Greater small nerve fibre damage in the skin and cornea of type 1 diabetic patients with painful compared to painless diabetic neuropathy
Funding information
This research was funded by awards from the Juvenile Diabetes Research Foundation International (27-2008-362).
Abstract
Background and aim
Damage to small nociceptive fibres may contribute to painful diabetic neuropathy. We aimed to compare large and small nerve fibre measurements together with skin biopsy and corneal confocal microscopy in patients with type 1 diabetes and painful or painless diabetic neuropathy.
Methods
We have assessed the McGill pain questionnaire, neuropathy disability score, vibration perception threshold, warm and cold sensation thresholds, electrophysiology, corneal confocal microscopy and skin biopsy in participants with type 1 diabetes and painful (n = 41) or painless (n = 50) diabetic neuropathy and control subjects (n = 50).
Results
The duration of diabetes, body mass index, glycated haemoglobin (HbA1c), blood pressure and lipid profile did not differ between subjects with painful and painless neuropathy. Neuropathy disability score and vibration perception threshold were higher and sural nerve conduction velocity was lower, but sural nerve amplitude, peroneal nerve amplitude and conduction velocity and cold and warm sensation thresholds did not differ between patients with painful compared to painless diabetic neuropathy. However, intraepidermal nerve fibre density, corneal nerve fibre density, corneal nerve branch density and corneal nerve fibre length were significantly lower in subjects with painful compared to painless diabetic neuropathy.
Conclusions
There is evidence of more severe neuropathy, particularly small fibre damage in the skin and cornea, of patients with painful compared to painless diabetic neuropathy.
INTRODUCTION
Approximately 20% of patients with diabetic peripheral neuropathy (DPN) develop painful neuropathy which is associated with significant morbidity and reduced quality of life.[1] The efficacy of current therapies is highly variable,[2, 3] which may in part reflect diverse etiological mechanisms operating at the peripheral nociceptor, spinal and supraspinal levels in painful DPN.[4] It has been suggested that phenotyping patients based on their symptom complex and identifying the primary site and mechanism of their symptoms may allow a more tailored therapeutic approach and improved response to treatment.[5] However, sensory testing, skin punch biopsy and brain imaging have been deemed to have insufficient evidence to support their use as biomarkers in pain clinical trials.[6] Furthermore, a retrospective analysis of seven clinical trials showed a limited impact of sensory phenotyping on predicting the efficacy of drugs to relieve neuropathic pain.[7]
Given that painful diabetic neuropathy is considered to arise from dysfunction and damage to nociceptive C-fibres and autonomic fibres,[8, 9] it is no surprise that standard neurophysiology does not differentiate diabetic patients with painful compared to painless neuropathy.[10] Indeed, whilst neurophysiological parameters such as the F-wave and H-reflex identify patients with subclinical diabetic neuropathy, they cannot differentiate patients with and without painful neuropathy.[11, 12] However, a recent meta-analysis has shown a greater abnormality in heat pain thresholds in patients with painful compared to painless diabetic neuropathy.[13] But, a skin biopsy study demonstrated no difference in intraepidermal nerve fibre density (IENFD) in patients with and without painful neuropathy.[14] Similarly, another detailed immunohistochemical skin biopsy study found no difference in IENFD, but patients with painful diabetic neuropathy had an increased density of dermal peptidergic fibres containing substance P and calcitonin gene-related peptide compared to patients with painless DPN and healthy controls.[15] We have previously shown a reduction in intraepidermal nerve fibre length in patients with painful compared to painless diabetic neuropathy.[16] A recent study has shown increased axonal regeneration and swelling,[17] whilst another study has shown no relationship between the severity of axonal swelling [18] and painful diabetic neuropathy. Furthermore, exercise reduced painful neuropathic symptoms but was associated with an increase in IENF branch density.[19]
Corneal confocal microscopy (CCM) is a rapid, non-invasive technique that can be used to image and quantify small fibre pathology [20] and is comparable to IENFD for the diagnosis of DPN.[21-23] We have shown a significant reduction in corneal nerve fibre length in patients with painful compared to painless diabetic neuropathy [16] and more recently we have shown a reduction in central and inferior whorl corneal nerve fibre length in patients with painful diabetic neuropathy.[24] Furthermore, in a recent study from China there was greater corneal nerve fibre damage in diabetic patients with painful compared to painless diabetic neuropathy.[25]
In the present study, we compared detailed measures of large and small fibre function together with skin biopsy and CCM in a large cohort of patients with type 1 diabetes and painful or painless diabetic neuropathy.
