities). Govone Flora received grants from Roche. Immovilli Paolo received fees for speaking or advising from Roche, Biogen, Sanofi, Bristol Squibb Meyers, Novartis and Merck. Iodice Rosa reports personal fees from Merck, Biogen, Teva, Sanofi Genzyme, Roche, Almirall, Viatris. Landi Doriana received travel funding from Biogen, Merck-Serono, Sanofi-Genzyme, Teva, speaking or consultations fees from Sanofi-Genzyme, Merck-Serono, Teva, Biogen, Roche; Research sponsorship from Roche. Lorefice Lorena received honoraria for consultancy or speaking from Biogen, Novartis, Sanofi, Genzyme, Serono and Teva and Almirall. Lus Giacomo received personal compensation for activities with Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis Pharmaceuticals, Teva neuroscience as a consultant and speaker and received research support from Biogen Idec, Merck Serono, and Novartis. Marfia Girolama Alessandra received speaking or consultation fees from Almirall, Bayer-Schering, Biogen, Genzyme, Merck-Serono, Novartis, Teva, Sanofi-Genzyme. Pasquali Livia received personal compensations for speaking or consultancy from Sanofi, Novartis, Merck, Alexion, Biogen; and supporting for attending meetings from Sanofi, Merck. Repice Anna Maria has received honoraria for speaking and for participating to advisory board from Merck, Biogen-Idec, Sanofi-Genzyme, Novartis, Roche, Bristol Mayer. Schiavetti Irene received consulting fees from Horizon, Hippocrates Research, Eyepharma, Hoya Holding N.V., DMG Italia and DreamsLab. Signori Alessio received consulting fees from Horizon, Chiesi and Sanofi-Genzyme outside of this work. Signoriello Elisabetta received personal compensation from Almirall, Biogen, Genzyme, Novartis, and Teva fortraveling and advisory boards. Sormani MP received consulting fees from Roche, Biogen, Merck, Novartis, Sanofi, Celgene, Immunic, Geneuro, GSK, Medday; received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from Roche, Biogen Merck, Novartis, Sanofi, Celgene; participated on a Data Safety Monitoring Board or Advisory Board for Roche, Sanofi, Novartis, Merck. Zecca Chiara received consulting fees, or research grants from Almirall, Biogen Idec, Bristol Meyer Squibb, Lundbeck, Merck, Novartis, Sanofi, Teva Pharma, Roche [Ente Ospedaliero Cantonale (employer) received compensation for Zecca Chiara's speaking activities]. Other authors have nothing to disclose.
APPENDIX A: CladStop Study Group
| Abbadessa, Gianmarco | I Division of Neurology–University of Campania “Luigi Vanvitelli,” Naples, Italy |
| Albanese, Angela | Department of Health Sciences, University of Genoa, Genoa, Italy |
| Bonavita, Simona | Dipartimento di Scienze Mediche e Chirurgiche Avanzate, Università della Campania Luigi Vanvitelli, Naples, Italy |
| Cardi, Martina | Department of Biomedical Metabolic and Neurosciences, University of Modena and Reggio Emilia, Modena, Italy |
| Cassano, Emanuele | Clinica Neurologica, DSNRO Università Federico II di Napoli, Naples, Italy |
| Centonze, Diego | Unit of Neurology, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Neuromed, Pozzilli, Italy. Tor Vergata University, Department of Systems Medicine, Rome, Italy |
| Cocco, Eleonora | Centro Sclerosi Multipla Ospedale Binaghi Cagliari, Azienda Sanitaria Locale (ASL) Cagliari, Italy. Dipartimento Scienze Mediche e Sanità Pubblica, Università di Cagliari, Cagliari, Italy |
| Conte, Antonella | Centro Sclerosi Multipla Policlinico Umberto I, Rome, Italy |
| Cordioli, Cinzia | Centro Sclerosi Multipla, Azienda Socio Sanitaria Territoriale (ASST) Spedali Civili di Brescia, Montichiari, Italy |
| Di Filippo, Massimiliano | Clinica Neurologica, Dipartimento di Medicina e Chirurgia Università di Perugia, Perugia, Italy |
