Cardiovascular health and chronic axonal polyneuropathy: A population-based study
Abstract
Background and purpose
Chronic axonal polyneuropathy is a common, usually multifactorial, disease for which there is no treatment yet available. We investigated the association between cardiovascular health, defined by the health score of the American Heart Association, and chronic axonal polyneuropathy.
Methods
Between June 2013 and January 2017, we investigated participants of the Rotterdam Study, a population-based cohort study. Participants were screened for polyneuropathy and categorized as having no, possible, probable or definite polyneuropathy. The cardiovascular health score (range 0–14; higher score reflecting better health) consisted of four health behaviours (diet, physical activity, smoking and body mass index) and three health factors (blood pressure, serum cholesterol and fasting glucose level).
Results
We included 1919 participants, of whom 120 (6.3%) had definite polyneuropathy. The median (interquartile range [IQR]) age was 69.0 (58.6–73.7) years and 53.4% were women. A higher cardiovascular health score was associated with a lower prevalence of definite polyneuropathy (per point increase: odds ratio [OR] 0.90, 95% confidence interval [CI] 0.84–0.96). Optimal cardiovascular health (score≥10) was strongly associated with a lower prevalence of definite polyneuropathy (OR 0.55, 95% CI 0.32–0.90). An increase in health factors and health behaviour scores separately was associated with a lower prevalence of polyneuropathy (per point increase: OR 0.82, 95% CI 0.71–0.95 and OR 0.86, 95% CI 0.78–0.96, respectively). The association between a lower cardiovascular health score and lower sural nerve amplitude was not significant after correction for covariates (difference 0.07µV, 95% CI −0.02–0.17).
Conclusions
Better cardiovascular health, consisting of both modifiable health behaviours and health factors, is associated with a lower prevalence of chronic axonal polyneuropathy.
INTRODUCTION
Chronic axonal polyneuropathy is a common disease in the elderly that leads to considerable morbidity [1]. The majority of the risk factors for polyneuropathy have been identified in clinical settings and among patients with longstanding and clinically apparent disease. The most common risk factors are diabetes mellitus, vitamin deficiencies, alcohol abuse and other toxic factors such as chemotherapy [2]. In recent years, there has been more emphasis on the association between metabolic syndrome and chronic axonal polyneuropathy [3-6] and additionally on the effect of lifestyle factors such as diet [7, 8]. However, in clinical settings, risk factors are not evident in up to 30% of patients with chronic axonal polyneuropathy and this percentage is even higher in the general population [9, 10]. Furthermore, in patients with a known risk factor, other risk factors may also be present, and therefore, chronic axonal polyneuropathy is considered to be a multifactorial disease. This indicates that multiple risk factors with modest effects in conjunction may lead to nerve damage and, consequently, to polyneuropathy.
The cardiovascular health score of the American Heart Association (AHA) combines the effect of risk factors, namely, metabolic syndrome and modifiable lifestyle factors. The AHA developed this score to promote health, rather than solely treating the disease, and to improve communication between individuals and healthcare providers [11]. A higher cardiovascular score has been associated with a lower risk of stroke, coronary heart disease and death [12, 13]. In the present study, we aimed to investigate the association between the cardiovascular health score and polyneuropathy. Specifically, we separate the impact of health factors; including blood pressure, serum cholesterol and fasting glucose levels, from modifiable behaviour factors, including smoking, body mass index (BMI), physical activity and diet. Our hypothesis is that health factors and modifiable behaviour factors are both associated with the prevalence of polyneuropathy.
METHODS
Study population
This study was embedded in the Rotterdam Study, an ongoing population-based cohort study to investigate chronic diseases [14]. The cohort started in January 1990 (RS-Ⅰ) and was extended in 2000 (RS-Ⅱ) and 2006 (RS-Ⅲ). From June 2013 until 2017, 2069 participants of subcohorts RS-Ⅰ, RS-Ⅱ and RS-Ⅲ were screened for polyneuropathy. From this group, 150 participants were excluded because of insufficient screening. The remaining 1919 participants were used for analysis. Sural sensory nerve action potential (SNAP) amplitude was available in 1416 of the included participants.