RESEARCH DESIGN AND METHODS
Study participants
Ninety-one participants with type 1 diabetes mellitus and 50 age-matched healthy control subjects were studied. The study was approved by the North Manchester Research Ethics committee and written informed consent was obtained from all participants. This research adhered to the tenets of the Declaration of Helsinki. Patients with a history of any systemic disease apart from diabetes associated with neuropathy were excluded from the study. In addition, patients with any history of systemic or ocular diseases associated with corneal involvement were excluded from the study.
Clinical and peripheral neuropathy assessments
All participants underwent a complete medical history and an assessment of body mass index (BMI), blood pressure, glycated haemoglobin (HbA1c), cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglycerides. Symptoms of neuropathy were assessed using the neuropathy symptom profile (NSP) and short form McGill pain questionnaire (SF-MPQ).[26]
Detailed assessment of neuropathy included the neuropathy disability score (NDS), vibration perception threshold (VPT) on the hallux of both feet using a Neurothesiometer (Horwell; Scientific Laboratory Supplies Ltd, Wilford, Nottingham, UK), cold and warm sensation threshold (CST and WST) and cold- and heat-induced pain threshold (CIP and HIP) using a TSA-II NeuroSensory Analyser (Medoc Ltd, Ramat-Yishai, Israel) on the dorsolateral aspect of the left foot. A Dantec ʻKeypointʼ system (Dantec Dynamics Ltd, Bristol, UK) was used to measure sural nerve action potential amplitude (SNAP) and conduction velocity (SNCV) and peroneal nerve action potential amplitude (PMNAP) and conduction velocity (PMNCV).
Skin biopsy and IENFD assessment
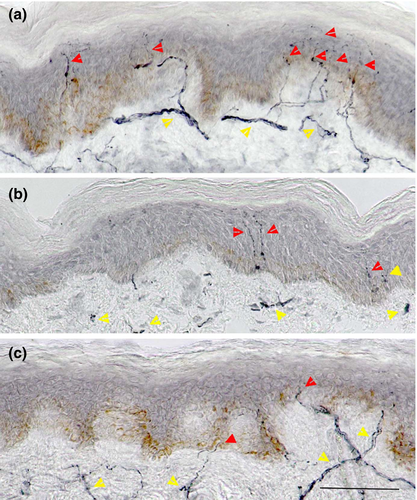
Two 3 mm punch skin biopsies were taken from the dorsum of the dominant foot, 2 cm above the second metatarsal head under local anaesthesia (1% lidocaine). The biopsies were immediately fixed in 4% paraformaldehyde, cryoprotected in graded solutions of sucrose, frozen and cut on a cryomicrotome (HM450, Microm International GmbH, Walldorf, Germany). Six 50 μm sections per biopsy were immunostained using anti-human PGP 9.5 antibody (Abcam, Cambridge, UK), and nerve fibres were demonstrated using SG chromogen (Vector Laboratories, Peterborough, UK).[27] IENFD was quantified in accordance with established criteria and expressed as a number of nerve fibres per millimetre length of epidermis.[28]
Corneal confocal microscopy
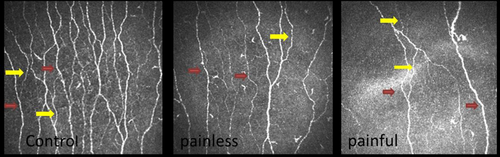
The central cornea of all study participants was scanned using laser in vivo corneal confocal microscopy (IVCCM) (Heidelberg Retinal Tomograph III Rostock Cornea Module (HRT III RCM); Heidelberg Engineering GmbH, Heidelberg, Germany).[29] The examination was performed according to our previously published protocol, by highly experienced optometrists, and took approximately 5 min for both eyes of each participant.[30] Six images (three from each eye) from the corneal subbasal nerve plexus were exported and used for image analysis. Two experienced examiners analysed all the images manually using CCMetrics (MA Dabbah; Imaging Science and Biomedical Engineering, University of Manchester, Manchester, UK), while being masked from the outcome of medical and peripheral neuropathy assessments. Corneal nerve morphological parameters including corneal nerve fibre density (CNFD), the number of main nerve fibres/mm2; corneal nerve branch density (CNBD), the number of branch points on the main nerves/mm2; corneal nerve fibre length (CNFL), the total length of nerves mm/mm2; and corneal nerve fibre tortuosity (CNFT), the tortuosity coefficient (TC) of main nerves were measured for each image and the average of the results from six images was used for data analysis.
