Delirium REduction after administration of melatonin in acute ischemic stroke (DREAMS): A propensity score–matched analysis
Funding information
This study was supported by Tübinger Forschungsförderung (A.Mengel) (2459-0-0). The authors are thankful for the support of the Open Access Publishing Fund of the University of Tuebingen.
Abstract
Background and purpose
Poststroke delirium (PSD) comprises a common and severe complication after stroke. However, treatment options for PSD remain insufficient. We investigated whether prophylactic melatonin supplementation may be associated with reduced risk for PSD.
Methods
Consecutive patients admitted to the Tübingen University Stroke Unit, Tübingen, Germany, with acute ischemic stroke (AIS), who underwent standard care between August 2017 and December 2017, and patients who additionally received prophylactic melatonin (2 mg per day at night) within 24 h of symptom onset between August 2018 and December 2018 were included. Primary outcomes were (i) PSD prevalence in AIS patients and (ii) PSD risk and PSD-free survival in patients with cerebral infarction who underwent melatonin supplementation compared to propensity score–matched (PSM) controls. Secondary outcomes included time of PSD onset and PSD duration.
Results
Out of 465 (81.2%) patients with cerebral infarction and 108 (18.8%) transient ischemic attack (TIA) patients, 152 (26.5%) developed PSD (median time to onset [IQR]: 16 [8–32] h; duration 24 [8–40] h). Higher age, cerebral infarction rather than TIA, and higher National Institutes of Health Stroke Scale score and aphasia on admission were significant predictors of PSD. After PSM (164 melatonin-treated patients with cerebral infarction versus 164 matched controls), 42 (25.6%) melatonin-treated patients developed PSD versus 60 (36.6%) controls (odds ratio, 0.597; 95% confidence interval, 0.372–0.958; p = 0.032). PSD-free survival differed significantly between groups (p = 0.027), favoring melatonin-treated patients. In patients with PSD, no between-group differences in the time of PSD onset and PSD duration were noted.
Conclusions
Patients prophylactically treated with melatonin within 24 h of AIS onset had lower risk for PSD than patients undergoing standard care. Prospective randomized trials are warranted to corroborate these findings.
INTRODUCTION
Delirium represents a state of acute brain dysfunction that develops over a short period of time and is characterized by fluctuating disturbances of attention, awareness, and cognition [1 It frequently affects patients treated in intensive care units (ICUs), with a prevalence of ~20% in nonintubated [2 up to ~83% in mechanically ventilated patients [3. Poststroke delirium (PSD) has been reported to affect 25% of acute stroke patients [4, whereas PSD is clearly associated with lower functional outcome, longer hospital stay, and higher in-hospital mortality and mortality at 12 months [5, 6.
Currently, the neurobiological mechanisms underlying delirium remain poorly understood. A multitude of predisposing (e.g., higher age, cognitive impairment, and dementia) and precipitating factors (e.g., infections, medication, and hydroelectrolytic disorders) indicate a multifactorial origin [7. In the intensive care unit (ICU), disturbances of sleep and circadian rhythm are proven significant risk factors for delirium [8. Also, mechanisms pertinent to acute brain injury, hypoperfusion, and neurotransmitter imbalances have been implicated in the pathogenesis of PSD [7. To date, no pharmacological intervention has been proven effective in delirium prevention [9.
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone secreted by the pineal gland, which holds multifaceted roles in the regulation of the sleep–wake cycle [10. Recent studies suggest that melatonin provides a link between delirium and the disruption of sleep–wake regulation [11, with significant associations found between melatonin levels and delirium occurrence (i.e., with urinary melatonin metabolite levels being normal in patients without delirium, low in hyperactive delirium, and elevated in hypoactive delirium) [12. Neuroprotective effects of melatonin have been additionally demonstrated in acute ischemic stroke (AIS), as melatonin has been shown to regulate the postischemic cerebral blood flow and diminish neuroinflammation, excitotoxicity, and ischemia-reperfusion injury [13-15.
