Cutaneous manifestations in Moyamoya angiopathy: A review
Abstract
Background and purpose: Moyamoya angiopathy (MA) is a progressive cerebrovascular disease with a poorly understood pathophysiology. It is mainly characterized by progressive bilateral stenosis of the terminal intracranial part of the supraclinoid internal carotid arteries and the proximal parts of the middle and anterior cerebral arteries. This results in early-onset ischemic or hemorrhagic strokes. The disease may be idiopathic (known as Moyamoya disease) or associated with other heritable or acquired conditions, including type 1 neurofibromatosis or other RASopathies, sickle cell disease, Down syndrome, or autoimmune disorders (known as Moyamoya syndrome). Apart from the brain, other organ manifestations including cutaneous ones have also been described in MA patients.
Materials and methods: A literature research on PubMed was performed for articles mentioning the cutaneous association in MA and published between 1994 and October 2020.
Conclusion: The present review summarizes the cutaneous associations as well as the coincidental dermatological findings seen in MA patients. Those include changes in the epidermis, dermis, or skin appendages for example café-au-lait spots, hypomelanosis of Ito, livedo racemosa, hemangiomas, premature graying of hair, chilblains etc.
Abbreviations
-
- AGS
-
- Aicardi-Goutière syndrome
-
- BRCC3
-
- BRCA1/BRCA2-containing complex subunit 3
-
- C1q
-
- complement component 1q
-
- CFC
-
- cardiofaciocutaneous syndrome
-
- CLS
-
- café-au-lait spots
-
- CMN
-
- congenital melanocytic nevi
-
- CNS
-
- central nervous system
-
- GNAQ
-
- guanine nucleotide-binding protein G(q) subunit alpha protein
-
- GNA11
-
- guanine nucleotide-binding protein G(q) subunit alpha-11 protein
-
- GUCY1A3
-
- guanylate cyclase soluble subunit alpha-3 enzyme
-
- IKBKG
-
- inhibitor of kappa beta kinase gamma
-
- IP
-
- incontinentia pigmenti
-
- MA
-
- Moyamoya angiopathy
-
- MAPK
-
- mitogen-activated protein kinase
-
- MGDA
-
- morning glory disc anomaly
-
- MOPD II
-
- Majewski osteodysplastic primordial dwarfism type II
-
- MRA
-
- magnetic resonance angiography
-
- MRI
-
- magnetic resonance imaging
-
- MRVI1
-
- murine retrovirus integration site 1 homolog
-
- MTCP1
-
- mature T-cell proliferation 1
-
- MTCP1NB
-
- mature T-cell proliferation 1 neighbor protein
-
- NF1
-
- neurofibromatosis type 1
-
- PCNT
-
- pericentrin protein
-
- PHACE
-
- posterior fossa anomalies, hemangioma, arterial lesions, cardiac abnormalities/coarctation of the aorta, eye anomalies
-
- PPV
-
- phakomatosis pigmentovascularis
-
- Rasopathies
-
- syndromes associated with Ras pathway mutations
-
- RNF213
-
- RING finger protein 213
-
- SAMHD1
-
- SAM and HD domain containing deoxynucleoside triphosphate
-
- SHOC2
-
- leucine-rich repeat scaffold protein
-
- SLE
-
- systemic lupus erythematosus
-
- SPRED1
-
- Sprouty-related EVH1 domain-containing 1
-
- SS
-
- Sneddon syndrome
-
- STA-MCA
-
- superficial temporal artery to middle cerebral artery
-
- YAP
-
- Yes-associated protein (transcriptional regulator)
INTRODUCTION
Moyamoya angiopathy (MA) is a cerebrovascular condition characterized by progressive bilateral stenosis of the terminal part of the supraclinoid internal carotid arteries and proximal branches of the middle and cerebral arteries, as well as the development of a fragile collateral network [1]. The diagnosis is based on clinical and radiological criteria which include the presence of bilateral internal carotid artery occlusions on cerebral angiography. The diagnosis should be supported by cerebral magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) [2]. The pathogenesis of MA is still not fully understood. A genetic component is plausible, given the observed variations of the RING finger 213 gene (RNF213) in East Asian MA patients, the ethnic differences of the diseased population, the association with several monogenic disorders, and the high familial rate [3]. Nevertheless, endogenous factors, such as blood flow, or exogenous factors, such as autoimmune disorders and infections, also seem to play a role in disease etiology. An imbalance of angiogenic factors has also been implicated [4]. MA can be idiopathic, also called Moyamoya disease, or part of a systemic syndrome (Moyamoya syndrome).