Corneal sensation was measured in the centre of the cornea using non-contact corneal aesthesiometry (NCCA) (Glasgow Caledonian University, Glasgow, UK).[31]
Study definition of painful neuropathy
All 91 patients with type 1 diabetes were confirmed to have diabetic neuropathy based on an abnormality in at least two of five neurological measures including IENFD (<3.3), CNFD (<24.4), CST (<23.8), VPT (>16.5) or PMNCV (<40) defined by results lying 2 SD outwith the mean of control subjects. Patients were divided into two groups based on the SF-MPQ and McGill pain index (PI), with a score >1/5 considered to have painful diabetic neuropathy and those with a score 0/5 considered to have painless diabetic neuropathy.
Statistical analysis
SPSS Statistics for Windows v19.0 (IBM Corp., Armonk, NY, USA) for Windows was used to compute the results. Analysis included descriptive and frequency statistics and all data are presented as mean ± SD. Shapiro−Wilk test and Q-Q plots were used to evaluate whether the data were normally distributed or not. To evaluate the difference between the two groups, the independent samples t-test (Mann−Whitney U test for non-parametric) and to evaluate the difference among the groups, one-way ANOVA with Bonferroni adjustment were used.
RESULTS
Demographic and clinical findings
Ninety-one patients with type 1 diabetes mellitus, 41 with painful neuropathy and 50 with painless neuropathy and 50 age-matched controls were studied. There was no difference in age, gender, ethnicity, smoking, alcohol consumption, BMI, lipid profile, blood pressure and HbA1c, but height was significantly lower (p = 0.02) in patients with painful compared to painless diabetic neuropathy (Table 1).
| Parameter | Control | Type 1 diabetes painless neuropathy | Type 1 diabetes painful neuropathy |
|---|---|---|---|
| Subjects (n) | 50 | 50 | 41 |
| Age (years) | 51.5 ± 12.7 | 47.6 ± 14.4 | 52.7 ± 14.4 |
| Gender (F/M) | 26/24 | 20/30 | 24/17 |
| Ethnicity (Asian/European) | 18/32 | 4/46 | 3/38 |
| Duration of diabetes (years) | – | 30.8 ± 17.0 | 33.6 ± 16.1 |
| Smoking (n/day) | 0.7 ± 2.4 | 1.8 ± 4.9 | 1.7 ± 5.5 |
| Alcohol consumption (units/week) | 5.1 ± 7.6 | 5.6 ± 8.1 | 3.2 ± 5.9 |
| HbA1c (%) | 5.6 ± 0.3 | 8.2 ± 1.2* | 8.5 ± 1.7* |
| HbA1c-IFCC (mmol/mol) | 37.7 ± 3.6 | 64.7 ± 18.1* | 69.6 ± 18.5* |
| BMI (kg/m2) | 26.7 ± 4.5 | 26.7 ± 4.1 | 27.03 ± 4.8 |
| Height (cm) | 166.0 ± 10.7 | 171.6 ± 9.2* | 166.1 ± 9.4# |
| Waist circumference (cm) | 90.3 ± 14.9 | 92.3 ± 13.5 | 89.8 ± 18.9 |
| Cholesterol (mmol/L) | 5.0 ± 0.7 | 4.4 ± 0.9 | 4.2 ± 0.7 |
| HDL (mmol/L) | 1.6 ± 0.5 | 1.6 ± 0.4 | 1.7 ± 0.5 |
| Triglycerides (mmol/L) | 1.4 ± 0.7 | 1.1 ± 0.6 | 1.2 ± 0.7 |
| LDL (mmol/L) | 2.7 ± 0.7 | 2.3 ± 0.9* | 2.1 ± 0.4* |
| BP Sys/Dia (mmHg) | 128.7 ± 17.9/73.0 ± 9.9 | 133.6 ± 20.5/70.4 ± 7.8 | 137.7 ± 24.6/72.1 ± 11.2 |
Note
- Data are presented as mean ± SD. All symbols indicate statistically significant difference, *p < 0.01 compared to controls and #p < 0.05 compared to patients with painless neuropathy.
- Abbreviations: BMI, body mass index; BP, blood pressure; Dia, diastole; F, female: HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; IFCC, International Federation of Clinical Chemistry; LDL, low-density lipoprotein; M, male; Sys, systole.
Neuropathy assessments
SNCV, SNAP, PMNCV, PMNAP, CST and IENFD were significantly (p < 0.01) lower and NSP, NDS, VPT and WST were significantly higher (p < 0.01) in patients with type 1 diabetes compared to control subjects.
