Antiherpetic medication and incident dementia: Observational cohort studies in four countries
Christian Schnier and Janet Janbek are joint first authors.
Funding information
We gratefully acknowledge financial support (grant VIRADE 2019) from the Benter Foundation, Pittsburgh, Pennsylvania, USA.
Abstract
Background and purpose
Several epidemiological studies from Taiwan, all using the same data resource, found significant associations between herpes virus infection, antiherpetic medication, and subsequent dementia. We conducted a multicenter observational cohort study using health registry data from Wales, Germany, Scotland, and Denmark to investigate potential associations between antiherpetic medication and incident dementia, and also to comprehensively investigate such associations broken down according to medication type and dose, type of herpes virus, and dementia subtype.
Methods
A total of 2.5 million individuals aged 65 years or more were followed up using linked electronic health records in four national observational cohort studies. Exposure and outcome were classified using coded data from primary and secondary care. Data were analyzed using survival analysis with time-dependent covariates.
Results
Results were heterogeneous, with a tendency toward decreased dementia risk in individuals exposed to antiherpetic medication. Associations were not affected by treatment number, herpes subtype, dementia subtype, or specific medication. In one cohort, individuals diagnosed with herpes but not exposed to antiherpetic medication were at higher dementia risk.
Conclusions
Short-term antiherpetic medication is not markedly associated with incident dementia. Because neither dementia subtype nor herpes subtype modified the association, the small but significant decrease in dementia incidence with antiherpetic administration may reflect confounding and misclassification.
INTRODUCTION
The role of infections in the development of dementia has been widely debated [1]. Attention has focused on herpes viruses infection in part because variants at the APOE locus are not only associated with Alzheimer disease (AD; e.g., Saunders et al. [2]) but also govern the extent of damage caused by infection with herpes viruses (e.g., Itzhaki et al. [3]). Moreover, infections with herpes simplex virus (HSV) and the related varicella zoster virus (VZV) are known to induce deposition of amyloid-β [4-6].
Several epidemiological studies from Taiwan, all using the same data resource, found significant associations between herpes virus infection, antiherpetic medication, and dementia. First, individuals diagnosed with herpes zoster ophthalmicus (caused by VZV) had an increased (hazard ratio [HR] = 3) rate of incident dementia within 5 years of diagnosis [7]; information on antiherpetic medication was not available. Second, patients with herpes zoster infection had a slightly increased risk of incident dementia (HR = 1.1) compared to controls [8], and the subgroup treated with antiherpetic medication had a reduced risk of dementia (HR = 0.6) compared to untreated HSV-positive patients. Third, HSV infection was reported to increase the risk of incident dementia (HR = 2.5) [9], and treatment with antiherpetic medication markedly reduced the risk (HR = 0.1 vs. untreated HSV-positive controls). Moreover, the association was greater in those treated for longer times. In addition, a study from South Korea reported that patients with VZV infection had an increased rate of incident dementia (HR = 1.1), but this was reduced (HR = 0.8 vs. untreated VZV) by antiherpetic medications [10]. These results were surprising, given the brevity of antiherpetic persistence in the human body.
Despite these reports, a recent meta-analysis into associations of human herpesvirus infections with dementia or mild cognitive impairment concluded that evidence to date for an association is insufficient [11].
We therefore conducted a multicenter observational cohort study using health registry data from Wales, Germany, Scotland, and Denmark to investigate potential associations between antiherpetic medication and incident dementia, and also to comprehensively investigate such associations broken down according to medication type and dose, type of herpes virus, and dementia subtype.
METHODS
The primary objective was to study the association between oral antiherpetic medication and incident dementia. Secondary objectives were to study whether that association was modified by (i) number of treatments, (ii) herpes subtype, (iii) type of antiherpetic medication, and (iv) dementia subtype.
Data sources
We utilized linked routinely collected health data (RCHD) from four different sources: (i) the Secure Anonymised Information Linkage Databank (SAIL), Wales (1995–2018); (ii) the IMS Disease Analyzer, Germany (1992–2017); (iii) the Danish National Registries (DNR; 2000–2015); and (iv) the Electronic Data Research and Innovation Service (eDRIS) of Public Health, Scotland (2004–2020).