The most common disease features are cerebrovascular events, both ischemic and hemorrhagic [5, 6]. Although MA primarily affects the vessels of the brain, a systemic involvement has also been observed, affecting, for example, the kidneys (renal artery stenosis), eyes (optic nerve malformations) [7], musculoskeletal system (facial dysmorphia) [8], and skin. Cutaneous manifestations often go unnoticed by physicians. They may represent either a known association or, in some cases, a mere coincidence. In cases where disease involvement goes beyond the brain vessels, the term Moyamoya syndrome is traditionally used. However, recent studies have shown that, even in cases previously thought to be idiopathic, systemic signs, in addition to brain vessel involvement, can be observed [8, 9]. This leads to the assumption that the disease probably involves more organs than previously thought. In the present review, we outline the dermatological manifestations associated with MA. The recognition of such lesions may be useful in identifying misdiagnosed syndromes and should lead to the initiation of proper treatment.
METHODS
We performed a literature search in PubMed for articles published between 1994 and October 2020 using the search terms “Moyamoya”, “Moyamoya syndrome”, “Moyamoya disease”, “Moyamoya arteriopathy” AND “cutaneous manifestations”, “skin lesions”, “neurofibromatosis type 1”, “neurofibroma”, Raynaud's”, “PHACE syndrome”, “loose skin”, “malar rash”, “Becker nevus”, “chilblain”, “livedo racemosa”, “hemangioma”, “Café-au-lait”, “hypomelanosis of ito”, “phakomatosis pigmentovascularis”, “hair graying”, “melanocytic nevi” and “capillary malformation”.
Established cutaneous manifestations in Moyamoya syndromes
Café-au-lait macules, neurofibromas and axillary/inguinal freckling
Neurofibromas are benign tumors of nerves and skin presenting as skin-colored papules or nodules (Figure 1). Café-au-lait spots (CLS) are areas of hyperpigmentation that typically include pale or light brown macules. Both, along with freckling in intertriginous areas such as the axillary (Figure 2) and inguinal regions, represent diagnostic criteria of neurofibromatosis type 1 (NF1). NF1 is an autosomal dominant neurocutaneous disorder caused by a mutation of a gene on chromosome 17 coding for a protein called neurofibromin. The diagnosis of NF1 is based on clinical criteria established by the National Institute of Health [10]. The skin changes in NF1 are often evident in the first year of life and tend to increase in size and number over time.
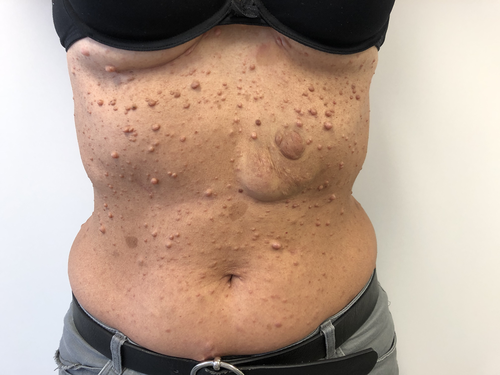
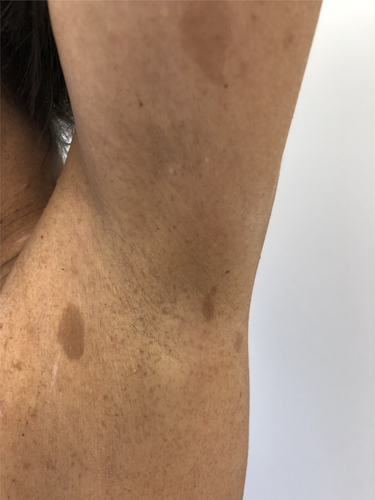
The association between MA and NF1 has been reported in several cases. The prevalence of MA in NF1 pediatric patients is approximately 0.6% [11, 12]. Furthermore, MA was found in almost 50% of patients with both cerebral vasculopathy and NF1 [3]. Despite the proximity of the NF1 gene (17q11.2) to the RNF213 gene (17q25) involved in familial Moyamoya disease; the role of the NF1 in the occurrence of MA is controversial [13]. Nevertheless, it was suggested that the lack of neurofibromin expression may account for the proliferation of vascular smooth muscle cells, which is responsible for the development of obstructive vascular diseases. Variants of the murine retrovirus integration site 1 homolog (MRVI1) gene might represent a genetic susceptibility factor for MA in NF1 patients of European ancestry [14]. CLS have also been reported in Majewski osteodysplastic primordial dwarfism type II (MOPD II), a pediatric disease caused by loss of function of the pericentrin protein gene (PCNT). In this case, CLS appear more frequently with age and could reach up to 2 cm in diameter [15]. They have also been described in Legius syndrome in association with MA. Legius syndrome shares clinical features of both NF1 regarding skin manifestations and Noonan syndrome regarding macrocephaly and facial dysmorphism. It is associated with Sprouty-related EVH1 domain-containing 1 (SPRED1) mutations involved in growth factor-regulating cascades [16].