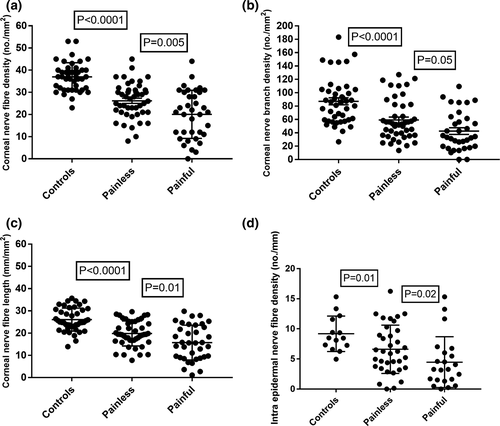
NSP (p = 0.0001), NDS (p = 0.003) and VPT (p = 0.001) were higher and SNCV (p = 0.009) was lower in patients with painful compared to painless neuropathy. IENFD was significantly lower (p = 0.02) in patients with painful compared to painless neuropathy (Figure 1). There was no correlation between NDS, VPT, quantitative sensory testing and neurophysiology with the severity of painful neuropathic symptoms using the McGill visual analogue index (Table 2).
| Parameter | Control | Type 1 diabetes painless neuropathy | Type 1 diabetes painful neuropathy |
|---|---|---|---|
| McGill VAS (0–10) | 0 | 0.04 ± 0.2 | 5.51 ± 2.7*# (p < 0.0001) |
| NSP (0–38) | 0.3 ± 0.8 | 1.6 ± 2.9 | 8.2 ± 6.4# (p = 0.0001) |
| NDS (0–10) | 0.6 ± 1.2 | 2.6 ± 1.4 | 4.5 ± 2.2# (p = 0.003) |
| VPT (V) | 6.5 ± 5.0 | 12.4 ± 10.7 | 21.1 ± 14.1# (p = 0.001) |
| CST (°C) | 28.2 ± 2.2 | 25.3 ± 5.3* | 22.3 ± 8.2* |
| WST (°C) | 36.9 ± 3.1 | 39.8 ± 3.5* | 41.6 ± 4.9* |
| CIP (°C) | 11.8 ± 9.4 | 7.3 ± 7.2* | 7.4 ± 8.9 |
| HIP (°C) | 44.9 ± 3.2 | 45.9 ± 3.3 | 47.3 ± 3.1* |
| SNCV (m/s) | 50.2 ± 4.5 | 42.7 ± 6.0* | 38.5 ± 7.9*# (p = 0.009) |
| SNAP (µV) | 19.4 ± 9.6 | 9.8 ± 7.7* | 6.6 ± 6.8* |
| PMNCV (m/s) | 48.6 ± 4.1 | 40.3 ± 7.3* | 37.7 ± 8.7* |
| PMNAP (mV) | 5 ± 2.2 | 3.7 ± 2.5 | 2.7 ± 4.1* |
| IENFD (n/mm) | 9.1 ± 2.9 | 6.6 ± 4* | 4.4 ± 4.2*# (p = 0.02) |
| NCCA (mbars) | 0.5 ± 0.3 | 0.7 ± 0.5 | 1.5 ± 1.8*# (p = 0.003) |
| CNFD (n/mm2) | 37.0 ± 6.3 | 26.2 ± 8.0* | 20.2 ± 10.7*# (p = 0.005) |
| CNBD (n/mm2) | 87.1 ± 34.4 | 58.1 ± 30.0* | 46.4 ± 32.5*# (p = 0.05) |
| CNFL (mm/mm2) | 26.1 ± 5.2 | 19.8 ± 5.6* | 15.7 ± 7.8*# (p = 0.01) |
| CNFT (TC) | 15.6 ± 3.6 | 19.2 ± 6.9* | 17.5 ± 8.6 |
Note
- Data are presented as mean ±SD. All symbols indicate statistically significant difference, *p < 0.01 compared to controls and #p < 0.05 compared to the patients with painless neuropathy.
- Abbreviations: CIP, cold-induced pain threshold; CNBD, corneal nerve branch density; CNFD, corneal nerve fibre density; CNFL, corneal nerve fibre length; CNFT, corneal nerve fibre tortuosity; CST, cold sensation threshold; HIP, heat-induced pain threshold; IENFD, intraepidermal nerve fibre density; NCCA, non-contact corneal aesthesiometry; NDS, neuropathy disability score; NSP, neuropathy symptom profile; PMNAP, peroneal nerve action potential amplitude; PMNCV, peroneal motor nerve conduction velocity; SNAP, sural nerve action potential amplitude; SNCV, sural nerve conduction velocity; TC, tortuosity coefficient; VAS, visual analogue scale; VPT, vibration perception threshold; WST, warm perception threshold.