SAIL holds anonymized health data from ~80% of Welsh general practitioner practices linked to national datasets including inpatient records, death records, and Welsh Index of Multiple Deprivation [12]. The IMS Disease Analyzer database holds unlinked and anonymized information on drug prescriptions, diagnoses, and basic medical and demographic data from ~3% of all outpatient medical practices in Germany [13]. DNR comprise linked RCHD from the entire Danish population; registries used in this study were the Danish Civil Registration System [14], the Danish National Patient Registry [15], and the Danish National Prescription Registry [16]. The eDRIS database holds countrywide linked anonymous RCHD on hospital admissions, prescriptions, mortality, and national health service registration data [17]. All four datasets are representative of the general population in the relevant country; however, individuals with poor quality/missing identifier data or reduced access to health care (e.g., migrants) are likely to be underrepresented.
Study populations
The study populations in SAIL, IMS Disease Analyzer, and eDRIS comprised all individuals with follow-up data from age 60 years onward who had no dementia-related record before their 65th birthday, were alive at their 65th birthday, and for whom no information on any of the covariates in the analysis was missing (Figure 1). The study population in DNR comprised all individuals born in or before 1950, alive and residing in Denmark, with no dementia-related RCHD before start of follow-up. In IMS Disease Analyzer, individuals were excluded for whom a dementia-related prescription but no dementia-related diagnosis could be found or who received antiherpetic medication during follow-up without any known herpes-related diagnosis. Start of follow-up in cohorts from SAIL, IMS Disease Analyzer, and eDRIS was the 65th birthday (midmonth in datasets where only month and year of birth were available, or midyear where only year of birth was available). In the DNR cohort, start of follow-up was the later of 1 January 2000 and 65th birthday. End of follow-up was at the date of the first dementia-related code or the date of deregistration, death, or the end of the study period. In the unlinked data from IMS Disease Analyzer, mortality data were not available; however, a death would have been recorded as deregistration (Table 1).
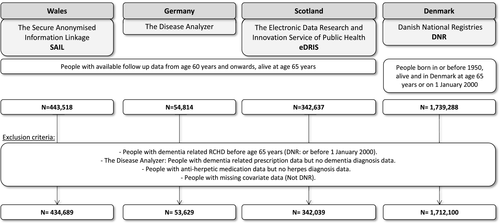
| SAIL | Disease Analyzer | DNR | eDRIS | |
|---|---|---|---|---|
| Total N (PY) | 434,689 (3,255,540) | 53,629 (550,852) | 1,712,100 (13,798,349) | 342,637 (961,666) |
| Follow-up in PY, median (IQR) | 6.7 (3.3–11.3) | 8.8 (4.5–14.5) | 7.4 (3.8–12.2) | 2.7 (1.4–4.2) |
| Deaths, n (%) | 73,802 (17%) | NAa | 600,610 (35%) | 14,994 (4%) |
| Year of birth, median (IQR) | 1945 (1940–1949) | 1941 (1937–1947) | 1936 (1926–1944) | 1952 (1950–1953) |
| Sex, n (%) | ||||
| Female | 220,539 (51%) | 29,031 (54%) | 925,393 (54%) | 171,022 (50%) |
| Herpes diagnosis, n (%) | ||||
| Total | 53,981(12%) | 19,650 (37%) | NA | NA |
| Herpes simplex | 7344 (2%b/14%c) | 7,737 (19%/39%) | NA | NA |
| Herpes zoster | 37,439 (9%/69%) | 13,643 (29%/69%) | NA | NA |
| Antiherpetic medication, n (%)d | ||||
| Total | 39,997 (9%/74%) | 10,352 (19%/53%) | 169,989 (8%) | 8625 (3%) |
| T+ | 33,395 (8%/83%) | 8603 (16%/83%) | 106,185 (6%/76%) | 7336 (2%/85%) |
| T++ | 3725 (1%/9%) | 1151 (2%/11%) | 22,476 (1%/13%) | 678 (0.2%/8%) |
| T+++ | 2877 (1%/7%) | 598 (1%/6%) | 20,664 (1%/12%) | 581 (0.2%/7%) |
| Acyclovir and valacyclovir | 35,940 (8%) | 6173 (12%) | 167,633 (8%) | 8453 (2%) |
| Famciclovir | 4720 (1.1%) | NA | 3096 (0.14%) | 172 (0.05%) |
| Brivudine | NA | 4140 (8%) | NA | NA |
| Dementiae | ||||
| Total, n (rate) | 15,640 (48) | 4244 (77) | 129,662 (94) | 1314 (14) |
| AD, n (rate) | 6607 (21) | 767 (18) | NA | NA |
| VD, n (rate) | 5020 (16) | 1024 (23) | NA | NA |
| Age at first diagnosis, median years (IQR) | 75 (71–79) | 74 (69–78) | 82 (77–87) | 67 (66–68) |
- Abbreviations: AD, Alzheimer disease; DNR, Danish National Registries; eDRIS, Electronic Data Research and Innovation Service; IQR, interquartile range; NA, not available; PY, person-years; SAIL, Secure Anonymised Information Linkage Databank; VD, vascular dementia.