Hypomelanosis of Ito
Hypomelanosis of Ito is a neurocutaneous condition characterized by bizarre, swirly hypopigmentations along the Blaschko skin lines of the trunk and occasionally the extremities. These lines represent those of normal cell devolvement in the skin. Hypomelanosis of Ito is associated with neurological and musculoskeletal disorders in approximately 33%–94% of patients [17]. The most common neurological abnormalities are autistic behavior, seizures, and mental retardation. Craniofacial abnormalities such as a high forehead, a depressed nasal bridge, and hypertelorism have also been described. Hypomelanosis of Ito has been sporadically associated with MA in young female patients [18, 19]. Karyotyping is recommended in patients presenting with this neurocutaneous disorder as it has been associated with chromosomal aberrations including trisomy 18 [20].
Livedo racemosa and Raynaud's phenomenon
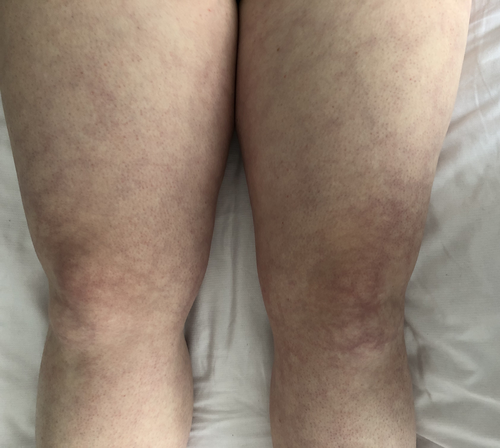
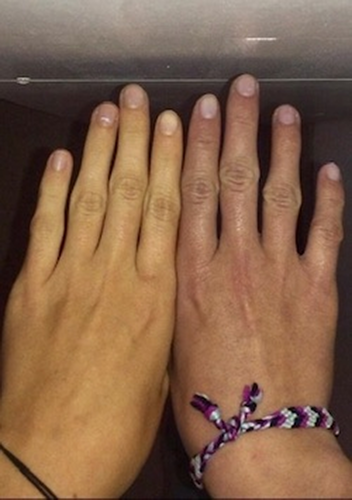
Livedo racemosa is a pathological, netlike violaceous skin pattern that persists in warmth due to an intraluminal or vessel wall pathology [21] (Figure 3). It has an incidence of up to 12.8% in MA patients, thus reflecting a possible sign of global vasomotor dysfunction [22, 23]. Livedo racemosa is also one of the hallmarks of Sneddon syndrome (SS) both in association with phospholipid antibodies or not. SS is a rare vasculopathy with infrequent reports of MA in affected patients [24, 25]. At present, it is not known whether there is a common pathogenic mechanism for both SS and MA or whether their association is merely coincidental. It has been hypothesized that a chronic state of hypoxia, consequent to SS vasculopathy, causes dynamics alterations leading to Moyamoya vessel pattern [24]. Although not specifically observed in MA, NOTCH3 gene mutations have been found in SS familial cases with pediatric strokes, supporting the hypophysis that the loss of NOTCH3 signaling may be an underlying disease mechanism for SS [26]. Other syndromes known to be associated with MA, such as MOPD II, can also present with livedo racemosa [27]. Raynaud's phenomenon is a condition in which a decrease in blood flow often leads to whitish then bluish discolorations of the fingers and/or toes. Another disease in which Raynaud's phenomenon and livedo are simultaneously found is autosomal recessive and characterized by loss of function mutations in GUCY1A3, the gene encoding α1β1 soluble guanylate cyclase, a major nitric oxide receptor. Here, Raynaud's phenomenon is sometimes diffuse and can involve whole fingers, hands, toes, and feet (Figure 4). No significant abnormality is detected upon a capillaroscopic examination. The GUCY1A3 mutation commonly leads to MA and achalasia [22]. The latter occurs very early in life and often requires surgery. The penetrance of MA in this disease is close to 50%, causing childhood-onset strokes. The fact that Raynaud's phenomenon and livedo are observed could suggest the presence of microvascular dysfunction.