The first evidence of the beneficial effects of exogenous supplementation with melatonin or the melatonin receptor-agonist ramelteon in delirium prevention has recently been provided by small randomized controlled trials (RCTs) in elderly patients [16 and patients treated in the general ICU [17. Similarly, beneficial effects of melatonin in reducing early postoperative cognitive decline were shown in a recent RCT in surgical patients [18, although one previous RCT in older surgical patients (mean age 84 years) had shown no efficacy of melatonin in preventing postoperative delirium [19. To the best of our knowledge, the only published study concerning PSD treatment is a noncontrolled case-series study of five elderly patients with AIS, who received ramelteon for established delirium, with significant clinical improvement reported in all patients [20.
The present study aims to investigate possible associations between exogenous melatonin substitution and PSD occurrence. We hypothesized that prophylactic melatonin supplementation within the first 24 h after AIS onset would be associated with a reduced risk for PSD.
METHODS
Study design and regulations
We conducted a propensity score–matched (PSM) analysis including patients with AIS who were prophylactically treated with melatonin for PSD prevention versus nontreated AIS controls. The study was approved by the institutional ethics committee, protocol number 752/2018BO2. Individual informed consent was waived for this study, as use of routine treatment data for research purposes is covered by a clinic-wide consent.
Patient population
We identified adult patients with AIS, who were consecutively admitted to the stroke unit (SU) of the University Hospital of Tübingen within two periods (see below). AIS was defined in line with the International Classification of Diseases, Tenth Revision (ICD-10) criteria to encompass the diagnoses of cerebral infarction (ICD-10 I63) and transient ischemic attack (TIA) (ICD-10 G45.9).
All patients acutely admitted to the SU between August 1, 2018 and December 31, 2018 were treated with melatonin within the first 24 h of AIS onset (single dose of 2 mg/day at 8 p.m.) [21, according to our standard operating procedure (SOP) for PSD prevention, and were eligible for inclusion in the study. The SOP for PSD prevention was put into effect on August 1, 2018, due to the high prevalence of PSD among AIS patients and the increasing SU staff awareness of the role of early strategies for PSD prevention, complementing previous SOPs by adding prophylactic melatonin supplementation (single dose of 2 mg/day at 8 p.m.) within the first 24 h of AIS onset and until discharge from the SU in patients aged ≥55 years. Previous versions of this SOP had already established structured early-PSD screening, using the Richmond Agitation-Sedation Scale (RASS) and Intensive Care Delirium Screening Checklist (ICDSC) on an 8-h basis during SU stay, along with nonpharmacological prevention strategies [22. The reasons for implementing the use of melatonin in the SOP for PSD prevention were (i) no alternative treatments for PSD prevention currently available; (ii) level I evidence from RCTs in favor of high efficacy of melatonin in delirium prevention in the elderly [16 and general ICU patients [17; (iii) level I evidence from RCTs in support of an excellent tolerability and safety profile of melatonin [16, 17. Because the study SU is an intermediate care unit, none of the patients was on mechanical ventilation, in shock, or had severe liver or renal insufficiency. Melatonin was administered orally, unless patients had severe dysphagia, in which case medication was administered through a nasogastric tube. Exclusion criteria for the present study comprised (i) duration of SU stay <24 h; (ii) treatment with antipsychotics, α2-receptor agonists, or benzodiazepines on admission; (iii) RASS level of −5 or −4 for the majority (>50%) of the stay; (iv) missing records of National Institutes of Health Stroke Scale (NIHSS), RASS, or the ICDSC scores for >50% of the SU stay.
To define the control cohort, an automated electronic database search was performed to identify adult patients with AIS who were consecutively admitted to the SU and underwent standard care between August 1, 2017 and December 31, 2017 (i.e., prior to the implementation of the latest SOP for PSD prevention) and selecting the same seasonal period as in the melatonin cohort to account for possible seasonal variation in the occurrence of delirium [23. Exclusion criteria were the same as for the melatonin cohort, but in addition, included documented melatonin treatment.