- a In Disease Analyzer, death was recorded as end of follow-up, which included other reasons such as deregistration.
- b Percentage of the total study population.
- c Percentage of those diagnosed.
- d Number of treatments: T+, treated once; T++, treated twice; T+++, treated thrice or more.
- e Rate per 10,000 PY.
Dementia classification
In all datasets, we used coded information to classify individuals with dementia, using the date of the first code as date of diagnosis. Table S1 lists codes used for dementia classification. In SAIL, individuals were classified as having dementia if they had a dementia-related diagnostic code from primary care, hospital admission, or mortality records (underlying or contributing cause). In the unlinked data from IMS Disease Analyzer, individuals were classified as having dementia if a dementia-related diagnosis was documented from primary care data. In DNR and eDRIS, individuals were classified as having dementia if they had a dementia-related prescription or dementia-related hospitalization. In cohorts with available diagnostic codes from primary care (SAIL and IMS Disease Analyzer), diagnostic codes were also used to subtype dementia into AD, vascular dementia (VD), and "other/unknown." Individuals with codes for both subtypes were counted as both AD and VD ("mixed dementia" is not coded in the RCHD). The positive predictive value (PPV) of RCHD (diagnostic codes from primary care, hospitalization, and mortality records) to classify dementia and dementia subtypes has recently been studied in Scotland, and the data showed good PPV for classification of dementia and AD, but relatively low PPV for classifying VD [18]. Similarly, the PPV for classifying dementia using data from secondary care settings in DNR was 86% [19].
Antiherpetic drugs
In all datasets, exposure to antiherpetic medication was restricted to oral antiherpetic medication; topical applications can be purchased without prescription and are therefore underreported in RCHD. Exposure classification was also restricted to prescriptions from primary care, because those from hospital admissions were not available. However, in all our study populations, herpes-associated hospital admissions were rare events. In the DNR, of the 1,712,100 people in the cohort, there were 11,720 people who had any herpes-associated hospital admissions during the study period. Of these, only 4115 people had herpes-associated hospital admissions, and there were never any prescriptions. In all four countries, prescriptions from primary or secondary care are necessary for access to oral antiherpetic medication; however, medication can be prescribed over the internet, in which case exposure would not be recorded in our databases. Table S1 lists codes used for antiherpetic medication classification.
Herpes classification
In SAIL and IMS Disease Analyzer, codes from primary care diagnosis and hospital admissions were used to classify herpes infection and herpes subtypes (Herpes simplex and H. zoster), and the date of the first diagnostic code was taken as the date of diagnosis. Individuals with diagnostic codes for both subtypes were classified according to the diagnostic code entered at first diagnosis (diagnostic codes are given in Table S1). Although a large proportion of the general population is subclinically infected with herpes virus, people were classified to be infected in our study only if they had had a herpes-related diagnosis in primary or secondary care; the diagnosed population therefore represents only herpes-infected people with clinical signs severe enough to seek medical help.
