Respiratory onset of amyotrophic lateral sclerosis in a pregnant woman with a novel SOD1 mutation
Funding information
This work was supported by a grant from FWO-Vlaanderen (Flemish Fund for Scientific Research, T003519N to P.V.D.) and C1 internal funds from KU Leuven (C14-17-107 to P.V.D. and DRT). P.M. has a research fellowship from the European Academy of Neurology (no award/grant number). T.G.M. is supported by an FWO postdoctoral fellowship (1246821N). K.F. received funding from the Ulla-Carin Lindquist ALS Foundation and Västerbotten County Council (ALF). P.M.A. received funding from the Swedish Brain Foundation (2020-0353), the Swedish Research Council (2017-03100), and the Knut and Alice Wallenberg Foundation (2012.0091, 2014.0305, 2020.0232). D.R.T. received funding from FWO (G0F8516N, G065721N) and Vlaamse Impulsfinanciering voor Netwerken voor Dementie Onderzoek (Innovatie door Wetenschap en Technologie 135043). P.V.D. is a senior clinical investigator for FWO-Vlaanderen and is supported by the ALS Liga België and the KU Leuven funds Een Hart voor ALS, Laeversfonds voor ALS Onderzoek, and Valéry Perrier Race Against ALS
Abstract
Background and purpose
With the advent of gene therapies for amyotrophic lateral sclerosis (ALS), the importance of gene testing in ALS is increasing. This will likely lead to the identification of new variants for which the pathogenicity is not established. We aimed to study the pathogenicity of a newly identified variant in superoxide dismutase 1 (SOD1).
Methods
Gene testing was performed using Sanger sequencing. SOD1 activity in erythrocytes was measured using spectrophotometry. Postmortem brain and spinal cord sections were stained with antibodies against phospho-TDP-43 and SOD1.
Results
We identified a novel c.416G>T (p.Gly139Val) mutation in SOD1, which caused a rapidly progressive respiratory onset form of ALS. The mutation resulted in a 50% drop of SOD1 activity. Postmortem examination confirmed the absence of TDP-43 pathology and displayed typical SOD1 inclusions in remaining motor neurons, confirming the pathogenic nature of the mutation.
Conclusions
Novel variants of unknown pathogenicity will be identified as a result of a surge in gene testing in people with ALS. An in-depth study of a newly identified p.Gly139Val mutation in SOD1 confirmed the pathogenicity of this mutation. Future patients with this particular mutation should qualify for SOD1 silencing or editing therapies.
INTRODUCTION
A 34-year-old woman presented with respiratory onset amyotrophic lateral sclerosis (ALS) starting in the 6th month of pregnancy. Initially, the dyspnea was considered to be due to the pregnancy. After an uneventful vaginal delivery, the symptoms progressively worsened with increasing dyspnea, vocal weakness, and paresis in both legs, initially most pronounced in proximal muscles. The muscle wasting subsequently spread to the arms. Initial neurological examination, 6 months after onset, revealed dysphonia, dyspnea, and muscle weakness (a mild paresis of upper limb abduction, hip flexion, knee flexion, and ankle flexion and extension on both sides). Deep-tendon reflexes were brisk in all four limbs, with a positive Hoffmann–Trömner sign on the right side, but down-going plantar reflexes. Muscle tone in the extremities was normal. No muscle atrophy or fasciculations were noted. There were no sensory abnormalities. The gait was slightly unsteady, and walking on her heels and toes was difficult.
Electrodiagnostic testing revealed reduced compound muscle action potential amplitudes upon stimulation of the tibial nerve, with abnormal F-wave latencies and normal sensory conduction studies, but signs of denervation and chronic neurogenic changes in different upper and lower limb muscles, as well as in thoracic paravertebral muscles upon needle electromyography. Magnetic resonance imaging of the brain and spinal cord were normal. Laboratory tests including muscle-specific kinase antibodies were negative. Phosphorylated neurofilament heavy chain and neurofilament light chain in the cerebrospinal fluid were 5.009 pg/ml and 20.584 pg/ml, respectively. A diagnosis of ALS was made, and treatment with riluzole was initiated.
The ALS Functional Rating Scale–Revised score was 38 at 9 months after symptom onset and continued to decline at a rate of 2.66 points per month thereafter. At first presentation, the forced vital capacity was 56% in sitting position and 46% in supine position. Her respiratory function continued to decline, and noninvasive ventilation was started 9 months after onset. Because of progressive dysphagia, a percutaneous endoscopic gastrostomy was inserted. At the age of 35 years, 13 months after disease onset, she became permanently ventilator-dependent. She opted not to receive invasive ventilation through a tracheostomy and deceased from respiratory failure 20 months after symptom onset.
METHODS AND RESULTS
Sanger sequencing revealed a heterozygous mutation GGA>GTA in SOD1 predicting a substitution of glycine to valine in codon 139 (p.Gly139Val). Mutations in FUS, TDP-43, and C9orf72 were excluded. According to the American College of Medical Genetics and Genomics criteria and online ALS databases, this variant is classified as likely pathogenic. A GGA>GAA (p.Gly139Glu) missense mutation has previously been reported [1, 2]. This region is a mutational hotspot, with two pathogenic mutations in codon 138, two in codon 139, four in codon 140, and five in codon 142. Like the previously reported p.Gly139Glu mutation, this new p.Gly139Val mutation appears to be associated with rapidly progressive ALS. However, the penetrance of the p.Gly139Val appears to be incomplete, as evident from the pedigree (Figure 1a).