Data collection and analyses
Baseline characteristics and in-hospital clinical parameters were recorded for all patients and automatically retrieved from the clinical information system (Intellispace Critical Care and Anesthesia information system; Philips Healthcare). Diagnosis of cerebral infarction was based on clinical symptoms and neuroimaging findings from computed tomography (CT) and/or magnetic resonance imaging (MRI) studies. All patients with TIA underwent brain CT or MRI imaging on admission. However, as follow-up MRI to detect delayed neuroimaging abnormalities is not systematically performed at the study SU [24, TIA diagnosis was based on clinical diagnostic criteria [25, including symptom duration <24 h. To mitigate the well-known biases and limitations related to clinical TIA diagnosis [24, 25, patients with TIA were only included in the analysis of prevalence, risk factors, and clinical characteristics of PSD, but were excluded from further analyses of PSD in association with melatonin treatment. Delirium assessment for the control and melatonin cohort was based on the ICDSC, which was administered on admission and every 8 h (morning, afternoon, and night shifts) during the whole duration of the stay in the SU. In accordance with published literature, an ICDSC score of ≥4 points for nonaphasic [26 and 5 points for aphasic patients [27 was considered indicative of delirium. PSD onset was defined based on the first ICDSC score ≥4 or 5 points for nonaphasic and aphasic patients, respectively. The ICDSC score was assessed by neurocritical care nurses trained in its use as part of routine clinical assessment. The agreement of the ICDSC ratings with the Diagnostic and Statistical Manual of the American Psychiatric Association (Fifth Edition) (DSM-5) diagnostic criteria in stroke patients was validated in a previous study [27. For all patients, PSD diagnosis was confirmed in accordance with the DSM-5 criteria by an independent neurologist who was blinded for the ICDSC scores. Motor subtypes of delirium were defined using RASS scores as previously described [28. RASS and NIHSS assessments were performed on admission and then every 6 h for the whole duration of SU stay by stroke neurologists who were blinded for test results of the ICDSC.
Primary and secondary endpoints
Primary endpoints of the study were (i) prevalence, risk factors. and characteristics of PSD (defined as ISDSC ≥4 for nonaphasic and ≥5 for aphasic patients) among AIS patients and (ii) risk for PSD and PSD-free survival among patients with cerebral infarction treated with melatonin compared to matched controls who underwent standard care. Secondary endpoints comprised time of PSD onset and PSD duration, evaluated in the subgroup of patients with cerebral infarction who developed PSD. Also, safety outcomes potentially associated with melatonin treatment were assessed by review of clinical records.
Statistical analyses
Group differences between baseline demographics and clinical characteristics were assessed using χ2 tests or two-tailed independent-sample Mann-Whitney U tests depending on data characteristics (i.e., categorical vs. continuous), respectively. In the first part of the analyses, all observations were included. Univariate binary logistic regression models were used to examine clinical factors as predictors for the development of PSD among AIS patients.
In the second part of the analyses, among patients with cerebral infarction, baseline differences in clinical covariates between patients treated with melatonin and controls were balanced using a PSM analysis [29. Propensity scores were calculated via a logistic regression analysis for baseline differences. Treated patients and controls were matched 1:1 based on their propensity scores using nearest-neighbor matching, with a matching tolerance of 0.01%. Standardized differences were calculated to compare patient features before and after PSM, with imbalance being defined as an absolute value greater than 0.10 (small effect size) [29. After PSM, delirium-free survival curves for the melatonin and control cohorts were generated using the Kaplan-Meier method, and between-group differences were assessed using a log-rank test.
In the third part of the analyses, differences between group characteristics (i.e., melatonin vs. control cohort) were examined in the subgroup of patients with cerebral infarction who developed delirium. With respect to secondary endpoints, linear regression analyses were performed using melatonin treatment as predictor after logarithmic transformation of the dependent variables (i.e., time of PSD onset and duration) due to right-skewed distribution of the standardized residuals. The significance level for all procedures was determined as p < 0.05. All statistical analyses were computed with IBM SPSS Statistics version 23 (IBM). Results are reported in accordance with the TREND (Transparent Reporting of Evaluations with Nonrandomized Designs) guidelines [30.