Facial hemangiomas in PHACE syndrome
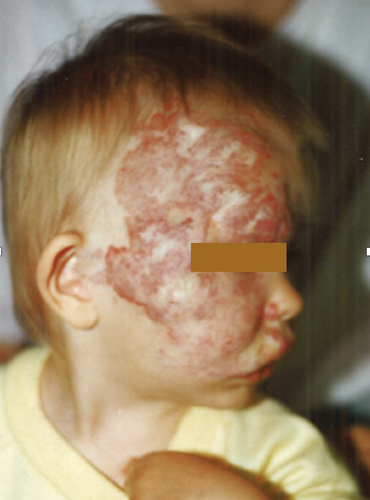
Large infantile hemangiomas of the face, neck, and/or scalp, that are associated with developmental defects, are characteristic of PHACE syndrome (posterior fossa anomalies, hemangioma, arterial lesions, cardiac abnormalities/coarctation of the aorta, eye anomalies) [28]. The larger the hemangioma, the higher the risk of having PHACE syndrome [29]. An example of a violaceous, well-circumscribed congenital plaque on a child with MA and PHACE syndrome can be seen in Figure 5. Congenital cerebral vascular malformations are the most frequent extracutaneous manifestations of this syndrome [30]. Despite the numerous cases in the literature, only a few with MA have been reported so far. Progressive arterial occlusion and stenosis have been reported in approximately one-fifth of cases with PHACE syndrome [31]. Structural brain abnormalities, congenital heart disease, aortic arch as well as brachiocephalic abnormalities can also be seen. RNF213 heterozygous variants in a child with PHACE and MA have been reported, suggesting a possible role of RNF213 also in the etiology of PHACE syndrome [32]. However, Hadisurya et al. [30] did not find RNF213 variants in a German patient with PHACE syndrome.
Melanocytic lesions in phakomatosis pigmentovascularis
Birthmark lesions, including Mongolian spots (bluish-black lesions predominantly on the lumbosacral area), nevus spilus (congenital nevus with a speckled appearance), and nevus anemicus (a localized area of pale skin) are, along with vascular malformations, the characteristics of a congenital syndrome known as phakomatosis pigmentovascularis (PPV). It is classified into four types (I to IV) depending on the presence of certain skin lesions [33]. The presence of an extracutaneous manifestation confers the letter “b” to the disease type. Tsuruta et al. [34] described the case of a Japanese baby with MA, nevus flammeus, and nevus spilus, classified as PPV Type III b. A true association between PPV and MA has been postulated because of the vascular abnormalities present in both. Recent genetic studies showed mutations in GNA11 and GNAQ genes encoding for G proteins in patients with PPV and MA. Interestingly, transgenic mosaic zebrafish models expressing mutant GNA11 developed dermal melanocytosis [35].
Thin, loose anagen hair in Noonan-like syndrome
Loose anagen hair is a hair disorder characterized by loose, thin hair that can be easily and painlessly plucked from the scalp. Loose anagen hair has been observed both in MA and in Noonan syndrome, which is an autosomal dominant disorder characterized by typical facial features (deep groove in the area between the nose and the mouth, widely spaced eyes, low set ears, and micrognathia), short stature skeletal anomalies, heart defects, mental retardation, and undescended testis [36]. Noonan syndrome has been reported in a few MA cases [37]. Noonan-like syndrome with loose anagen hair as well as MA has been associated with mutations in the SHOC2 (leucine-rich repeat scaffold protein) gene [38]. Along with loose hair, those patients tend to have other cutaneous manifestations such as hyperpigmentations and hairless skin [39]. Another syndrome with thin hair, characterized by growth failure, ectodermal abnormalities, and heart defects is cardiofaciocutaneous syndrome (CFC). The latter has also been associated with MA. Although there is a phenotypic overlap between Noonan syndrome and CFC, neck abnormalities and familial occurrences are rare in CFC [40].