Corneal sensation and nerve fibre pathology
The corneal sensation threshold was significantly higher in patients with painful neuropathy compared to control subjects (p < 0.001) and painless diabetic neuropathy (p = 0.003). CNFD, CNBD and CNFL were significantly lower (p < 0.001) in patients with type 1 diabetes compared to healthy controls and CNFD (p = 0.005), CNFL (p = 0.01) and CNBD (p = 0.05) were significantly lower in patients with painful compared to painless diabetic neuropathy (Figures 2 and 3). CNFT was significantly higher (p = 0.03) in patients with painless neuropathy compared to controls but there was no difference between patients with painful and painless diabetic neuropathy. There was no correlation between corneal nerve parameters and the severity of neuropathic pain based on the McGill visual analogue index.
DISCUSSION
The present study confirms our previous studies demonstrating that CCM can identify neuropathy in patients with diabetes.[32-34] Small fibre neuropathy is considered to underlie neuropathic pain;[35] however, symptom assessment tools[36] as opposed to measures of small fibre dysfunction or damage are advocated in the diagnosis of neuropathic pain.[37] This is because there is controversy as to whether specific features of small fibre pathology in skin biopsies[38, 39] may be related to painful neuropathy.[17, 18, 40, 41]
We demonstrate a more severe neuropathy in patients with painful neuropathy, which is consistent with our data from a large population-based study[1] and from two recent detailed phenotyping studies.[42] Most neurophysiological measures and thermal thresholds did not differ between patients with painful and painless diabetic neuropathy, in agreement with previous data.[9, 16] However, there was evidence of a greater reduction in IENFD, CNFD, CNBD and CNFL in patients with painful compared to painless diabetic neuropathy.[16, 43] The lower CNBD suggests lower nerve regeneration, which contrasts with the results of a recent study showing increased axonal regeneration and axonal swelling in patients with painful diabetic neuropathy.[17]
Identifying small fibre pathology may help in the diagnosis and identification of patients who may respond optimally to a particular therapy. Indeed in two randomized, placebo-controlled studies, the best response to treatment with oxcarbazepine[44] and lignocaine 5% patch[45] was achieved in those patients with an irritable nociceptor phenotype. We have recently shown that patients with altered rate-dependent depression, indicating reduced spinal inhibition, and a reduction in CNFD may respond optimally to therapies such as duloxetine.[43]
This is a cross-sectional study which prevents us from concluding a causal relationship between small nerve fibre damage in the skin and cornea in patients with painful diabetic neuropathy. The study was also only undertaken in patients with type 1 diabetes and the findings may not be applicable to the majority of patients with type 2 diabetes; although greater corneal nerve fibre damage has been shown in patients with type 2 diabetes and painful compared to painless neuropathy.[25] In addition, previous studies have assessed patients with type 1 and type 2 diabetes and shown greater small nerve fibre damage in painful compared to painless neuropathy.[9, 16, 24]
Corneal confocal microscopy represents a rapid, non-invasive, ophthalmic imaging technique to objectively quantify small fibre pathology and differentiate patients with painful from painless diabetic neuropathy.
ACKNOWLEDGEMENTS
This research was facilitated by the Manchester Biomedical Research Centre, the Greater Manchester Comprehensive Local Research Network and the Wellcome Trust Research Facility. Dr Mitra Tavakoli undertook corneal confocal microscopy and Dr Hassan Fadavi undertook clinical assessment and quantitative sensory testing in a proportion of the patients and control subjects.
CONFLICT OF INTEREST
There is no conflict of interest related to this work for any of the authors.
AUTHOR CONTRIBUTIONS
Maryam Ferdousi: Data curation (equal); Formal analysis (lead); Investigation (equal); Methodology (equal); Writing-original draft (lead). Shazli Azmi: Data curation (equal); Investigation (equal); Writing-review & editing (equal). Alise Kalteniece: Investigation (equal); Writing-review & editing (equal). Ioannis Petropoulos: Investigation (equal). Georgios Ponirakis: Investigation (equal). Omar Asghar: Investigation (equal). Uazman Alam: Investigation (equal). Andrew Marshall: Investigation (equal). Andrew J M Boulton: Writing-review & editing (supporting). Nathan Efron: Writing-review & editing (supporting). Handrean Soran: Writing-review & editing (supporting). Maria Jeziorska: Investigation (equal); Writing-review & editing (supporting). Rayaz Malik: Conceptualization (lead); Funding acquisition (lead); Resources (lead); Supervision (lead); Writing-review & editing (lead).
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.