RESULTS
Prevalence, risk factors, and clinical characteristics of PSD
The database interrogation yielded a total of 573 patients with AIS (465 [81.2%] with cerebral infarction vs. 108 [18.8%] with TIA), of whom 152 (26.5%) developed PSD. Patients’ baseline characteristics are presented in Table 1. Motor subtypes of PSD were hypoactive in 33 (21.7%), hyperactive in 16 (10.5%), and mixed in 103 (67.8%) patients. Out of the 152 patients, 112 (73.7%) developed PSD within the first 24 h of admission, whereas the vast majority of patients (96.7%, corresponding to 147 patients) developed PSD within the first 72 h (Figure 1). Median of PSD onset was 16 h (interquartile range [IQR], 8–32), and its median duration was 24 h (IQR, 8–40).
| Patient characteristics | All patients, n = 573 | Patients with PSD, n = 152 | Patients without PSD, n = 421 | P value |
|---|---|---|---|---|
| Age, years, mean (SD) | 74 (14) | 80 (10) | 72 (15) | <0.001a,* |
| Sex | ||||
| Female, n (%) | 264 (46.1) | 68 (44.7) | 196 (46.6) | 0.70b |
| Type of AIS | ||||
| Cerebral infarction, n (%) | 465 (81.2) | 137 (90.1) | 328 (77.9) | 0.001b,* |
| Baseline parameters | ||||
| NIHSS o/a, median (IQR) | 3 (1–8) | 6 (3–13) | 2 (0–6) | <0.001a,* |
| Aphasia o/a, n (%) | 154 (26.9) | 64 (42.1) | 90 (21.4) | <0.001b,* |
- Abbreviations: AIS, acute ischemic stroke; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; o/a, on admission; PSD, poststroke delirium; SD, standard deviation.
- a Mann-Whitney U test.
- b χ2 test.
- * Denotes significance at p < 0.05.
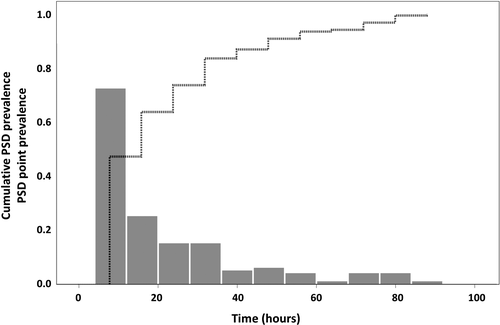
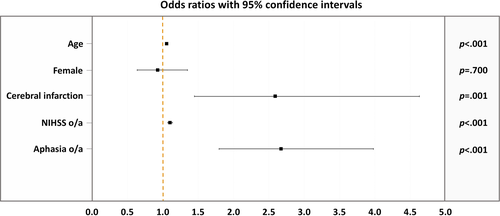
Univariate logistic regression analyses showed that higher age was related to an increased risk for PSD (odds ratio [OR], 1.055; 95% confidence interval [CI], 1.036–1.074; p < 0.001). Also, cerebral infarction (OR, 2.590; 95% CI, 1.449–4.628; p < 0.001), higher NIHSS on admission (OR, 1.102; 95% CI, 1.069–1.136; p < 0.001), and presence of aphasia on admission (OR, 2.675; 95% CI, 1.798–3.980; p < 0.001) were associated with an increased risk for PSD. Contrarily, sex was not associated with the risk for developing PSD (OR, 0.929; 95% CI, 0.640–1.349; p = 0.70) (Figure 2).
Safety outcomes potentially associated with melatonin treatment in PSD
Out of the 573 AIS patients, 300 (52.4%) underwent melatonin treatment versus 273 (47.6%) controls. No patient discontinued melatonin during the study period. Rates of antibiotic use as a surrogate marker for infections were similar between treated patients (13%) compared to controls (17.9%, p = 0.101). There were no records of anaphylactic reactions in patients undergoing melatonin supplementation.