Covariates
Linked RCHD were used to classify year of birth and sex. We used several proxies for socioeconomic status (SES). In SAIL and eDRIS, we adjusted for multiple deprivation; in IMS Disease Analyzer, we adjusted for type of insurance (private insurance being indicative of higher disposable income); and in DNR, we adjusted for highest education and civil status at start of follow-up. In DNR, we additionally adjusted for comorbidities using the Charlson Comorbidity Index (excluding dementia).
Type of herpes infection
To investigate in SAIL and IMS Disease Analyzer whether the association of antiherpetic medication with incident dementia was affected by herpes subtype, we conducted a subset analysis that included only individuals with either H. zoster infection or without any herpes diagnosis; individuals with a diagnosis of H. simplex were excluded. We then repeated the analysis including only individuals with H. simplex diagnosis and removed those with H. zoster infection. Coinfections were not considered. To study the effect of herpes subtype and associated treatment on dementia incidence in DNR and eDRIS, individuals were classified by the first prescribed drug (only aciclovir) into those with high drug strength (800 mg) and low drug strength (200 or 400 mg), which are indicative of H. zoster and H. simplex infection according to national prescribing guidelines in the two countries.
Type of dementia
To investigate in SAIL and IMS Disease Analyzer whether the association of antiherpetic medication with incident dementia was only seen in a particular type of dementia, we again explored a subset of the study population that included only individuals either with a diagnosis of AD or without any dementia, and excluded individuals with any other or "unknown" dementia. We then repeated the analysis with VD.
Type of antiherpetic medication and number of treatments
For medication-specific analysis, we included aciclovir and its prodrug, valaciclovir (combined), famciclovir (not IMS Disease Analyzer), and brivudine (only IMS Disease Analyzer). The original analysis included ganciclovir and valganciclovir; however, we excluded them because very few individuals in each cohort received these medications. In eDRIS, from the entire cohort of elderly individuals, only 105 received any antiherpetic medication other than aciclovir or valaciclovir; therefore, medication-specific analysis was not performed. To study the effect of type of antiherpetic medication in SAIL and IMS Disease Analyzer, we tested the effect of each drug by excluding individuals from the study population who were exclusively exposed to another antiherpetic medication. The number of prescriptions (one, two, or three or more) was analyzed independently of the time between prescriptions.
Statistical analysis
Data analysis employed multivariate survival analysis. In all cohorts, sequential prescriptions of antiherpetic medication were included as time-dependent variables, increasing from zero prescriptions (unexposed controls) to three or more prescriptions. For cohorts with information on primary care diagnosis (SAIL and IMS Disease Analyzer), we additionally included herpes diagnosis as a time-dependent variable. In DNR, we used Poisson regression to fit piecewise exponential survival models with the logarithm of person-years at risk as an offset variable (SAS software, version 9.4 of the SAS System for Windows) [20]. To control for effects of age and year in these models, incremental years were included as time-dependent variables. In all other cohorts, we used Cox proportional hazard models (R, package "survival" [21]). To control for the effect of calendar year, we adjusted for birth-year categories; the effects of age were accounted for by following up every individual in the cohort from their 65th birthday. For cohorts with information from primary care, we included the practice number as random effect to control for correlation between patients of the same practice (R package "coxme" [22]).
Ethics and governance
Analysis of data from SAIL and eDRIS was granted under information governance review panel (IGRP) 0938 and NHS scotland public benefit and privacy panel (PBPP) 1819-0297, respectively. Analysis of data from IMS Disease Analyzer and DNR did not require specific governance approval.
