Sirolimus leads to rapid and sustained clinical improvement of motor deficits in a patient with inclusion body myositis
Marc Pawlitzki and Christopher Nelke contributed equally to this work.
Abstract
Background and purpose
To provide further evidence for sirolimus, a mammalian target of rapamycin inhibitor, as a treatment strategy for patients with inclusion body myositis (IBM).
Methods
We acquired longitudinal clinical data and immunological assessments of CD8+ T-cell subsets in peripheral blood for evaluation of potential anti-inflammatory treatment effects of sirolimus.
Results
Therapy with sirolimus 2 mg/day by mouth led to rapid and sustained clinical improvement of motor symptoms for an observation period of more than 1 year. Treatment was well tolerated, with no occurrence of adverse effects. We did not observe a meaningful alteration of CD8+ T-cell subsets in our patient after 9 and 12 months compared to baseline.
Conclusions
The significant and persistent clinical improvement highlights the use of sirolimus as a potential treatment option in patients with IBM. In light of the lack of immunological treatment effects observed for cytotoxic CD8+ T cells, further studies should investigate the potential myoprotective effects of sirolimus.
INTRODUCTION
Efficacious treatment strategies for inclusion body myositis (IBM) remain an unmet need. Although histopathological features of IBM contain inflammatory patterns such as CD8+ T-cell infiltration within the skeletal muscle tissue [1], previous immunosuppressive approaches were not clinically significant for ameliorating IBM.
A recently published phase II trial of sirolimus, a mammalian target of rapamycin inhibitor, showed no evidence of slowing disease progression with regard to muscles strength. However, significant changes were observed for secondary outcome measures such as 6-min walking distance [2]. We took this as an opportunity for therapy with sirolimus in a severely affected IBM patient. Surprisingly, we observed a substantial clinical improvement within 1 year, which persisted for the further observation period (currently 24 months).
CASE REPORT
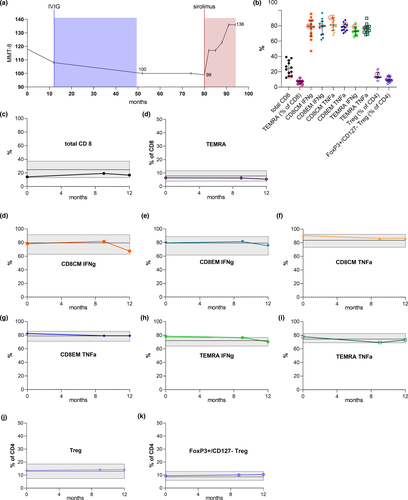
A 66-year-old White female was diagnosed with anti-NT5c1A antibody negative clinico-pathologically defined IBM [3]. She presented with characteristic progressive weakness and atrophy in the muscles of the hand, forearm, knee extensors (Manual Muscle Testing 8 [MMT8] score 110/150), and severe dysphagia over 12 months. Magnetic resonance imaging of both legs showed extensive muscle atrophy with persistent signs of inflammation. Muscle biopsy of the tibialis anterior muscle revealed typical histopathological features of ragged red fibers, rimmed vacuoles, and CD8+ T-cell infiltration within major histocompatibility complex class I–expressing muscle fibers. She received intravenous immunoglobulins (120 g over 5 days) every 3 months for 4 years without stabilization of her disease. Midsevere tetraparesis (MMT8 99/150) persisted, and she was mobile only with assisted walking (IBM Functional Rating Scale [IBMFRS] 19/40). Treatment with sirolimus 2 mg/day by mouth was initiated. After 9 months, the patient improved substantially (MMT8 123/150). Three months later, we did not notice significant weakness in the proximal muscles of the limbs except for the quadricep muscles. The patient was able to walk without assistance (MMT8 136/150, IBMFRS 29/40; Figure 1a, Figure S1). Analysis of dysphagia by functional endoscopic swallowing revealed no progression. Treatment was well tolerated, with no occurrence of adverse effects. Sirolimus blood level was 9.4 ng/ml [4–10]. We performed a detailed immunological assessment of CD8+ T-cell subsets in peripheral blood for evaluation of potential anti-inflammatory effects of sirolimus. Our patient presented similar distribution of cytotoxic CD8+ T-cell subsets compared with 11 other treatment-naïve IBM patients (Figure 1b). At 9 and 12 months follow-up, we did not observe a meaningful alteration of CD8+ T-cell subsets in our patient compared to baseline (Figure 1c,f–i). Only interferon-γ–producing CD8+ T cells were marginally reduced at month 12 (Figure 1d,e).
DISCUSSION
IBM continues to impose a substantial burden of disease on affected patients and leads to loss of ambulation in most patients. To date, no effective treatment exists, potentially due to the neurodegenerative aspects that accompany the inflammatory drivers of the disease [4]. Immunologically, IBM patients are characterized by an increased rate of highly differentiated effector CD8+ T cells both in the peripheral blood and in muscle tissue [1]. Moreover, the presence of anti-NT5c1A antibodies in a relevant part of IBM cases suggest the role of B-cell immunity and its potential impact on protein degradation in myofibers [4]. However, previous immunosuppressive approaches (i.e., prednisolone or alemtuzumab) displayed only marginal beneficial effects [5]. Sirolimus also has immunosuppressive effects mediated via inhibition of the interleukin-2 pathway, leading to a decrease in CD8+ effector T-cell subpopulations in the peripheral blood of IBM patients [2]. However, we did not detect meaningful changes in immune cell subpopulations despite clinical improvement, suggesting therapeutic effects of sirolimus beyond immunological modulation. The degenerative viewpoint of IBM is supported by histopathological studies showing protein deposits within muscle fibers, which may contribute to the myopathic changes in IBM [4]. Interestingly, previous experimental studies support myoprotective features of sirolimus, which might induce autophagic pathways of misfolded protein aggregates and rimmed vacuoles in IBM [6]. The substantial clinical improvement without immunological treatment effects recorded for cytotoxic CD8+ T cells as observed in our patient might argue for treatment strategies harnessing myoprotective effects. Interestingly, in contrast to recent therapeutic approaches in IBM, sirolimus exerts both immunosuppressive and myoprotective effects. As such, we speculate that the negative outcome of arimoclomol in IBM might be attributed to the lack of immunosuppression, arguing for a dual-treatment strategy.
As a limitation, it should be mentioned that only changes in the peripheral blood of one patient were investigated. Therefore, we cannot confirm that changes in CD8+ T cells can occur in IBM patients receiving sirolimus, and we do not provide information on T-cell changes in muscles. A current phase III trial will provide further insight into the potential of sirolimus in IBM.
CONFLICT OF INTEREST
The authors declare no conflict of interest in relation to the content of this article.
AUTHOR CONTRIBUTIONS
Marc Pawlitzki: Conceptualization (equal), data curation (equal), investigation (equal), writing–original draft (equal). Christopher Nelke: Data curation (equal), formal analysis (equal), writing–original draft (equal). Melanie Korsen: Data curation (equal), writing–review & editing (equal). Sven Meuth: Data curation (equal), writing–review & editing (equal). Tobias Ruck: Conceptualization (equal), investigation (equal), supervision (equal), writing–review & editing (equal).
ACKNOWLEDGMENT
Open Access funding enabled and organized by Projekt DEAL.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