Association of prophylactic melatonin treatment and PSD
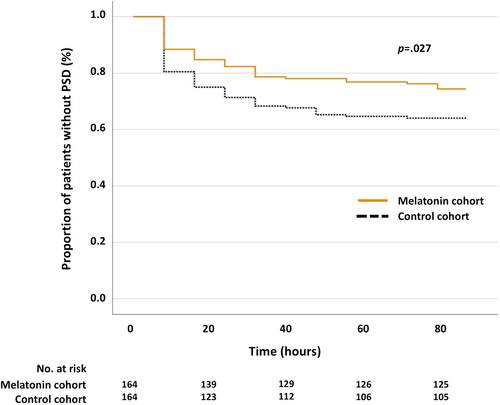
Baseline characteristics of patients with cerebral infarction are presented in Table 2. By matching the two groups for NIHSS on admission and aphasia 1:1, 328 patients were included in the analysis; 164 (50%) patients were treated with melatonin versus 164 (50%) controls. After PSM, no between-group differences in demographical and clinical characteristics were noted (Table 2). Forty-two (25.6%) patients in the melatonin cohort developed PSD versus 60 (36.6%) patients in the control cohort (OR, 0.597; 95% CI, 0.372–0.958; p = 0.032) (Table 3). In all treated patients, melatonin administration preceded PSD onset. The PSD-free survival distributions were plotted for both cohorts and are illustrated in Figure 3. The log-rank test revealed significant differences between the two cohorts (χ2[1] = 4.915, p = 0.027).
| Patient characteristics | Unmatched comparisons | Matched comparisons | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All, n = 465 | Control cohort, n = 235 | Treated cohort, n = 230 | p value | SMDa | All, n = 328 | Control cohort, n = 164 | Treated cohort, n = 164 | p value | SMD | |
| Age, years, mean (SD) | 74 (14) | 74 (15) | 73 (14) | 0.175b | 0.07 | 74 (14) | 73 (15) | 74 (13) | 0.871b | 0.068 |
| Sex | ||||||||||
| Female, n (%) | 207 (44.5) | 115 (48.9) | 92 (40.0) | 0.053c | 0.09 | 142 (43.3) | 74 (45.1) | 68 (41.5) | 0.504c | 0.037 |
| Baseline parameters | ||||||||||
| NIHSS o/a, median (IQR) | 4 (1, 10) | 6 (2, 12) | 3 (1, 6) | <0.001b,* | 0.45 | 4 (1, 9) | 4 (1, 9) | 4 (1, 9) | 1.0b | 0 |
| Aphasia o/a, n (%) | 148 (31.8) | 93 (39.6) | 55 (23.9) | <0.001c,* | 0.17 | 90 (27.4) | 45 (27.4) | 45 (27.4) | 1.0c | 0 |
Note
- Patients who received melatonin are denoted as treated cohort versus untreated patients as the control cohort.
- Abbreviations: IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; o/a, on admission; SD, standard deviation; SMD, standardized mean difference.
- a SMD refers to Cohen's d and Cramer's V for continuous and categorical variables, respectively.
- b Mann-Whitney U tests.
- c χ2 tests.
- * Denotes significance at p < 0.05.
| Patient characteristics | All, n = 102 | Control cohort, n = 60 | Treated cohort, n = 42 | p value |
|---|---|---|---|---|
| Age, years, mean (SD) | 79 (11) | 79 (11) | 80 (11) | 0.563a |
| Sex | ||||
| Female, n (%) | 38 (37.3) | 22 (36.7) | 16 (38.1) | 0.883b |
| Baseline parameters | ||||
| NIHSS o/a, median (IQR) | 6 (3, 12) | 6 (3, 11) | 7 (4, 13) | 0.152a |
| Aphasia o/a, n (%) | 43 (42.2) | 24 (40) | 19 (45.2) | 0.598b |
| Applied treatments after PSD diagnosis | ||||
| High-potency antipsychotics, n (%) | 15 (14.7) | 7 (11.7) | 8 (19.0) | 0.201b |
| Low-potency antipsychotics, n (%) | 9 (8.8) | 5 (8.3) | 4 (9.5) | 0.154b |
| Benzodiazepines, n (%) | 35 (34.3) | 24 (40) | 11 (26.2) | 0.832b |
| α2-agonists, n (%) | 3 (2.9) | 1 (1.7) | 2 (4.8) | 0.363b |
| Antibiotics, n (%) | 28 (27.5) | 16 (26.7) | 12 (28.6) | 0.148b |
Note
- Patients who received melatonin are denoted as treated cohort versus untreated patients as control cohort.
- Abbreviations: IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; o/a, on admission; PSD, poststroke delirium; SD, standard deviation.
- a Mann-Whitney U tests.
- b χ2 tests.