RESULTS
Study populations and drug prescription
There was considerable heterogeneity in the composition of the four study populations (Table 1), notably in mean follow-up time, ranging from 2.7 years (eDRIS) to 9 years (IMS Disease Analyzer). The longer follow-up time was associated with higher proportions of individuals being recorded as receiving antiherpetic medication and diagnoses of herpes virus infection and dementia. Similarly, including individuals with a start date later than their 65th birthday in DNR increased the average age of the DNR cohort and increased the rate of dementia diagnosis and death. The proportion diagnosed with herpes viral infection was higher in IMS Disease Analyzer than in SAIL, and H. zoster was diagnosed more often than H. simplex in both cohorts. In all four cohorts, aciclovir and valaciclovir were the most commonly prescribed antiherpetics; however, in IMS Disease Analyzer, many prescriptions were for brivudine, a drug that was not prescribed in any of the other cohorts. In all four cohorts, antiherpetic medication was most commonly prescribed only once during follow-up, and at most 1% of all individuals in the cohorts were exposed to three or more doses of any antiherpetic medication. In both cohorts where dementia could be subtyped (SAIL and IMS Disease Analyzer), the number of individuals with dementia coded as AD was similar to the number coded as VD. In all cohorts, women were more likely to be exposed to antiherpetic medication than men (Table S2). Figure S1 in the supplement presents a comparison of the different cohorts with the study populations of Tzeng et al. [9] and Bae et al. [22].
Dementia incidence
Exposure to one or more doses of antiherpetic medication was significantly associated with a slight decrease in dementia incidence in DNR (adjusted hazard ratios [HRs] ranged from HR = 0.89, 95% confidence interval [CI] = 0.83–0.95 to HR = 0.93, 95% CI = 0.88–0.98) and in SAIL for a single exposure to antiherpetic medication (adjusted HR = 0.91, 95% CI = 0.86–0.97). It was not significantly associated in the other cohorts (Figure 2a; Tables S3–S6). Notably, in DNR (the largest cohort included in our study), all levels of exposure were associated with a small but significantly reduced incidence of dementia. Increased treatment numbers were not associated with differences in effects of exposure in any of the cohorts. Adjustment for covariates in the different cohorts did not have a large effect on any of the estimated HRs (Tables S3–S6). In IMS Disease Analyzer, individuals diagnosed with herpes virus infection but not exposed to any antiherpetic medication had an increased incidence of dementia compared to those not diagnosed (HR = 1.18, 95% CI = 1.09–1.28; Figure 2a; Table S4). This increased rate was not seen in the other cohort that had information on diagnosis (SAIL), where individuals diagnosed but not treated had a nonsignificant lower incidence (HR = 0.95, 95% CI = 0.88–1.02; Figure 2a; Table S3).
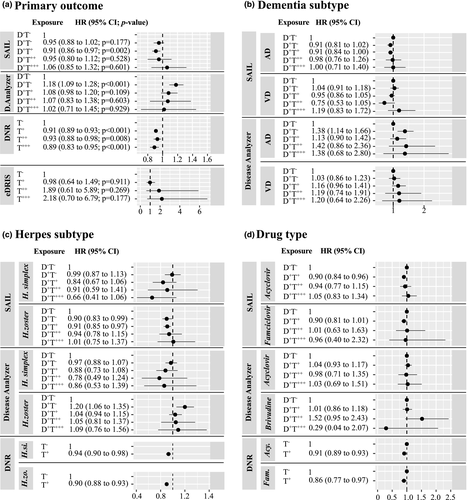
In all cohorts, effects of exposure to antiherpetic medications were independent of medication type (Figure 2d). In IMS Disease Analyzer, individuals diagnosed with H. zoster and not exposed to any antiherpetic medication had a higher rate of dementia (HR = 1.2, 95% CI = 1.06–1.35) compared to those undiagnosed, whereas dementia incidence in those diagnosed with H. simplex and not exposed was unchanged (HR = 0.97, 95% CI = 0.88–1.07; Figure 2c). This trend toward higher HRs in individuals diagnosed with H. zoster and lower HRs in those diagnosed with H. simplex could also be observed in people exposed to antiherpetic medication; however, these differences were not significant. In SAIL and DNR, herpes subtype had no effect on the association between exposure to antiherpetic medication and dementia. Finally, in both SAIL and IMS Disease Analyzer, the association between exposure to antiherpetic medication and dementia incidence was similar across dementia subtypes (Figure 2b; Tables S4 and S5).