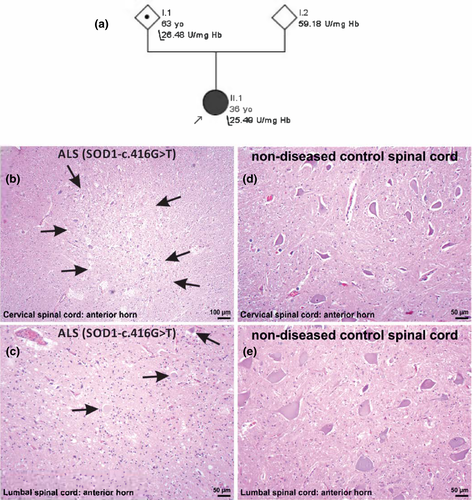
Segregation analysis revealed that one of the parents, who was in good health and older than 60 years, carried the mutation. To explore the functional effects of the genetic variant, SOD1 enzymatic activity was analyzed in erythrocytes from the patient and her parents (measured in triplicate). The enzymatic activity was 25.49 U/mgHb (normal activity is 55 U/mgHb) in the patient, and was 26.48 and 59.18 U/mgHb in the carrier and noncarrier parent, respectively. These results suggest that the mutation results in a completely unstable SOD1 mutant protein devoid of enzymatic activity (Figure 1a). Why such deleterious mutations with effect on SOD1 stability do not result in fully penetrant ALS remains unknown, but this has been reported for other SOD1 mutations as well [3].
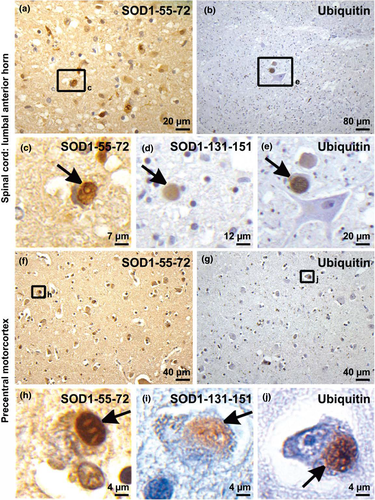
At autopsy (Supplementary Material S1), severe motor neuron loss was observed in the spinal cord (Figure 1b,c), whereas the motor cortex was less severely affected. No abnormal TDP-43 staining or inclusions were seen using a phospho-TDP-43 antibody. Using antibodies raised against human SOD1-targeting amino acids 131–153 and amino acids 57–72 (Supplementary Material S2, Table S3), we observed the formation of threadlike or round neuronal cytoplasmic inclusions throughout the anterior horn of the spinal cord and in Layer IV neurons of the motor cortex (Figure 2a,c,d,f,g,i; Supplementary Material S2, Tables S1 and S2), which were also positive for ubiquitin (Figure 2b,e,g,j,i). These findings confirmed the pathogenicity of the SOD1 mutation.
DISCUSSION
Mutant SOD1 probably causes neurodegeneration by the gain of a toxic function that may involve the formation of prionlike species [4]. Emerging evidence suggests that the SOD1 monomer is the substrate for aggregate formation. Hence, greatly lowering the expression of SOD1 is a promising therapeutic strategy for SOD1-mediated ALS [5]. Both intrathecal infusion of antisense oligonucleotides, and adeno-associated virus delivering anti-SOD1 microRNA are presently being evaluated in clinical trials [6, 7]. Our results shows that the p.Gly139Val mutation is causing rapidly progressive ALS. Future patients carrying the SOD1 p.Gly139Val mutation should qualify for SOD1 gene silencing or editing therapies.
ACKNOWLEDGMENTS
We are thankful to the family for their invaluable contribution to this research.
CONFLICT OF INTEREST
D.R.T. has received speaker honorarium from Novartis Pharma Basel (Switzerland) and Biogen (USA), and travel reimbursement from GE Healthcare (UK), and UCB (Belgium), and has collaborated with GE Healthcare (UK), Novartis Pharma Basel (Switzerland), Probiodrug (Germany), and Janssen Pharmaceutical Companies (Belgium). All other authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Pegah Masrori: Conceptualization (lead), formal analysis (equal), investigation (lead), methodology (equal), project administration (lead), resources (equal), visualization (equal), writing–original draft (lead), writing–review & editing (equal). Simona Ospitalieri: Formal analysis (equal), investigation (equal), writing–review & editing (supporting). Karin Forsberg: Formal analysis (supporting), resources (equal), writing–review & editing (supporting). Thomas G. Moens: Writing–review & editing (supporting). Koen Poesen: Investigation (supporting), resources (equal), writing–review & editing (supporting). Valerie Race: Formal analysis (supporting), investigation (supporting), writing–review & editing (supporting). Thomas Brännström: Resources (supporting), writing–review & editing (supporting). Peter M. Andersen: Conceptualization (equal), formal analysis (equal), investigation (equal), methodology (equal), supervision (equal), writing–review & editing (equal). Dietmar R. Thal: Formal analysis (equal), investigation (equal), methodology (equal), resources (equal), supervision (equal), visualization (lead), writing–review & editing (equal). Philip Van Damme: Conceptualization (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (lead), supervision (lead), writing–review & editing (equal).
ETHICS APPROVAL
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of the University Hospital in Leuven (S60803). The patient and her relatives gave written informed consent.
Open Research
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article or uploaded as Supplementary Information.