Standard protocol approvals, registrations and patient consent
The Rotterdam Study was approved by the Medical Ethics Committee of Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, licence number 1071272-159521-PG). The Rotterdam Study Personal Registration Data collection is filed with the Erasmus MC Data Protection Officer under registration number EMC1712001. The Rotterdam Study has been entered into the Netherlands National Trial Register and into the World Health Organization International Clinical Trials Registry Platform under shared catalogue number NTR6831. All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians.
Assessment of the components of the cardiovascular health score
The data of the determinant was collected in subcohort RS-I, RS-II and RS-III (examination rounds 5, 3 and 1, respectively), one visit before the screening of the outcome polyneuropathy. The mean (standard deviation [SD]) time difference between assessment of the cardiovascular health components and polyneuropathy screening was 5.3 (0.7) years.
The AHA cardiovascular health score consisted of seven components: smoking status, BMI, physical activity level, diet, blood pressure, serum cholesterol level, and fasting serum glucose level (Table S1) [11]. Smoking habits were determined during a structured home interview. Physical activity in minutes of moderate and/or vigorous intensity was assessed using the LASA physical activity questionnaire, and dietary habits with a validated 389-item food frequency questionnaire as described elsewhere [15]. Blood pressure, BMI, and fasting serum glucose and cholesterol levels were measured during the visit to our research centre. BMI was calculated as body weight/height-squared in kg/m2. Blood pressure was measured in the sitting position and the average of two measurements was used. The use of antihypertensive drugs, and glucose- and lipid-lowering medications was assessed during the home interview and review of pharmacy records.
The AHA's recommendations regarding cut-off values and medication use were used to categorize BMI, physical activity, blood pressure, and serum cholesterol and glucose levels [11] (Table S1). Based on the available data in our study, we changed smoking status to never, past or current, and the cut-off values for advised dietary intake for vegetables or fruit, fish, wholegrains, sugar-containing beverages and sodium intake were based on the Dutch dietary guidelines [15] (Table S1). All components were scored in three categories (poor = 0, intermediate = 1, and ideal = 2) [11]. The cardiovascular health score was calculated as the sum of all seven components, ranging from 0 to 14, in which a higher score represents better cardiovascular health. Participants with prevalent stroke, heart failure or coronary heart disease could reach a maximum cardiovascular health score of 7 as each component score was subtracted by 1 point [11]. As an optimal cardiovascular health score is thought to be most beneficial, the total cardiovascular health score was dichotomized into two groups based on the 75th percentile of the interquartile range (IQR): an optimal (score ≥10) versus nonoptimal score (0–9). The health behaviour score was the sum of the scores for BMI, diet, smoking and physical activity, ranging from 0 to 8. The health factors score was the sum of the scores for blood pressure, and fasting serum glucose and cholesterol levels, ranging from 0 to 6.
Polyneuropathy screening
The screening to diagnose chronic axonal polyneuropathy consisted of three components: a symptom questionnaire, neurological examination of the legs, and nerve conduction studies of the sural nerves [10]. The questionnaire assessed the presence of 12 symptoms that occur bilaterally for at least 3 months and could be answered with never, sometimes or (almost) continuously [16]. Symptoms involved tingling, burning sensations, cotton-wool feeling, muscle cramps, muscle pain not related to exercise, stabbing pain, weakness, numbness, tightness and allodynia of the feet or legs. The participants were also asked if they were ever diagnosed with polyneuropathy. Neurological examination consisted of bilateral examination of the legs including vibration and superficial pain sensation, tendon reflexes and dorsal flexion of the feet. To quantify axonal degeneration, nerve conduction studies of the sural nerves were performed with a NicoletTM Viking Quest (Natus Medical Incorporated, San Carlos, CA, USA). The sural nerve was antidromically measured with surface electrodes, and SNAP amplitudes were measured from baseline to peak according to a predefined protocol [10]. The sural nerve response was measured bilaterally and the highest SNAP amplitude after stimulation of both sural nerves was used for analyses. Sural SNAP amplitude <4.0 µV was considered abnormal [17].