DISCUSSION
We analyzed associations between antiherpetic medication and dementia incidence in four large national observational cohorts. We report evidence for a low negative association between exposure and outcome; however, results were heterogeneous.
The negative association could be related to genuine protective effects of antiherpetic medication against neuropathologic consequences of herpes infection. In IMS Disease Analyzer, individuals diagnosed with herpes infection but not treated were at higher risk of dementia compared to both those undiagnosed and those diagnosed with H. zoster and treated. This result resembles observations from Taiwan and South Korea [7, 9, 10]. However, there is evidence against this interpretation. First, the effect could not be seen in SAIL, the only other cohort with available diagnostic information. Second, the associations between antiherpetic medication and dementia in SAIL and IMS Disease Analyzer were for both AD and VD, diseases that have different neuropathological manifestations. Finally, the association was independent of the herpes subtype (H. simplex vs. H. zoster), whereas possible associations of herpes infection with dementia have mostly been described for H. simplex and less for H. zoster [11].
Given that in cohorts other than IMS Disease Analyzer we did not see a dose–response effect, a direct neuroprotective effect of the antiherpetic medication seems unlikely. However, the great majority of patients in all four cohorts received single prescriptions of antiherpetic medication (typically for administration over a period of 1–2 weeks), and we cannot exclude the possibility that longer-term exposure might have greater effects.
The small negative association could also be related to indirect effects of exposure to antiherpetic medication. Given the nature of the observational cohorts, we could not study whether associations were affected by APOE genotype. Because a specific variant at the APOE locus, ε4 (APOE4), is associated with susceptibility to both dementia and herpes virus infection, a positive association between dementia, herpes infection, and antiherpetic medication would be expected. Of note, the Danish population in general has a slightly higher proportion of APOE4 carriers than the other populations [23], which might have contributed to the observed heterogeneity.
Finally, the observed negative association could reflect residual confounding, reverse causation, and misclassification. There is clear evidence in all our study populations of a negative association between SES and dementia incidence. SES could also be correlated with access to antiherpetic medication and medical care [24]; however, adjusting for SES in the statistical models did not change the estimated HR (Tables S3–S6). Similarly, controlling for the effects of comorbidities in DNR did not modify the significant negative association between exposure and dementia incidence (Table S5). Control for comorbidities is problematic, because a considerable number of comorbidities of dementia are early signs of dementia; by "controlling" for the effect of these, we risk losing the true effect of antiherpetic medication. Additionally, the effects of comorbidities are time-dependent; those increasing the risk to be diagnosed with herpes (e.g., with shingles) infection but not associated independently with dementia should not be considered as confounders. Vaccination against VZV (shingles vaccine) has been reported to be associated with reduced dementia incidence [25]. However, the major effect of VZV vaccination would be to reduce herpes diagnosis and thereby exposure to antiherpetic medication; therefore, vaccination would not act as a confounder in our study. If preclinical dementia were to be associated with an increased risk of herpes diagnosis and antiherpetic medication, we would have falsely attributed the dementia diagnosis to the antiherpetic medication (reverse causation). This would mean that the true negative association was slightly larger than the observed HR. However, only less than 5% of all first antiherpetic medications were prescribed less than 6 months before dementia diagnosis. Finally, individuals in all cohorts might have been misclassified as unmedicated if they had received antiherpetic medication purchased outside the national public health system. However, our study population was restricted to the elderly population (≥65 years old), and we feel that exposure misclassification is less likely to have been significant.