Constellation of loose and dark skin with deep palmar and plantar creases
Costello syndrome has also been associated with MA. It has similar features to Noonan syndrome and CFC [41]. Nevertheless, low-set ears with large/thick lobes, thick lips, nasal papilloma, loose skin on hands and feet, dark skin, deep palmar and plantar creases, and hyperkeratotic palms and soles are frequently present in Costello syndrome and infrequently in the other two [42]. Loose and dark skin is pathognomonic for Costello syndrome. Because of the reduced expression of elastin messenger ribonucleic acid, a disturbed elastogenesis has been implicated in this disease [43].
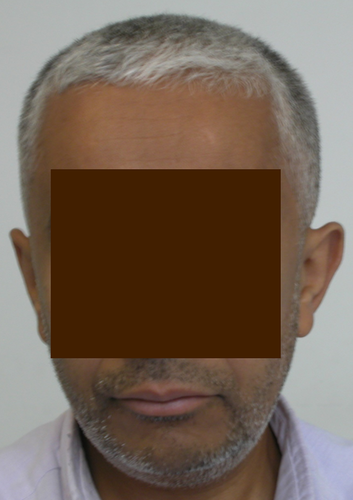
Canities
Canities or premature graying of hair has been observed in patients with an X-linked Moyamoya syndrome characterized by Xq28 deletions. These overlapping deletions remove mature T-cell proliferation 1 (MTCP1)/mature T-cell proliferation 1 neighbor protein (MTCP1NB) and BRCA1/BRCA2-containing complex 3 (BRCC3). BRCC3 is a deubiquitinating enzyme involved in angiogenesis. This Moyamoya syndrome is characterized by the association of MA with hypergonadotropic hypogonadism, short stature, dilated cardiomyopathy, premature coronary heart disease, early bilateral acquired cataract, and a stereotyped facial dysmorphism. Over 75% of these patients display significant graying of the hair around the age of 25 years (Figure 6). Further studies are needed to confirm that this observation is not an incidental finding, since the molecular and genetic etiology of premature graying of hair is poorly studied, its pathophysiology in this Moyamoya syndrome remains unknown [3].
Vesicles, verrucae, hyper- then hypopigmentation: the stages of incontinentia pigmenti
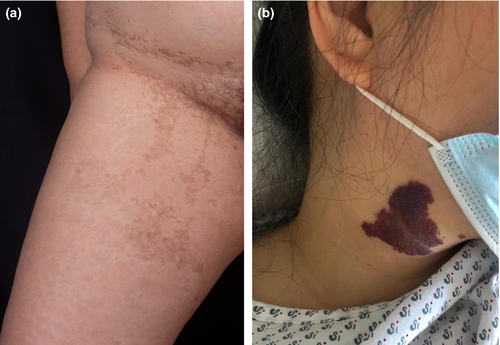
Incontinentia pigmenti (IP) is an X-linked genodermatosis caused by mutations in the inhibitor of kappa beta kinase gamma gene (IKBKG) on locus Xq28 [44]. This disease is typically lethal in males. Its first manifestations are dermatologic and develop in four stages, along the Blaschko lines. The stages usually appear sequentially, however, they may overlap [45]. In the first few weeks of life, the vesiculobullous stage starts, followed by the verrucous, the hyperpigmented (Figure 7a), and finally the hypopigmented stages.
Neurologic manifestations include seizures, learning disabilities, motor delay, hemiplegia, cerebellar ataxia, microcephaly, encephalomyelitis, and pediatric ischemic strokes [46]. MA and IP share several clinical similarities. Specifically, corpus callosum abnormalities, mainly hypoplasia, are seen in both diseases [23]. However, in IP, collateral vessels are not noted, resembling MA in Suzuki stage I [47]. Interestingly, a reported MRA of a 4-year-old patient with IP demonstrated partial occlusion of the left middle cerebral artery [48], indicating a Moyamoya-like pattern. Other patients with IP and MA have been described [49]. Minić et al. [50] hypothesized that the central nervous system (CNS) lesions in IP are distributed in lines analogous to the Blaschko skin lines.