All participants were individually discussed in an expert panel led by a neuromuscular specialist (P.D.) and included a neurophysiology specialist (J.D.) and two physicians trained in epidemiology with a special interest in neuromuscular diseases (N.T. and R.H.). Based on the level of abnormalities in the three components of the screening, participants were categorized as having ‘no’, ‘possible’, ‘probable’ or ‘definite’ chronic axonal polyneuropathy, irrespective of the cause [10]. After categorizing participants, their medical records were reviewed for diagnosis of chronic axonal polyneuropathy by a neurologist, as this diagnosis was considered superior to our screening. Participants were excluded if ≥2 of the three components of the screening were missing [10].
Assessment of covariates
Covariates were assessed at the same time as measurement of determinant and included age, sex and education level. Education level was assessed during the home interview and participants were categorized according to the UNESCO classification into four groups ranging from low (primary) to high (higher vocational) education, reflecting socioeconomic status.
Statistical analysis
Population characteristics were described using means (SD) or median (IQR) for continuous measures based on their distribution. Categorical measures were described as number (%).
To assess the association between cardiovascular health score and polyneuropathy, we used logistic regression analysis. The different categories of polyneuropathy were separately compared to ‘no polyneuropathy’ (reference category) to avoid misclassification. Furthermore, we performed a logistic regression analysis to assess the association between the group with optimal (score ≥10) versus non-optimal cardiovascular health score and polyneuropathy (each category vs. the reference category). Linear regression analysis in all participants was used to determine the association between cardiovascular health score (continuous scale) and sural SNAP amplitude. Furthermore, we split the cardiovascular health score into a health behaviour score and a health factors score to investigate the effect sizes for the presence of polyneuropathy as well as the sural SNAP amplitude.
All analyses were adjusted for age, sex, education level and time between determinant and outcome. We also performed sensitivity analyses to investigate the effect of diabetes mellitus and alcohol on our outcomes. We additionally adjusted for prevalent diabetes mellitus and alcohol intake in g/day by adding these variables separately to the model. We furthermore stratified by prevalent diabetes mellitus. Diabetes mellitus was defined as a fasting serum glucose level ≥ 126 mg/dl and/or the use of glucose-lowering drugs.
The percentage of missing values was between 1.3% (smoking status) and 14.0% (physical activity). To maximize statistical power, missing values were imputed using multiple imputations (five times) of the determinant, and covariates based on the outcome, the determinants and the covariates (IBM SPSS Statistics, version 25). Analyses were performed and figures were created with R, CRAN version 3.6.1 and GraphPad Prism, version 5.03. Statistical significance was considered at an alpha level for two-tailed tests on p <0.05.
RESULTS
We included 1919 participants with a median (IQR) age of 69.0 (58.6–73.7) years, and 53.4% of participants were women. The median (IQR) cardiovascular health score was 8.0 (7.0–10.0). Two participants had ideal cardiovascular health (score of 14) and 61 had a score of 0 (3.2%). Definite, probable and possible polyneuropathy were present in 120 (6.3%), 142 (7.4%) and 375 participants (19.5%), respectively (Table 1). Participants with definite polyneuropathy had more often experienced a cardiovascular event (20.0%) than the group without polyneuropathy (8.2%). Optimal cardiovascular health score (score≥10) was less prevalent in the group with definite polyneuropathy (16.7%) compared to probable (23.2%), possible (25.6%) and no polyneuropathy (32.8%). Participants with optimal cardiovascular health were more often female and were slightly younger (median [IQR] age 66.5 [55.3–72.6] years).