Some of the heterogeneity could be explained by differences in study design and study populations. In DNR and eDRIS, the nonexposed cohorts included diagnosed but untreated individuals; in the other cohorts, these were included as a separate group. Including a mixture of diagnosed and untreated individuals and those undiagnosed and untreated could mask a true negative association if the association between herpes infection and incident dementia holds true; the risk of dementia would be unequal in the exposed and unexposed groups in these cohorts (DNR and eDRIS) in our study. By contrast, we have no explanation for the (nonsignificant) trend toward increased dementia risk in individuals exposed to antiherpetic medication in IMS Disease Analyzer. Interestingly, the proportion of individuals diagnosed with herpes and the proportion who were treated were much higher in IMS Disease Analyzer than in SAIL, even though individuals in the two cohorts were followed up for similar periods. Although brivudine was only used in IMS Disease Analyzer, the effects of brivudine exposure were similar to those of aciclovir/valaciclovir, and it seems unlikely that the type of antiherpetic medication might explain the heterogeneity. Heterogeneity in study design and populations could also explain the differences between our results and those from studies from Taiwan and South Korea. Length of antiherpetic treatment was significantly longer in the study from Tzeng et al. [9]. However, that study also found an approximately 40% reduction in subsequent dementia development for subjects receiving medications for less than 30 days, which is not in line with our results. In addition, although we did not have direct information on the length of treatment, we did analyze the effect of the number of treatments (one, two, and three or more) and found no trend in greater effect in those exposed to higher number of treatments. Number of treatments and treatment duration are not directly comparable; however, patients with repeated prescriptions tend to be those with longer treatment durations. Because Tzeng et al. [9] classified patients HSV-positive only if they have had three or more outpatient visits within 1 calendar year, patients classified as herpes-positive in our study might have been different in regard to severity of symptoms.
Studies on dementia etiology require large study populations that are followed up over long periods of later life. Our study benefitted from the high quality of RCHD in the four nations containing reliable information on exposure, outcome, and covariates for a total of >2.5 million individuals aged 65 years or more. The size of the study populations allowed us to control for the single most important risk factor for dementia, age, by using a common age at the start of follow-up (IMS Disease Analyzer, SAIL, and eDRIS) or by using age as a time-dependent variable (DNR); in addition, we were able to include both repeated exposure to antiherpetic medication and herpes diagnosis as time-dependent covariates. However, our study has some limitations. Disease and exposure classification were based on imperfect information, especially regarding the validity of dementia subtypes, and the classification of herpes subtypes using drug strength as a proxy (in DNR). However, we would not expect misclassification to be differential. More importantly, because only information on the dates of dementia diagnosis and/or first dementia drug exposure was available, we could not ascertain whether exposure preceded or followed dementia development. In the case of AD, the pathology is likely to be present more than 10 years before symptom onset, so follow-up time in our cohorts would have been insufficient. We could have excluded individuals with exposures to antiherpetic medication for some period prior to dementia diagnosis; nevertheless, any time period would have been arbitrary and follow-up times in individuals without dementia impossible to calculate.
In conclusion, results from four large national cohorts indicate that short-term antiherpetic medication is not markedly associated with reduced subsequent dementia incidence. Because neither type of dementia nor type of herpes infection modified the association, the small but significant decrease in dementia incidence with antiherpetic administration may reflect unmeasured confounding and misclassification.
CONFLICT OF INTEREST
The authors declare no financial or other conflicts of interest.
AUTHOR CONTRIBUTIONS
Christian Schnier: Conceptualization (equal), data curation (lead), formal analysis (lead), methodology (lead), writing–original draft (lead), writing–review and editing (equal). Janet Janbek: Conceptualization (equal), data curation (equal), formal analysis (equal), methodology (equal), writing–original draft (equal), writing–review and editing (equal). Linda Williams: Conceptualization (equal), data curation (equal), formal analysis (equal), writing–review and editing (equal). Tim Wilkinson: Conceptualization (equal), writing–review and editing (equal). Thomas M. Laursen: Data curation (equal), formal analysis (equal), methodology (equal), writing–review and editing (equal). Gunhild Waldemar: Conceptualization (equal), project administration (equal), supervision (supporting), writing–review and editing (equal). Hartmut Richter: Conceptualization (equal), data curation (equal), formal analysis (equal), writing–review and editing (equal). Karel Kostev: Data curation (supporting), resources (supporting), writing–review and editing (supporting). Richard Lathe: Conceptualization (equal), funding acquisition (equal), methodology (equal), writing–review and editing (equal). Jürgen Haas: Conceptualization (equal), funding acquisition (equal), supervision (equal), writing–review and editing (equal).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from SAIL, eDRIS, IQVIA, and DNR. Restrictions apply to the availability of these data under (national) data protection legislation.