Incidental dermatological associations
Hemangioma and capillary malformations
Hemangioma is a benign vascular tumor caused by the proliferation of vascular endothelial cells. It appears clinically as an asymptomatic firm, blue, red or purple plaque or papule. Its most common form is infantile. Several cases of facial hemangiomas in association with MA and morning glory disc anomaly (MGDA) have been described. MGDA is a congenital condition characterized by an abnormal optic disc with whitish glial tissue at the center and radial blood vessels emerging from the rim of the disc [51]. An embryonic origin, mainly mesectodermal dysgenesis, has been postulated as the culprit of extensive capillary hemangiomas seen in association with MGDA [52].
Interestingly, similarly to PPV, mutations in GNAQ or GNA11 have also been reported in congenital hemangiomas as well as those that arise later in life, leading to the constitutive activation of mitogen-activated protein kinase (MAPK) and/or Yes-associated protein (YAP) signaling [53]. Nevus flammeus or port-wine stains are the most common head and neck vascular malformation, with a similar clinical appearance to infantile hemangioma, however, they do not blanch under pressure [54]. A case of MA in the setting of Sturge-Weber syndrome accompanied by a large port-wine stain on the face has also been described [55]. An example of nevus flammeus in a young patient with MA can be seen in Figure 7b.
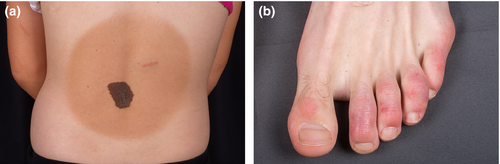
Congenital melanocytic nevi and Becker's nevus
Congenital melanocytic nevi (CMN) are benign proliferations of cutaneous melanocytes (example in Figure 8a). They are usually present at birth and are caused by abnormal growth, development, or migration of melanoblasts. Their incidence among newborns is approximately 1% [56]. Puri et al. [57] described a case of a young man with Hirayama disease (or juvenile muscular atrophy of the distal upper extremity), Moyamoya vessel pattern, and a CMN on the neck. The coexistence of these three manifestations was postulated to represent an unknown syndrome. In the case of large CMN, brain and spinal MRI should be performed to rule out neurocutaneous melanosis [58]. Becker's nevus is a late-onset epidermal nevus mainly found on the torso and shoulders. It has also been described in a Japanese girl with both MA and NF1. She had hairy pigmented macules in the form of Becker's nevus on the forearms [59].
Those melanocytic lesions are usually segmental and mainly present in the pediatric population. It is important to differentiate between isolated epidermal nevi and oculoneurocutaneous syndromes characterized by somatic cutaneous mosaicism. The mosaicism for mutations in genes involved in the Ras/MAPK signaling pathways is the cause of a group of disorders termed mosaic RASopathies, for example, oculoectodermal syndrome and Schimmelpenning-Feuerstein-Mims syndrome [60].
Malar rash
Malar rash, also known as the butterfly rash, is an erythematous rash on the cheeks and nose. This condition is mainly seen in patients with systemic lupus erythematosus (SLE) and has been described in SLE patients with MA. CNS vasculitis in the setting of lupus with malar rash, resulting in large vessel occlusions and MA, has been described in a handful of reports [61]. CNS manifestations of SLE include neuropsychiatric symptoms, seizures, or aseptic meningitis [62]. In the context of SLE, MA has been associated with complement component 1q (C1q) deficiency [63]. Interestingly, more than 90% of patients with C1q deficiency develop SLE [64].