No associations between melatonin treatment and PSD onset or PSD duration
Regression analyses were performed to determine whether treatment with melatonin could be associated with delayed PSD onset or reduced duration. The analyses revealed that both models were not significant (time of PSD onset F[1,100] = 1.177, p = 0.28, and PSD duration F[1,100] = 0.002, p = 0.97). Median time of PSD onset was 16 h (IQR, 8–32) for the melatonin cohort versus 8 h (IQR, 8–24) for the controls, whereas median PSD duration was 24 h (IQR, 14–40) for the melatonin cohort versus 20 h (IQR, 8–40) for the controls. Concerning PSD treatments, no significant between-group differences existed with respect to high-potency (haloperidol, risperidone) and low-potency (melperone, quetiapine, promethazine) antipsychotics, benzodiazepines, and α2-receptor agonists (Table 3). Also, rates of antibiotic use were similar between the treatment and control cohorts.
DISCUSSION
In the present study, we investigated the potential role of low-dose, prophylactic melatonin treatment in PSD. The main finding was that nightly supplementation with melatonin (single dose of 2 mg/day at 8 p.m.) was associated with a reduced risk for PSD as the primary endpoint, as indicated by the evidence of significant differences in PSD prevalence and PSD-free survival between patients with cerebral infarction treated with exogenous melatonin compared to matched controls.
Our study provides preliminary evidence that a pharmacological intervention might confer reduced susceptibility to PSD. Although causality cannot be safely inferred from the present findings, the evidence of reduced PSD risk in melatonin-treated patients compared to patients undergoing standard care implies a therapeutic potential of melatonin for PSD prevention. The associations noted in our study are also in line with even more impressive efficacy results of published RCTs that report odds ratios for delirium of 0.19 in elderly non-ICU patients [16, and 0.09 in general ICU patients [18, who were prophylactically treated with melatonin or ramelteon, respectively. Evidence of the neuroprotective effects of melatonin in stroke [13-15 could additionally support the therapeutic potential of melatonin in AIS. Thus, further research is warranted to provide insight on the full spectrum of neurobiological melatonin functions and melatonin treatment effects in PSD.
Regarding the clinical features of PSD and in line with published literature [6, 31, our analyses revealed that higher age, cerebral infarction (compared to TIA), higher NIHSS score on admission, and presence of aphasia on admission were significant predictors of PSD development. The observed PSD prevalence of 26.5% among AIS patients, the PSD duration with a median of 24 h (IQR, 8–40), and the findings of predominantly mixed (67.8%) and hypoactive (21.7%) PSD motor subtypes are in accordance with previous research [31-34. However, contrary to previous PSD studies, our results indicate that PSD occurred in 73.3% of patients within the first 24 h of admission, whereas the vast majority (96.7%) of PSD cases were noted within the first 72 h after AIS. The earlier onset of PSD in our study, as opposed to previously reported onsets within 4 to 7 days after AIS [6, 35, may be explained by the study design, which entailed frequent PSD screening (i.e., every 8 h) to capture early PSD symptoms after AIS [27. In addition to the importance of early PSD screening, these findings also suggest that early interventions after AIS could be efficient in PSD prevention.
Our results revealed no associations between prophylactic melatonin treatment and PSD onset as secondary outcome. The left-skewed distribution of PSD onset (Figure 1) implies a very short time window for therapeutic melatonin action, and might further argue for a therapeutic potential of melatonin supplementation as preventive rather than ad hoc treatment for PSD. Nonetheless, because the exact timing of melatonin administration was not documented for included patients, prospective randomized trials should corroborate our findings. We also found no evidence of association between melatonin treatment and PSD duration. Our findings are consistent with a line of evidence from delirium studies that points toward a superior efficacy of preventive over treatment strategies [16, 17, 36, 37.
With respect to safety considerations, melatonin supplementation was well-tolerated, and no patient discontinued melatonin during the study period. However, due to limitations associated with the study design, including the lack of structured adverse event monitoring and potential reporting biases, and constraints in disentangling side effects of melatonin treatment, including daytime sleepiness, headache, dizziness or other sleep-related adverse events [38, from AIS and PSD symptoms, safety outcomes should be rigorously evaluated in prospective RCTs. Nonetheless, the present findings are in keeping with previous research that supports the particularly safe pharmacological profile of melatonin, even in the elderly population and patients with multiple comorbidities [16, 17, 39, 40. Both the safety evidence, compared to side-effect profiles of other pharmacotherapies assessed for delirium prevention including antipsychotics [41, and the low cost of melatonin make it a particularly appealing drug for PSD prevention [16. These considerations are also highly relevant today, as the mounting evidence of a significant association between delirium and the spreading coronavirus disease (COVID-19) renders the need for delirium prevention strategies even more pressing [42, 43.