|
Total population N = 1919 |
|
|---|---|
| Female, n (%) | 1025 (53.4) |
| Age in years, median (IQR) | 69.0 (58.6–73.7) |
| Cardiovascular health score, median (IQR) | 8.00 (7.0–10.0) |
| Cardiovascular health score, n (%) | |
| 0 | 61 (3.2) |
| 1 | 47 (2.4) |
| 2 | 38 (2.0) |
| 3 | 34 (1.8) |
| 4 | 20 (1.6) |
| 5 | 43 (2.2) |
| 6 | 137 (7.1) |
| 7 | 245 (12.8) |
| 8 | 344 (18.0) |
| 9 | 371 (19.3) |
| 10 | 298 (15.5) |
| 11 | 174 (9.1) |
| 12 | 71 (3.7) |
| 13 | 24 (1.3) |
| 14 | 2 (0.1) |
| Polyneuropathy category, n (%) | |
| Definite polyneuropathy | 120 (6.3) |
| Probable polyneuropathy | 142 (7.4) |
| Possible polyneuropathy | 375 (19.5) |
| Sural SNAP amplitude in µvolt, median (IQR)a | 8.0 (5.0–11.0) |
| Education, n (%) | |
| Primary | 115 (6.0) |
| Low-intermediate | 694 (36.2) |
| Intermediate | 628 (32.7) |
| High | 482 (25.1) |
| Smoking, n (%) | |
| Never | 621 (32.4) |
| Past | 1007 (52.5) |
| Current | 291 (15.2) |
| Physical activity, median (IQR) | |
| Minutes moderate intensity per week | 390 (200–645) |
| Minutes vigorous intensity per week | 0 (0–150) |
| Adherence to dietary guidelines, median (IQR) | 4.0 (3.0–4.0) |
| BMI in kg/m2, mean ± SD | 27.2 ± 3.9 |
| Serum glucose levels in mg/dl, mean ± SD | 102.2 ± 21.4 |
| Use of antidiabetic medication, n (%) | 132 (6.9) |
| Serum cholesterol in mg/dl, mean ± SD | 211.0 ± 41.1 |
| Use of lipid-lowering medication, n (%) | 527 (27.5) |
| Systolic blood pressure in mmHg, mean ± SD | 143.5 ± 21.7 |
| Diastolic blood pressure in mmHg, mean ± SD | 84.5 ± 10.8 |
| Use of antihypertensive drugs, n (%) | 727 (37.9) |
| Prevalent cardiovascular disease, n (%) | |
| No | 1723 (89.8) |
| Yes | 196 (10.2) |
- Cardiovascular health score ranges from 0 (poor) to 14 (good). Adherence to dietary guidelines for vegetables or fruit, fish, whole grains, sugar-containing beverages and sodium intake (range 0–5).
- Abbreviations: IQR, interquartile range; N, number; SNAP, sensory nerve action potential.
- a Available in 1416 participants.
Better cardiovascular health, represented by a higher score, was associated with a lower prevalence of definite polyneuropathy. An optimal cardiovascular health score was associated with less prevalent definite polyneuropathy (odds ratio [OR] 0.55, 95% confidence interval [CI] 0.32–0.90). Similar effects, although attenuated and not statistically significant, were observed for probable (OR 0.75, 95% CI 0.48–1.14) and possible polyneuropathy (OR 0.83, 95% CI 0.63–1.09; Figure 1).
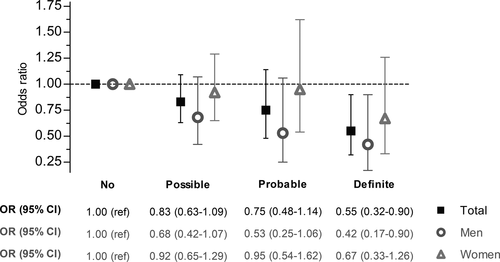
A one-point higher cardiovascular health score was associated with a lower prevalence of definite polyneuropathy versus the reference group (OR 0.90, 95% CI 0.84–0.96; Table 2). This effect was slightly more pronounced in men than women (OR 0.88, 95% CI 0.81–0.95 vs. OR 0.94, 95% CI 0.84–1.06). Similar results, albeit attenuated, were observed in probable and possible polyneuropathy (Table 2). Furthermore, we divided the cardiovascular health score into a health behaviour (with a maximum score of 8) and a health factors score (with a maximum score of 6). The effect of a better health factors score on lower prevalence of polyneuropathy was slightly larger than the effect of a better health behaviour score (OR 0.82, 95% CI 0.71–0.95 vs. OR 0.86, 95% CI 0.78–0.96), and this was also more pronounced in men than in women (Table 2).