Chilblains
Chilblains are an inflammatory skin condition presenting as painful or itchy erythematous or livedoid lesions after cold exposure, on the toes and fingers. After cold exposure, chilblain lesions have been described in many individuals affected by Aicardi-Goutière syndrome (AGS), which is a neurodevelopmental disorder characterized by a loss of function mutation in the SAMHD1 (SAM and HD domain containing deoxynucleoside triphosphate) gene on 20q11.3. MA is seen in AGS and is present in approximately 50% of mutation carriers [3]. SAMHD1 is implicated in immunoregulation and cerebral vascular hemostasis. Furthermore, SAMHD1 gene mutations have been described in an autosomal recessive condition with cerebral vasculopathy and a highly heterogeneous phenotype with a developmental disability, Moyamoya artheriopathy, acrocyanosis, Raynaud's, and chilblain lesions. A similar constellation of symptoms in association with SAMHD1 mutation was found in 14 Amish individuals without AGS [65]. An example of chilblain lesions is depicted in Figure 8b in a young patient with chilblain lupus erythematosus.
DISCUSSION AND CONCLUSION
Moyamoya angiopathy is the most common cause of stroke in Asian children and young adults. MA may be idiopathic or associated with coexistent or heritable diseases. These include NF1, Noonan syndrome, sickle cell anemia, polyarteritis nodosa, Down syndrome, Fanconi anemia, as well as autoimmune or inflammatory diseases. The identification of such disorders is important to implement correct therapy and prophylactic measures as well to provide genetic counseling for patients and their families in cases of heritable disorders.
The disorders associated with MA can be characterized by several systemic features including facial dysmorphisms, cardiac abnormalities, developmental delay, ophthalmologic disorders, short stature, macrocephaly, short neck, and various skin manifestations. The latter include pigmentation abnormalities, hairless skin, livedo racemosa, hemangiomas, malar rash, melanocytic nevi, hair graying, and neurofibromas. Herein, we reported the main cutaneous manifestations observed in MA, supporting physicians in the search and identification of possible underlying disorders in association with MA. The recognition of occult MA in patients manifesting with primary skin lesions may lead to stroke prevention through initiating early antiplatelet therapy or even indicating superficial temporal artery to middle cerebral artery (STA-MCA) bypass surgery [66-68].
For this reason, MA patients should undergo, not only a neurologic evaluation, but also a detailed medical history, physical examination including ophthalmic, skeletal, cardiac signs, but also an examination of the skin, hair and nails; both in the first visit as well as in follow-ups. MA patients with skin lesions, especially if associated with systemic features, should merit further investigations. For instance, MA patients with CLS should be investigated for neurofibromas, freckles in the axillary or inguinal regions, optic nerve glioma, Lisch nodules, or cortical thinning of the long bones. PHACE syndrome should be suspected in children with large segmental hemangiomas in the cervical-facial region. These subjects should undergo echocardiography, ophthalmological evaluation, radiographic imaging of the bones, and especially duplex ultrasonography or MRA to detect MA.
Livedo racemosa could support a coexistent SS or phospholipid antibodies syndrome and should, in the presence of neurological symptoms, raise the suspicion of MA [24]. A careful family history collection and investigation of similar skin signs in relatives is also essential to identify heritable traits. Contrarily, infants or children presenting with skin lesions, especially birthmarks, both in the presence or absence of associated systemic features, might require neuroimaging to exclude MA.
The exact etiological link between these skin lesions and MA is unknown. Since they are only reported in Moyamoya syndromes, they are likely more related to the syndrome itself then to MA. Although they might only represent an incidental finding, a possible association between those disorders and MA can be traced back to embryology. As previously mentioned, MGDA, which is often associated with Moyamoya, may be caused by mesenchymal dysgenesis of the cerebral vasculature which can also be responsible for hemangioma formation. Albrecht et al. [9] found a reduction of optic nerve head volume and of retinal nerve fiber layer in patients with idiopathic MA compared to control patients. The authors raised the question of whether the optic nerve anomalies observed in patients with idiopathic MA and MGDA could occur during embryogenesis as a primary mesenchymal defect or primary neuroectodermal dysgenesis with resulting mesodermal changes [69]. Minić et al. [50] hypothesized that the CNS lesions in IP are distributed in lines analogous to the Blaschko skin lines since MRI findings in IP point to the radial unit hypothesis in embryogenesis and not to vascular territories. Since there is a common embryonic origin of skin, CNS, and eyes; these authors also propose that CNS Blaschko line analogs, similar to those in the skin, represent the trace of the development of clones of neurons and glial cells arising from a cell with IKBKG mutation. Lastly, they argued that parenchymal abnormalities were most severe in patients with severe neonatal cutaneous lesions, especially if they were located on the scalp [45, 70]. Moreover, the extracutaneous “lines of Blaschko” have also been described in the brain and the eye, reinforcing the concept of mosaicism in the presentation of X-linked disorders [45, 50].