Limitations
We acknowledge some limitations of the present study. First, baseline differences, including the higher NIHSS scores on admission, along with the higher rates of aphasia noted in the control compared to the melatonin cohort, may have confounded our results. Baseline differences were accounted for by matching the groups prior to any comparisons for baseline confounder variables associated with AIS. However, given the multitude of predisposing (e.g., higher age, cognitive impairment, and dementia) and precipitating factors (e.g., infections, medication, and hydroelectrolytic disorders) of delirium, additional parameters may have hampered the comparability of our cohorts, even after PSM. Thus, the present findings require validation in prospective RCTs, which by accounting for imbalances in patient populations and biases in treatment assignment will evaluate the generalizability of our results. Second, as this study was conducted based on an automated retrieval of electronic records of patients discharged from the study SU, no mortality rates, as depicted in the Kaplan-Meier curves, or longitudinal clinical outcomes after discharge were available. Third, due to the limited number of cases per PSD motor subtype (i.e., hypoactive, hyperactive, and mixed), all PSD subtypes were jointly considered in the present analyses. However, as different neurobiological mechanisms may underly melatonin effects in different PSD subtypes [12, larger studies should assess possible differential melatonin effects in different PSD subtypes.
CONCLUSION
This study provides evidence that melatonin supplementation may be associated with reduced risk for PSD. Considering the current paucity of clinical guidelines for PSD prevention [6, 44, our results may have practical implications for the management of PSD in the neurological ICU setting, albeit prospective validation of these results is needed. Additionally, the finding of a high prevalence of early PSD within the first 24 h of AIS underlines the importance of early screening and early interventions for PSD prevention. These findings, in conjunction with the lack of association between melatonin supplementation and PSD onset or PSD duration, suggest a possible therapeutic potential of melatonin as a preventive rather than ad hoc treatment strategy. Nonetheless, the present findings should be regarded as exploratory and should be verified and expanded in future RCTs that will prospectively assess the neurobiological functions of melatonin and the full-spectrum of melatonin treatment effects in PSD.
ETHICS APPROVAL
The study was approved by the ethics committee of University Hospital Tübingen (protocol number 752/2018BO2).
ACKNOWLEDGMENTS
Open Access funding enabled and organized by Projekt DEAL.
WOA Institution: Universitatsklinikum Tubingen
Blended DEAL: Projekt DEAL
CONFLICT OF INTERESTS
A.Mengel was supported by Tübinger Forschungsförderung (A.Mengel) (2459-0-0). S.P. received speaker's honoraria and consulting honoraria from Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, and Werfen; reimbursement for congress traveling and accommodation from Bayer and Boehringer-Ingelheim; and research support from Bristol-Myers Squibb/Pfizer (significant); Boehringer-Ingelheim, Daiichi Sankyo (significant); and Helena Laboratories (all other contributions: modest). All competing interests are outside of the present work. U.Z. has received grants from the European Research Council, German Research Foundation, German Ministry of Education and Research, Biogen Idec GmbH, Servier, and Janssen Pharmaceuticals NV, all not related to this work; and consulting honoraria from Biogen Idec GmbH, Bayer Vital GmbH, Bristol Myers Squibb GmbH, Pfizer, CorTec GmbH, Medtronic GmbH, all not related to this work. The other authors have no competing interests to declare.
AUTHOR CONTRIBUTIONS
A.Mengel and S.P. conceived the present study. A.Mengel and M.-I.S. acquired the data, analyzed/interpreted the data, and drafted the manuscript. J.Z., C.B., V.S., and J.S.-P. acquired the data and analyzed/interpreted data. B.B. supervised the statistical analysis of the data and critically reviewed the manuscript. A.Meisel, R.F., U.Z., and S.P. critically reviewed the manuscript for important intellectual content. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.