| n/N |
No polyneuropathy OR (95% CI) |
n/N |
Possible polyneuropathy OR (95% CI) |
n/N |
Probable polyneuropathy OR (95% CI) |
n/N |
Definite polyneuropathy OR (95% CI) |
|
|---|---|---|---|---|---|---|---|---|
| Cardiovascular health score | 1282 | 1.00 (ref) | 375/1657 | 0.96 (0.92–1.01) | 142/1424 | 0.92 (0.87–0.98) | 120/1402 | 0.90 (0.84–0.96) |
| Men | 596 | 1.00 (ref) | 167/763 | 0.97 (0.91–1.03) | 66/662 | 0.91 (0.84–0.99) | 65/661 | 0.88 (0.81–0.95) |
| Women | 686 | 1.00 (ref) | 208/894 | 0.96 (0.90–1.02) | 76/762 | 0.94 (0.85–1.04) | 55/741 | 0.94 (0.84–1.06) |
| Behaviour score | 1282 | 1.00 (ref) | 375/1657 | 0.93 (0.87–1.00) | 142/1424 | 0.91 (0.82–1.00) | 120/1402 | 0.86 (0.78–0.96) |
| Men | 596 | 1.00 (ref) | 167/763 | 0.96 (0.87; 1.06) | 66/662 | 0.89 (0.76–1.01) | 65/661 | 0.84 (0.74–0.97) |
| Women | 686 | 1.00 (ref) | 208/894 | 0.91 (0.83; 1.01) | 76/762 | 0.93 (0.80–1.09) | 55/741 | 0.89 (0.76–1.06) |
| Factors score | 1282 | 1.00 (ref) | 375/1657 | 0.96 (0.88–1.05) | 142/145 | 0.84 (0.73–0.97) | 120/1402 | 0.82 (0.71–0.95) |
| Men | 596 | 1.00 (ref) | 167/763 | 0.93 (0.82–1.06) | 66/662 | 0.82 (0.68–0.99) | 65/661 | 0.73 (0.60; 0.88) |
| Women | 686 | 1.00 (ref) | 208/894 | 0.99 (0.86–1.13) | 76/762 | 0.88 (0.71–1.08) | 55/741 | 0.98 (0.77;−1.25) |
- All results are adjusted for age, sex, education level and time between measurement of determinant and outcome. Each polyneuropathy category was separately compared to the reference group (‘no polyneuropathy’, N = 1282), stratified by sex. A higher cardiovascular health score (ranging from 0 to 14) represents better health. Bold text: statistically significant (p < 0.05) findings.
- Abbreviations: CI, confidence interval; OR, odds ratio; ref, reference.
Additionally, we performed sensitivity analyses, which indicated that our results were not merely driven by diabetes mellitus or alcohol intake (Table S2). The results of the association between cardiovascular health score and chronic axonal polyneuropathy remained quite similar after adjusting (OR 0.93, 95% CI 0.87–0.99) or stratifying for prevalent diabetes mellitus (present: OR 0.93, 95% CI 0.75–1.02 and absent: OR 0.93, 95% CI 0.86–1.01; Table S2).
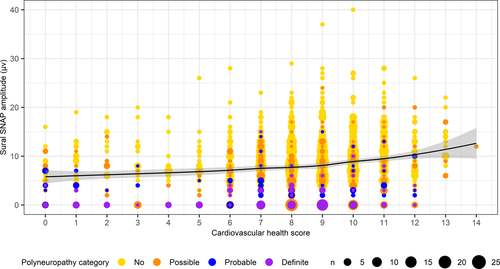
Figure 2 shows that a lower cardiovascular health score may be associated with a lower sural SNAP amplitude, even in the categories without definite polyneuropathy. However, when adjusted for covariates, this finding did not reach statistical significance (Table 3).