Notably, mild facial abnormalities and cutaneous signs were found in MA patients normally interpreted as idiopathic [8]. These findings argue for a wider spectrum of systemic symptoms in patients traditionally labeled as idiopathic MA having “only” bilateral intracranial stenoses.
At the molecular level, the association between cutaneous lesions and MA could be explained by the proliferation of smooth muscle cells and their migration from medial to intimal linings of the vessels, in MA. Elevated levels of basic fibroblast growth factors, soluble adhesion molecules, cellular retinoic acid-binding protein I, and hepatocyte growth factor were found in MA patients [6].
Common genetic pathways can also be hypothesized in heritable conditions associated with MA. Genetic studies showed that DNA maintenance and the DNA damage response/repair may also play a central role in MA and the development of associated heritable conditions [71, 72]. Pericentrin mutations in Seckel syndrome and MOPD II, both systemic diseases associated with MA, also argue for a crucial role of the DNA damage repair system in the pathophysiology of MA [72]. The above-mentioned Xq28 deletion in three families with MA results in loss of BRCC3, which results in defective angiogenesis in zebrafish [73]. Furthermore, the NFKB pathway could play a role since mutations in the IKBKG on Xq28 also cause the various cutaneous, CNS, bone, and ocular manifestations of incontinentia pigmenti [45]. The associations with other RASopathies (NF1, Noonan, Costello, Legius syndrome) support a possible role for dysregulation of the Ras/MAPK pathway in the pathogenesis of MA [16]. The role of the so-called “Moyamoya disease factor: RNF213” in Asian MA is still unclear. The RNF213 gene encodes a RING finger protein that possesses both ubiquitin ligase activity and ATPases associated with diverse cellular activities [74]. A founder mutation in this gene is mainly found in familial and sporadic patients with MA in Japan and Korea. Experimental data suggested multiple pathogenic mechanisms involving endothelial function, smooth muscle cell proliferation, inflammatory signaling pathways, hemostasis, angiogenesis, and vascular remodeling [74-77]. Currently, the exact role of RNF213 in vascular disorders remains controversial. Furthermore, it has not been shown to play a role in the pathology of skin lesions.
Recently, MRVI1 was identified as a potential susceptibility gene of MA in NF1 [14]. In response to nitric oxide, MRVI1 induces the relaxation of smooth muscle cells of the colon and aorta. MRVI1-knockout mice display a gastrointestinal phenotype that partially overlaps that observed in humans, intracranial vessels were not investigated in the study [78]. MRVI1 also plays an important role in platelet adhesion, secretion, and aggregation [79]. However, the exact role of MRVI1 gene in the pathophysiology of skin lesions is yet to be explored.
Overall, these pathophysiological mechanisms might also play a role in skin lesions and also systemic manifestations of MA. Although further studies are necessary to clarify the exact relationship between cutaneous lesions and MA, skin lesions widen the clinical spectrum of MA, making the multidisciplinary approach, including neurologists but also geneticists, dermatologists and cardiologists, crucial not only for a correct diagnosis but also for the management of all MA features.
ACKNOWLEDGMENTS
None. Open Access funding enabled and organized by Projekt DEAL.
WOA Institution: RUPRECHT KARLS UNIVERSITAET HEIDELBERG
Blended DEAL: Projekt DEAL
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Fouad Mitri: data curation (equal); methodology (equal); writing -- original draft (equal), writing -- review and editing (equal); Anna Bersano: data curation (equal), writing -- original draft (equal), writing -- review and editing (equal); Dominique Hervé: writing -- original draft (equal); Markus Kraemer: conceptualization (equal); methodology (equal); writing -- review and editing (equal).
ETHICAL APPROVAL
Formal institutional board approval was not needed, the review was performed using literature research tools. The principles outlined in the Declaration of Helsinki were followed.
CONSENT OF PUBLICATION
All authors agree to the publication of this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing does not apply to this article as no new data were created or analyzed in this study.