| Di Lemme, Sonia | Unit of Neurology, IRCCS Neuromed, Pozzilli, Italy |
| Di Sabatino, Elena | Clinica Neurologica, Dipartimento di Medicina e Chirurgia Università di Perugia, Perugia, Italy |
| Fantozzi, Roberta | Unit of Neurology, IRCCS Neuromed, Pozzilli, Italy |
| Ferraro, Diana | Department of Neurosciences, Ospedale Civile di Baggiovara, Azienda Ospedaliero–Universitaria di Modena, Modena, Italy |
| Ferrò, Maria Teresa | Neuroimmunology, Center for Multiple Sclerosis, Cerebrovascular Department, Neurological Unit, Azienda Socio Sanitaria Territoriale (ASST), Crema, Italy |
| Frau, Jessica | Centro Sclerosi Multipla Ospedale Binaghi Cagliari, Azienda Sanitaria Locale (–ASL) Cagliari, Italy. |
| Gabri, Nicoletti Carolina | Multiple Sclerosis Clinical and Research Unit, Department of Systems Medicine, Tor Vergata University, Rome, Italy |
| Gobbi, Claudio | Multiple Sclerosis Center, Neurocenter of Southern Switzerland, EOC, Lugano, Switzerland. Faculty of Biomedical Sciences, Università della Svizzera Italiana, Lugano, Switzerland |
| Govone, Flora | Centro SM–Neurologia di Mondovì, Mondovì (Cuneo), Italy |
| Grimaldi, Luigi | Fondazione Giglio di Cefalù, Cefalù (Palermo), Italy |
| Immovilli, Paolo | Neurology Unit, Emergency Department, Guglielmo da Saliceto Hospital, Piacenza, Italy |
| Iodice, Rosa | Clinica Neurologica, DSNRO Università Federico II di Napoli, Naples, Italy |
| Landi, Doriana | Multiple Sclerosis Clinical and Research Unit, Department of Systems Medicine, Tor Vergata University, Rome, Italy |
| Lavorgna, Luigi | I Division of Neurology–University of Campania “Luigi Vanvitelli,” Naples, Italy |
| Lorefice, Lorena | Centro Sclerosi Multipla Ospedale Binaghi Cagliari–ASL Cagliari, Cagliari, Italy |
| Lus, Giacomo | Centro Sclerosi Multipla, II Clinica Neurologica, Università della Campania Luigi Vanvitelli, Naples, Italy |
| Malimpensa, Leonardo | Mediterranean Neurological Institute Neuromed (IRCCS), Pozzilli, Italy |
| Marfia, Girolama | Multiple Sclerosis Clinical and Research Unit, Department of Systems Medicine, Tor Vergata University, Rome, Italy |
| Miele, Giuseppina | Dipartimento di Scienze Mediche e Chirurgiche Avanzate, Università della Campania Luigi Vanvitelli, Naples, Italy |
| Napoli, Francesca | Multiple Sclerosis Clinical and Research Unit, Department of Systems Medicine, Tor Vergata University, Rome, Italy |
| Pasquali, Livia | Department of Clinical and Experimental Medicine, Neurology Unit, University of Pisa, Pisa, Italy |
| Repice, Anna | Department of Neurology 2, Careggi University Hospital, Florence, Italy |
| Ruscica, Francesca | Fondazione Giglio di Cefalù, Cefalù (Palermo), Italy |
| Schiavetti, Irene | Department of Health Sciences, University of Genoa, Genoa, Italy |
| Signori, Alessio | Department of Health Sciences, University of Genoa, Genoa, Italy |
| Signoriello, Elisabetta | Centro Sclerosi Multipla, II Clinica Neurologica, Università della Campania Luigi Vanvitelli, Naples, Italy |
| Siniscalchi, Carmine | Clinica Neurologica, DSNRO Università Federico II di Napoli, Naples, Italy |
| Sormani, Maria Pia | Department of Health Sciences, University of Genoa, Genoa, Italy. IRCCS Ospedale Policlinico San Martino, Genoa, Italy |
| Tozza, Stefano | Clinica Neurologica, DSNRO Università Federico II di Napoli, Naples, Italy |
| Vitetta, Francesca | Department of Neurosciences, Ospedale Civile di Baggiovara, Azienda Ospedaliero–Universitaria di Modena, Modena, Italy |
| Zecca, Chiara | Multiple Sclerosis Center, Neurocenter of Southern Switzerland, EOC, Lugano, Switzerland |
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