|
Sural SNAP amplitude (total N = 1416) Differencea (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|
| n/N | No polyneuropathy | n/N | Possible polyneuropathy | n/N | Probable polyneuropathy | n/N | Definite polyneuropathy | |
| Cardiovascular health score | 946 | 0.00 (−0.14;0.09) | 281/1227 | 0.00 (−0.10;0.10) | 109/1336 | 0.05 (−0.05;0.14) | 80/1416 | 0.07 (−0.02;0.17) |
| Men | 419 | −0.04 (−0.16;0.08) | 117/536 | −0.03 (−0.15;0.09) | 53/589 | 0.01 (−0.11;0.12) | 47/636 | 0.06 (−0.05;0.17) |
| Women | 527 | 0.02 (−0.18;0.21) | 164/691 | 0.05 (−0.11;0.21) | 56/747 | 0.10 (−0.05;0.26) | 33/780 | 0.10 (−0.05;0.26) |
| Behaviour score | 946 | −0.08 (−0.26;0.09) | 281/1227 | −0.02 (−0.18;0.14) | 109/1336 | 0.05 (−0.01;0.20) | 80/1416 | 0.08 (-0.0470.23) |
| Men | 419 | −0.14 (−0.33;0.05) | 117/536 | −0.10 (−0.29;0.10) | 53/589 | −0.05 (−0.23;0.13) | 47/636 | 0.02 (−0.16;0.19) |
| Women | 527 | 0.02 (−0.27;0.31) | 164/691 | 0.10 (-0.15;0.34) | 56/747 | 0.18 (−0.05;0.42) | 33/780 | 0.19 (−0.05;0.43) |
| Factors score | 946 | 0.04 (−0.17; 0.26) | 281/1227 | 0.03 (−0.17;0.23) | 109/1336 | 0.10 (−0.09;0.29) | 80/1416 | 0.15 (−0.04;0.33) |
| Men | 419 | 0.06 (−0.17;0.29) | 117/536 | 0.02 (−0.22;0.26) | 53/589 | 0.08 (−0.14;0.31) | 47/636 | 0.19 (−0.03;0.42) |
| Women | 527 | 0.01 (−0.34;0.37) | 164/691 | 0.02 (−0.29;0.33) | 56/747 | 0.08 (−0.22;0.38) | 33/780 | 0.08 (−0.22;0.38) |
- All results are adjusted for age, sex, education level, time between measurements of determinant and outcome. Each polyneuropathy group is a sum of the other groups in ordinal sequence starting at no polyneuropathy, stratified by sex.
- Abbreviations: CI, confidence interval; SNAP, sensory nerve action potential.
- a Adjusted mean difference per 1-unit increase.
DISCUSSION
Better cardiovascular health, consisting of health behaviour and health factors, was associated with a lower prevalence of chronic axonal polyneuropathy. An optimal cardiovascular health score (defined as score ≥10) was most strongly associated with a lower prevalence of polyneuropathy, but interestingly, also an increase of only one point in cardiovascular health score was associated with a significantly lower prevalence of polyneuropathy. Furthermore, both higher health factors score as well as health behaviour score were associated with a lower prevalence of polyneuropathy. This is of particular interest as individuals can potentially modify their health behaviour.
We found a markedly low prevalence of ideal cardiovascular health. This low prevalence is similar to the prevalence in other studies [18-20]. A better cardiovascular health score reduced the risk of cardiovascular events such as stroke, heart disease and death. Moreover, better cardiovascular health is associated with a lower risk of cognitive decline and dementia [21-24], suggesting that modifying these factors may also benefit chronic diseases other than cardiovascular disease.
How cardiovascular risk factors precisely relate to chronic axonal polyneuropathy is not yet fully understood. It seems likely that chronic axonal polyneuropathy is a multifactorial disease and several pathways causing nerve ischaemia followed by axonal degeneration are proposed [25-28]. Several factors such as chronic inflammation due to oxidative stress causing microvascular damage of the neural vasculature, or structural changes of the blood vessel endothelium due to hypertension could possibly contribute to the development of chronic axonal polyneuropathy [25-28]. Considering this possible relation, we expected the cardiovascular health score to be associated with polyneuropathy. Interestingly, the results were not merely driven by diabetes mellitus or alcohol intake, which are known risk factors for polyneuropathy.
We found that both cardiovascular health score and health behaviour score were associated with polyneuropathy. This is of interest, as our previous work did not show a significant association between diet, a component of the health behaviour score, and polyneuropathy in this population [7]. As health behaviours are modifiable, changing lifestyle may be used for possible prevention, delay in progression or even treatment of chronic axonal polyneuropathy. Although optimal cardiovascular health was most beneficial, even small improvements such as improving only one of the seven components of the score was already significantly associated with the prevalence of polyneuropathy. Improving cardiovascular health by only one point, not necessarily to the ideal level, seems achievable to the general population and is therefore likely to be sustainable for a prolonged period of time. This health score is relatively easy to translate to the general population and therefore probably a good tool to provide more insight for both patients and healthcare professionals and may be useful for future prevention programmes.
We did not find a significant association between a lower cardiovascular health score and a lower sural SNAP amplitude after adjustment for covariates. Our previous study showed that metabolic syndrome and kidney dysfunction resulted in damage to the peripheral nerves, even when polyneuropathy was not yet clinically present [29]. Based on the effect sizes of the association between cardiovascular health score and sural SNAP amplitude, the results of our previous study [29] and the hypothesis of the AHA that health is a broader, more positive construct than just the absence of clinically evident disease [11], it would be interesting to restudy the (subclinical) effects of cardiovascular health on the peripheral nerves when power is increased by larger numbers of participants.
A limitation of this study is the use of questionnaires to assess dietary habits, smoking and physical activity as this is prone to measurement error. Furthermore, due to the structure of the visits for the Rotterdam Study, we used the determinants and covariates of the visit before the assessment of polyneuropathy. This might have led to immortal time bias and an underestimation of the results. However, the use of determinants of one visit before polyneuropathy assessment introduced a time component in our analysis, reducing, at least partly, the risk of possible reverse causation. We also have to take into account that the population we studied was mostly Caucasian and living in an urban area, limiting the generalizability to other ethnic origins. In the present study we investigated the presence of chronic axonal polyneuropathy and did not find indications for demyelinating or small-fibre neuropathies. Furthermore, notwithstanding the large effect sizes, a lack of power cannot be ruled out, particularly for the analyses for the outcome sural SNAP amplitude. Also, when stratifying on sex, the group of women with polyneuropathy was relatively small, resulting in statistically nonsignificant findings. Although the cardiovascular health score combines risk factors by which it accounts for the multifactorial nature of chronic axonal polyneuropathy, we also need to bear in mind the limitations of this score. Firstly, the different components of cardiovascular health score were assigned similar weights, although it is acknowledged that improving certain components would have a greater impact to reduce the risk for disease than other components. For instance, in previous studies, we did not find an association between diet, one of the health behaviour components, and polyneuropathy [7]. This could be because the cardiovascular health score accounts for the multifactorial nature of the disease by combining risk factors, or the effect of the health behaviour score is merely driven by other components. Secondly, potential interactions between the components, such as glucose levels and BMI, should be taken into account when using the health behaviour and health factors scores separately. Thirdly, it is conceivable that the effect of health behaviour on polyneuropathy is mediated by a change in health factors, for instance, a healthier diet may improve glucose levels that consequently reduces the risk of polyneuropathy. Therefore, the difference between the health behaviour score and health factors score, as defined by the AHA, may not always be clear in clinical practice. Finally, to determine if changing lifestyle and/or preventive medication such as lipid-lowering drugs lowers the risk for or progression of polyneuropathy in individuals, longitudinal studies are required.
In conclusion, better cardiovascular health was associated with a lower prevalence of chronic axonal polyneuropathy. Both health factors and health behaviours contributed to this overall effect. To accelerate strategies to possibly slow progression, or even prevent or treat chronic axonal polyneuropathy, further research is needed to understand the causality and complexity regarding cardiovascular risk factors and chronic axonal polyneuropathy.
ACKNOWLEDGEMENTS
We thank the study participants of the Ommoord district and their general practitioners and pharmacists for contributing to the Rotterdam Study.
DISCLOSURE OF CONFLICTS OF INTEREST
P. A. van Doorn and M. A. Ikram received a grant from Prinses Beatrix Spierfonds for neuromuscular diseases (grant number W.OR17-10) to conduct this study. N. E. Taams, F. Ahmadizar, R. Hanewinckel, J. Drenthen, T. Voortman and M. Kavousi report no disclosures relevant to the manuscript. The funding source had no role in study design, collection, analysis, interpretation of data, writing of the manuscript or decision to submit the article for publication.
Open Research
DATA AVAILABILITY STATEMENT
Data can be obtained on request. Requests should be directed toward the management team of the Rotterdam Study ([email protected]), which has a protocol for approving data requests. Because of restrictions based on privacy regulations and informed consent of the participants, data cannot be made freely available in a public repository.




